Abstract
Objective
To inverse-localize epileptiform cortical electrical activity recorded from severe traumatic brain injury (TBI) patients using electroencephalography (EEG).
Methods
Three acute TBI cases were imaged using computed tomography (CT) and multimodal magnetic resonance imaging (MRI). Semi-automatic segmentation was performed to partition the complete TBI head into 25 distinct tissue types, including 6 tissue types accounting for pathology. Segmentations were employed to generate a finite element method model of the head, and EEG activity generators were modeled as dipolar currents distributed over the cortical surface.
Results
We demonstrate anatomically faithful localization of EEG generators responsible for epileptiform discharges in severe TBI. By accounting for injury-related tissue conductivity changes, our work offers the most realistic implementation currently available for the inverse estimation of cortical activity in TBI.
Conclusion
Whereas standard localization techniques are available for electrical activity mapping in uninjured brains, they are rarely applied to acute TBI. Modern models of TBI-induced pathology can inform the localization of epileptogenic foci, improve surgical efficacy, contribute to the improvement of critical care monitoring and provide guidance for patient-tailored treatment. With approaches such as this, neurosurgeons and neurologists can study brain activity in acute TBI and obtain insights regarding injury effects upon brain metabolism and clinical outcome.
Keywords: electroencephalography, traumatic brain injury, localization, monitoring, outcome, epilepsy
Introduction
Electroencephalography (EEG) plays an important role in the treatment of critically ill patients [1, 2], in the monitoring of acute traumatic brain injury (TBI) [3, 4] and in the preoperative planning of epileptogenic focus removal [5–7]. The use of continuous EEG (cEEG) is particularly important in the neurointensive care treatment of patients with TBI and with status epilepticus, where cEEG can allow clinicians to determine treatment effectiveness in patients undergoing continuous infusion of antiseizure drugs [8]. The Neurocritical Care Society has suggested that cEEG, rather than serum drug levels, should guide therapy of refractory status epilepticus [8], which highlights the importance of this method in the acute care of patients with epileptic seizures.
Recent research on acute TBI pathophysiology has led to renewed interest in the potential use of cEEG to improve TBI outcomes [3, 9, 10]. When combined with physiologically driven decision making via multimodal brain monitoring, EEG can aid neurointensivists to determine when the brain is at risk for injury and whether clinical intervention is warranted to prevent permanent brain damage [11]. Unfortunately, though scalp EEG can provide much clinically useful information, its spatial resolution is too low for the task of resolving the detailed spatial patterns of electric activity in the hours and days following brain trauma. This makes it difficult to determine which specific brain locations exhibit TBI-related pathophysiology, which largely precludes the integration of EEG with structural neuroimaging methods such as magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) to improve clinical decision making.
In the context of the present article, inverse localization involves the process of computationally estimating the locations, orientations and strengths of the electric currents in the brain which generate EEG signals. As a consequence of being a noninvasive method for identifying the sources of brain activity, EEG inverse localization has been used extensively in the past to identify and to study the neurophysiological correlates of phenomena such as sleep, cognition and affect [12, 13].
Given the past and present usefulness of EEG in the context of acute TBI clinical care, the absence of neurological and neurosurgical insights derived from EEG inverse localization may equate to missed opportunities to track acute injury evolution both spatially and temporally, with possibly negative consequences upon the formulation of treatment decisions for TBI patients. Thus far, the use of inverse localization methods in TBI has been extremely limited because standard source localization techniques can generate inaccurate results in the presence of pathology. The anatomy of the TBI head and the spatial variations in its conductivity have previously been challenging to take into account, and EEG inverse localization has been used very seldom in the TBI research community, let alone the neurointensive care setting.
In this paper, we demonstrate the use of anatomically precise models derived from multimodal MRI to localize epileptiform electrical activity recorded noninvasively from severe TBI patients using scalp EEG. Our contribution illustrates a realistic, patient-specific approach to TBI source localization and the investigation itself can be appropriately conceptualized as a proof-of-concept study to assess the feasibility of the implemented method.
Materials and Methods
Participants included three males of ages 31, 25 and 45, respectively, from whom MRI volumes were acquired at 3.0 Tesla (1 mm3 voxel size, Siemens Trio TIM scanner, Erlangen, Germany) within 72 hours after injury. Although the Glasgow coma scale (GCS) scores of the three patients upon admission to the neurointensive care unit (NICU) were 9, 14 and 14, respectively, their Glasgow outcome scale (GOS) score upon transfer from the NICU was 3 for all patients, reflecting the severity of their injuries as well as the decline of their clinical condition subsequent to hospital admission. The study was approved by the Institutional Review Board of the School of Medicine at the University of California, Los Angeles, and signed informed consent was obtained from the patients’ legally authorized representatives prior to the performance of any procedure (UCLA IRB approval #10-000929 dated 11/8/2012). The three subjects are examples of TBI patients with progressive lesion loads and were selected for the study based on (1) the type, location and spatial extent of their lesions, as well as (2) the presence of epileptiform discharges in their cEEG recordings, as identified in the EEG recordings subsequent to their acquisition (see below).
The TBI neuroimaging protocol is described extensively elsewhere [14]. Briefly, acquired MRI sequences included magnetization prepared rapid acquisition gradient echo (MP-RAGE) T1-weighted imaging, fluid attenuated inversion recovery (FLAIR), turbo spin echo (TSE) T2-weighted imaging, gradient-recalled echo (GRE) T2-weighted imaging, and susceptibility weighted imaging (SWI). Conventional computed tomography (CT) scans were also acquired. Image alignment, bias field correction and skull stripping were performed using the LONI (Laboratory of Neuro Imaging) Pipeline (http://pipeline.loni.ucla.edu). White matter (WM), gray matter (GM), cerebrospinal fluid (CSF), cerebellar WM/GM and subcortical structures were segmented in FreeSurfer (FS) [15], and manual correction of tissue labeling errors was performed by three experienced users with training in neuroanatomy. TBI-related lesions were segmented from GRE/SWI/FLAIR volumes as detailed elsewhere [14], skin was segmented from T1 MRI, and hard bone was segmented from CT. Eyes, muscle, cartilage, mucus, nerves, teeth, and ventriculostomy shunts were segmented from T1/T2 MRI. 3D models and visualizations were created using 3D Slicer (slicer.org) [16].
Acquisition of cEEG recordings from each patient was performed in the NICU at 250 Hz over three consecutive days using a standard referential electrode montage. Because scalp EEG potentials are due to electrical currents within the apical dendrites of cortical pyramidal neurons [17], EEG generators were modeled as dipolar currents oriented perpendicular with respect to the cortical surface. A total of 25 tissue types with distinct conductivity values were modeled, including healthy-appearing and edematous skin, fat, hard and soft bone, cerebrospinal fluid (CSF), healthy-appearing and edematous GM, healthy-appearing and edematous WM, cerebellum, spinal cord, subcortical structures, epidural hemorrhages, connective tissue, muscle, eyes, cartilages, mucus, nerves, teeth, silicone polyurethane (the manufacturing material of the ventriculostomy shunts), and sinus air. After co-registering the head and all sensor locations, each head volume was discretized into volume elements from which finite element method (FEM) models were generated [18, 19]. For each subject, a regular grid-based mesh (~400,000 nodes, ~450,000 linear elements, ~2 mm average edge length) was created and the so-called forward matrix (the values of the electric potential at each sensor due to every cortical source) was computed [18].
The inverse localization technique employed has been widely used for the study of epilepsy [20–22] and its technical details have been comprehensively explored elsewhere [23, 24], particularly in our previous publication [25]. Briefly, source localization is performed using a minimum-norm inverse linear operator [26] which seeks to minimize the expected difference between the estimated and the true inverse solution. The localization accuracy of each model can be quantified using the localization error (LE) measure, defined as the distance from the estimated source location to the true source location [27]. Previous results on our TBI-specific implementation [25] indicate that the latter can localize cortical sources in the presence of brain injury with an approximate LE in the range of ~0.5–1.5 cm for relatively superficial sources (i.e. most gyri and sulci), and ~1.5–2.5 cm for deep sources (e.g. insula and cingulate cortex). To visualize the results of the source localization process, the inverse estimate of the cortical activity can be mapped onto the surface of a reference brain using t scores, such that the magnitude of t associated with some cortical location indicates the likelihood for that location to be electrically active. The locations most likely to be active have |t| > 4. The sign of t indicates whether the localized electric current is oriented out of (t > 0) or into (t < 0) the cortex (see Figures 2–3 for details and examples).
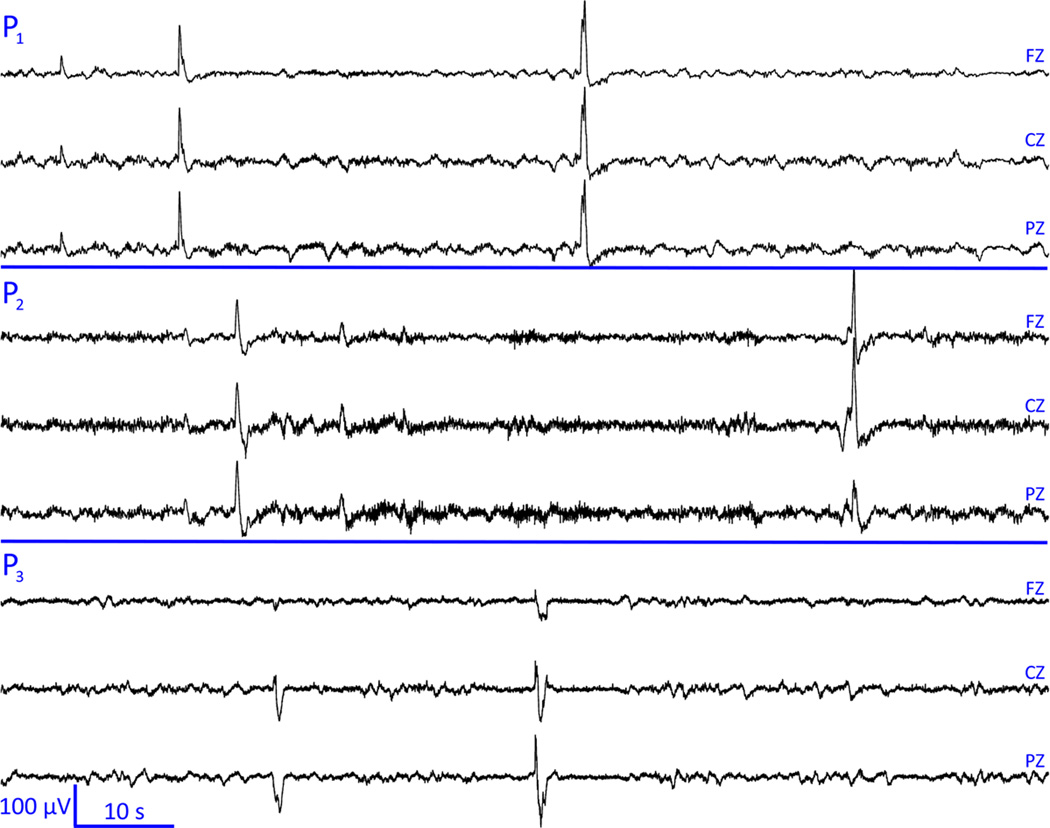
Figure 2.
Sample 60-second EEG recordings for three representative sensors (FZ, CZ and PZ) in each of the three patients denoted as P1, P2, and P3, respectively. Note, in each case, the aperiodic epileptiform spikes and their large magnitudes compared to the rest of the recording.
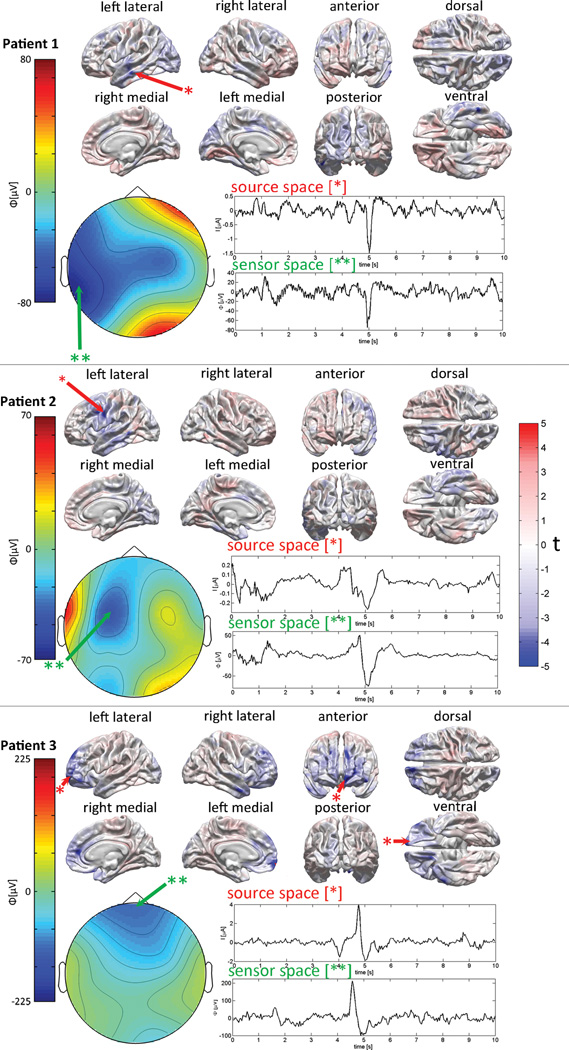
Figure 3.
Examples of inverse localization in three acute TBI patients. The localization technique is used to identify the cortical source responsible for the generation of an EEG waveform containing a large-magnitude deflection (often referred to as a “graphoelement” in the EEG literature). For each subject, the waveform of the EEG potential, Φ, being localized is shown for an interval of 10 s. Localization is illustrated at the time point with a latency of 5 s with respect to the beginning of the waveform. The EEG potentials recorded at this time point are mapped over the scalp using the interpolated values of the potentials measured at each sensor. The amplitudes of the potential Φ are measured in µV and different ranges are used for the topographic map of each subject to emphasize scalp differences in potential which are specific to each subject. The inverse estimate of the cortical activity responsible for the graphoelement is mapped onto the surface of a reference brain as t scores (see Methods section). The locations most likely to be active have |t| > 4. The sign of t indicates whether the localized electric current is oriented out of (t > 0, red hues) or into (t < 0, blue hues) the cortex. A single color map is used for t in all subjects, and color intensity indicates likelihood for the presence of electrical activity. In each subject, the scalp location which exhibits the largest negative values at a latency of 5 s is indicated by a beige arrow. Similarly, the cortical location with the most negative value of |t| corresponding to the spike generator is indicated by a red arrow. The waveform of the electric current (measured in µA) at the most likely location of each generator is depicted in addition to the sensor space waveform of the scalp potential (measured in µV).
Results
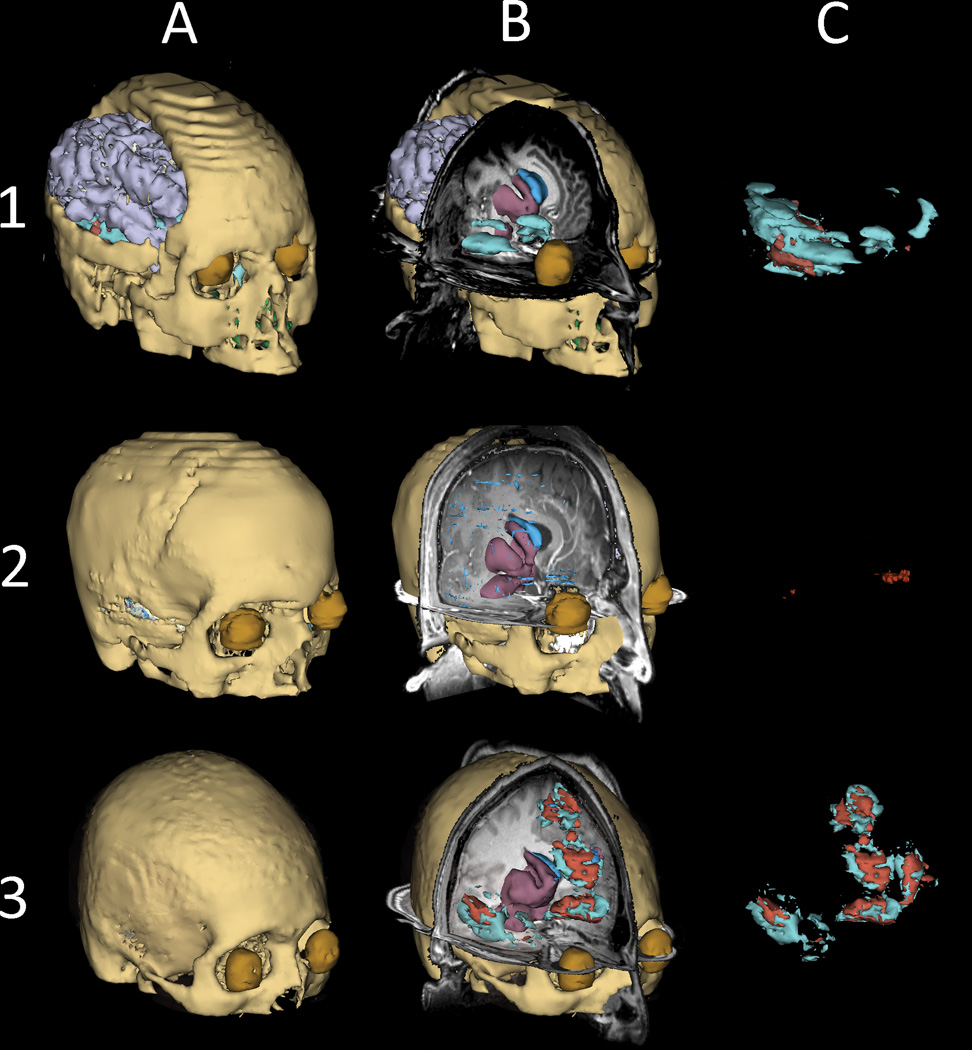
For each subject, each row of Figure 1 displays anatomical 3D models of the appropriate subject included in the study. The first column (A) displays the full model (except skin), while the second column (B) shows cross-sections through the head with MRI T1 superposed onto each FEM model. Also included in (B) are 3D models of subcortical structures, CSF and pathology where available. The third column (C) only displays brain pathology visible in the MR scans (edematous and hemorrhagic GM/WM, as segmented from GRE T2 and SWI imaging, see Methods section). As Figure 1 shows, each selected patient exhibits variable lesion loads and types of pathology; whereas fronto-temporal lesions and a large craniotomy are both visible in the right hemisphere of Patient 1, Patient 2 exhibits a comparably low lesion load. Patient 3, by contrast, has large lesions over both frontal and temporal cortices.
Figure 1.
Three-dimensional models of representative tissue types in three sample TBI patients. Models were generated in 3D Slicer [16] based on MRI volume segmentations. For each subject, the full model is shown in the first column (A), cross-sections through the head are shown in the second column (B), and TBI-related brain pathology is displayed in the third column (C). Each row corresponds to a patient. In (B), MRI T1 images are superposed onto each FEM model, and 3D models of subcortical structures, CSF and pathology are also shown available. Skin is omitted for convenience. Bone is shown in light brown, eyes in dark brown, gray matter in lilac, subcortical structures in purple, CSF in blue, edema in cyan and hemorrhage in red.
Figure 2 illustrates 60 seconds of EEG recordings from each patient, containing aperiodic epileptiform spikes, and Figure 3 shows examples of inverse localization for the three selected patients. Each subject’s illustration demonstrates the localization of the cortical source responsible for the generation of an interictal epileptiform discharge. In each patient, the EEG signal (i.e. sensor-space) waveform associated with the localized activity is shown, and the EEG potentials recorded at the time of the discharge are mapped over the scalp using the interpolated values of the potentials measured at each sensor (see figure caption for details). The inverse estimate of the cortical activity responsible for the epileptiform activity is mapped onto the surface of a reference brain as Student’s t scores, such that the magnitude of t associated with some cortical location indicates the likelihood for that location to be electrically active at the time of the spike. Thus, the locations most likely to be active have |t| > 4. The sign of t indicates whether the electric current at the location in question is oriented out of (t > 0) or into (t < 0) the cortex.
Comparing EEG topographic maps to cortical localization plots reveals that, for every patient, the epileptiform discharge was localized to the same cortical region below the scalp where the negative deflection in electric potential had been identified in the topographic map. Additionally, however, epileptiform activity generators were localized to specific gyri or sulci at previously unavailable resolution. In the first subject, the epileptiform generator is localized to the left middle temporal gyrus, whereas in the second subject it is localized to the precentral sulcus of the left hemisphere. In the third subject, as one might expect based on the scalp potential map, the most likely source responsible for the activity is identified in the frontopolar region of the left hemisphere. The sensor-space (scalp potential, in µV) and source-space (cortical current, in µA) waveforms are similar but not identical due to the physical effect of superposition, whereby the total scalp potential is the sum of potentials due to all active sources (many of which do not contribute to the recorded discharge).
Discussion
EEG monitoring as practiced for the critical care of acute TBI has typically focused on seizure identification in contrast to source localization. Reasons for this are manifold, including the complexity of structural changes affecting the TBI brain and the computational difficulty of modeling them accurately. However, because many clinical centers routinely obtain high-resolution CT and MRI data of the brain following admission to the NICU, leveraging such data to identify skin, skull, intact and injured brain in addition to hemorrhagic and edematous lesions can be used to look beyond the simple presence or absence of seizures towards precise localization of electrical sources. Currently, the relationship between the spatial configuration of epileptiform activity generators and that of brain atrophy is insufficiently understood [4], as is the manner in which these two factors jointly contribute to brain tissue fate [28, 29]. However, because epileptiform discharge frequency appears to be correlated with clinical outcome [10, 30], inverse localization of brain locations which initiate such discharges may reveal the spatiotemporal relationships between these factors, permitting the use of EEG to identify brain locations which are likely to experience atrophy in the months and years following injury.
Evidence[AI1] from multiple sources indicates that EEG-derived information on the spatial profile of neuronal activity after brain injury could provide insights on how acute pathophysiology influences clinical outcome. For example, acute EEG recordings from cerebral ischemia patients with poor outcome exhibit significantly more spreading depolarizations of gray matter cells than those of patients with good outcome [31]. Because the spatial pattern of neuronal activity in these patients correlates with both neurological deficits and with clinical symptoms [31], spatial information on such patterns obtained using EEG inverse localization could be clinically insightful. For example, studies on spreading depolarizations due to anoxia in the ischemic brain have led to some optimism that their detailed characterization could offer real-time insight and comprehensive indications of progressive ischemic injury when the latter phenomenon is known to occur [32–35]. It has even been suggested that acute therapeutic strategies might be tailored based on electrophysiological information provided by EEG to protect the ischemic penumbra from recruitment into the injury core [33]. Vespa et al. [36] have suggested that depolarization events could occur in perihematomal brain tissue and then lead to regional hyperglycolysis and metabolic crisis, and that ongoing injury occurring in metabolic penumbral tissue may be a new clinical target for pharmacological intervention to interrupt electric depolarization. In this context, it is plausible that inverse localization of EEG potentials could complement measures of metabolic dysfunction in the attempt to monitor and reduce the effects of brain ischemia. Because neuronal activity and cerebral blood flow are coupled, large decreases in the latter are associated with EEG frequency changes, and this is partly why intraoperative EEG monitoring has long been used in patients at high risk of cerebral ischemia [37]. Because cEEG can detect time windows in which surgical intervention can potentially prevent permanent brain damage [38, 39], EEG inverse localization could be useful to neurosurgeons by identifying brain regions at risk of permanent ischemic brain injury in acute TBI patients, thereby possibly also improving surgical efficacy. Because spreading depolarizations have been detected in human TBI only invasively via electrocorticography (ECoG) [34, 35], the prospects for deriving clinically useful information from such phenomena in routine TBI cases remain poor due to the need for invasive intervention. Consequently, the availability of inverse localization methods to detect and study TBI-related disruptions in brain function could ease ongoing translational efforts to use electrophysiological indicators as prognostic factors during acute TBI treatment.
Though anatomically faithful approaches have been used to model the TBI head in contexts ranging from transcranial current stimulation [40] to military blast simulations [41], brain source localization in the presence of brain lesions has been very infrequent. Even when it was performed [42], spherical head models were used most often despite being considerably less realistic than ours and therefore less likely to be appropriate for inverse localization. Given that increased forward model realism translates into improved accuracy when estimating the sources of electrical activity in the brain [43], the localization method applied here constitutes an appreciable improvement over existing methods for electric source localization in TBI patients.
While analysis techniques can be directly applied to recorded EEG signals without performing inverse localization, such methods are unlikely to provide accurate information regarding the spatial profile of cortical electrical activity during the hours and days following TBI. In addition, such methods do not take into account information pertaining either to the anatomy of the head or to spatial variations in its conductivity. Thus, the approach illustrated here has advantages over conventional analysis methods which are commonly used to study the uninjured brain, and anatomically constrained source modeling should be seen as a potentially valuable tool for the study of TBI-related pathophysiology in a clinical setting. For most cortical locations, the LEs to be expected from this approach are generally comparable to or lower than those of other methodologies which do not account for injury-related pathology [26, 27]. Nevertheless, improvements in localization accuracy can be achieved in this context by various means, such as by applying inverse algorithms with superior spatial accuracy [44], obtaining EEG measurements with higher SNR [43, 45], increasing the scalp density of EEG sensors [46], refining the forward model (e.g. by accounting for tissue anisotropies) [47, 48], combining EEG with other modalities such as functional MRI [49] or magnetoencephalography (MEG) [27], etc. In the case of the latter technique, the use of combined EEG/MEG recordings is likely to improve localization accuracy, although multiple challenges are likely to exist from the standpoint of acquiring such simultaneous recordings. One reason for this is related to the logistical difficulty of acquiring MEG recordings in a magnetically shielded room while simultaneously carrying out clinical monitoring and intervention. In addition, localization of cortical sources in TBI can involve the challenge of accounting for the magnetic noise due to (large) accumulations of blood iron in hemorrhagic areas. Despite these and other considerations, however, the use of simultaneous EEG/MEG recordings in the context of this study is likely to be benefic [26] and should be considered in facilities which possess the required instrumentation.
Conclusion
Inverse localization of electrical activity in acute TBI patients using anatomically-faithful models derived from multimodal MRI/CT may be useful for the localization of epileptogenic foci, the improvement of surgical efficacy, the enhancement of critical care monitoring and for guiding patient-tailored treatment. Such approaches might allow neurosurgeons and neurologists to study brain activity in acute TBI and to obtain insights regarding injury and surgical effects upon patient recovery. This implementation for inverse localization could also facilitate experimental studies to investigate the relationship between brain metabolism and electric pathophysiology in acute TBI [32]. Thus, because severe brain injury is believed to give rise to metabolic crisis and can have major effects upon the potential for outcome and recovery, accurate spatial mapping of electrical brain activity is important. Aside from its relevance to the study of pathophysiology in acute TBI, this source localization approach may also be of interest to the study of stroke, where the occurrence of cortical spreading depolarization and peri-infarct depolarization early after brain injury have been identified using subdural ECoG [32]. Compared to the latter, however, inverse localization of EEG potentials in TBI patients holds the advantage of being non-invasive, thereby allowing neurointensive care professionals to study electrical activity with more ease in advance of, or as an adjunct to, a surgical course of treatment.
Our primary aim in the foregoing has been to demonstrate a methodological approach which could be used to inform patient treatment and to improve TBI outcome scores, rather than focus on the type of novel information regarding TBI pathophysiology which can be obtained using this method. Importantly, our use of a highly detailed anatomical model to perform inverse localization in TBI patients is crucial for the purpose of obtaining highly accurate estimates of brain activity locations. Future studies in our laboratory will aim to incorporate the method presented here with our previous use of EEG to prognosticate TBI outcome [3, 4, 50–52] and to evaluate the extent to which the proposed methodology can be used throughout the clinical management of TBI patients with acute epileptiform discharges in order to improve their clinical outcome.
Acknowledgments
We gratefully acknowledge the dedicated staff of the Institute for Neuroimaging and Informatics at the University of Southern California.
Disclosure of funding. This work was supported by the National Alliance for Medical Image Computing (NA-MIC; www.na-mic.org), under NIH Roadmap Initiative grant 2U54EB005149, sub-award to J. D. V. H. and by the NINDS, grant P01NS058489 to P. M. V.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Industry affiliation and conflict of interest statement. The authors declare no potential conflicts of interest.
References
- 1.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–1748. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- 2.Abend NS, Dlugos DJ, Hahn CD, Hirsch LJ, Herman ST. Use of EEG monitoring and management of non-convulsive seizures in critically ill patients: a survey of neurologists. Neurocrit Care. 2010;12:382–389. doi: 10.1007/s12028-010-9337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vespa PM, Boscardin WJ, Hovda DA, McArthur DL, Nuwer MR, Martin NA, Nenov V, Glenn TC, Bergsneider M, Kelly DF, Becker DP. Early and persistent impaired percent alpha variability on continuous electroencephalography monitoring as predictive of poor outcome after traumatic brain injury. J Neurosurg. 2002;97:84–92. doi: 10.3171/jns.2002.97.1.0084. [DOI] [PubMed] [Google Scholar]
- 4.Vespa PM, McArthur DL, Xu Y, Eliseo M, Etchepare M, Dinov I, Alger J, Glenn TP, Hovda D. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology. 2010;75:792–798. doi: 10.1212/WNL.0b013e3181f07334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebersole JS. Non-invasive pre-surgical evaluation with EEG/MEG source analysis. Electroencephalogr Clin Neurophysiol Suppl. 1999;50:167–174. [PubMed] [Google Scholar]
- 6.Ebersole JS, Wade PB. Spike voltage topography identifies two types of frontotemporal epileptic foci. Neurology. 1991;41:1425–1433. doi: 10.1212/wnl.41.9.1425. [DOI] [PubMed] [Google Scholar]
- 7.Ebersole JS, Wade PB. Spike voltage topography and equivalent dipole localization in complex partial epilepsy. Brain Topogr. 1990;3:21–34. doi: 10.1007/BF01128858. [DOI] [PubMed] [Google Scholar]
- 8.Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, Laroche SM, Riviello JJ, Jr, Shutter L, Sperling MR, Treiman DM, Vespa PM. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 9.Steinbaugh LA, Lindsell CJ, Shutter LA, Szaflarski JP. Initial EEG predicts outcomes in a trial of levetiracetam vs. fosphenytoin for seizure prevention. Epilepsy Behav. 2012;23:280–284. doi: 10.1016/j.yebeh.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Lafrance WC, Jr, Deluca M, Machan JT, Fava JL. Traumatic brain injury and psychogenic nonepileptic seizures yield worse outcomes. Epilepsia. 2013;54:718–725. doi: 10.1111/epi.12053. [DOI] [PubMed] [Google Scholar]
- 11.Friedman D, Claassen J, Hirsch LJ. Continuous electroencephalogram monitoring in the intensive care unit. Anesth Analg. 2009;109:506–523. doi: 10.1213/ane.0b013e3181a9d8b5. [DOI] [PubMed] [Google Scholar]
- 12.Irimia A, Van Horn JD, Halgren E. Source cancellation profiles of electroencephalography and magnetoencephalography. NeuroImage. 2012;59:2464–2474. doi: 10.1016/j.neuroimage.2011.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halgren E, Sherfey J, Irimia A, Dale AM, Marinkovic K. Sequential temporo-fronto-temporal activation during monitoring of the auditory environment for temporal patterns. Human brain mapping. 2011;32:1260–1276. doi: 10.1002/hbm.21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irimia A, Chambers MC, Alger JR, Filippou M, Prastawa MW, Wang B, Hovda DA, Gerig G, Toga AW, Kikinis R, Vespa PM, Van Horn JD. Comparison of acute and chronic traumatic brain injury using semi-automatic multimodal segmentation of MR volumes. J. Neurotrauma. 2011;28:2287–2306. doi: 10.1089/neu.2011.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dale A, Fischl B, Sereno M. Cortical surface-based analysis - I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 16.Kikinis R, Pieper S. 3D Slicer as a tool for interactive brain tumor segmentation. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:6982–6984. doi: 10.1109/IEMBS.2011.6091765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dale A, Sereno M. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J. Cogn. Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- 18.Acar Z, Gencer N. An advanced BEM implementation for the forward problem of electro-magnetic source Imaging. Phys. Med. Biol. 2004;49:5011–5028. doi: 10.1088/0031-9155/49/21/012. [DOI] [PubMed] [Google Scholar]
- 19.Acar Z, Makeig S. Neuroelectromagnetic forward head modeling toolbox. J. Neurosci. Methods. 2010;190:258–270. doi: 10.1016/j.jneumeth.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiraishi H, Ahlfors SP, Stufflebeam SM, Takano K, Okajima M, Knake S, Hatanaka K, Kohsaka S, Saitoh S, Dale AM, Halgren E. Application of magnetoencephalography in epilepsy patients with widespread spike or slow-wave activity. Epilepsia. 2005;46:1264–1272. doi: 10.1111/j.1528-1167.2005.65504.x. [DOI] [PubMed] [Google Scholar]
- 21.McDonald CR, Thesen T, Hagler DJ, Jr, Carlson C, Devinksy O, Kuzniecky R, Barr W, Gharapetian L, Trongnetrpunya A, Dale AM, Halgren E. Distributed source modeling of language with magnetoencephalography: application to patients with intractable epilepsy. Epilepsia. 2009;50:2256–2266. doi: 10.1111/j.1528-1167.2009.02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka N, Cole AJ, von Pechmann D, Wakeman DG, Hamalainen MS, Liu H, Madsen JR, Bourgeois BF, Stufflebeam SM. Dynamic statistical parametric mapping for analyzing ictal magnetoencephalographic spikes in patients with intractable frontal lobe epilepsy. Epilepsy Res. 2009;85:279–286. doi: 10.1016/j.eplepsyres.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauk O, Wakeman DG, Henson R. Comparison of noise-normalized minimum norm estimates for MEG analysis using multiple resolution metrics. Neuroimage. 2011;54:1966–1974. doi: 10.1016/j.neuroimage.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gramfort A, Strohmeier D, Haueisen J, Hamalainen MS, Kowalski M. Time-frequency mixed-norm estimates: sparse M/EEG imaging with non-stationary source activations. Neuroimage. 2013;70:410–422. doi: 10.1016/j.neuroimage.2012.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irimia A, Goh SYM, Torgerson CM, Chambers MC, Kikinis R, Van Horn JD. Forward and inverse electroencephalographic modeling in health and in acute traumatic brain injury Clin. Neurophysiol. 2013 doi: 10.1016/j.clinph.2013.04.336. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu AK, Dale AM, Belliveau JW. Monte Carlo simulation studies of EEG and MEG localization accuracy. Human brain mapping. 2002;16:47–62. doi: 10.1002/hbm.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molins A, Stufflebeam SM, Brown EN, Hamalainen MS. Quantification of the benefit from integrating MEG and EEG data in minimum l2-norm estimation. Neuroimage. 2008;42:1069–1077. doi: 10.1016/j.neuroimage.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 28.Bouilleret V, Cardamone L, Liu YR, Fang K, Myers DE, O'Brien TJ. Progressive brain changes on serial manganese-enhanced MRI following traumatic brain injury in the rat. J. Neurotrauma. 2009;26:1999–2013. doi: 10.1089/neu.2009.0943. [DOI] [PubMed] [Google Scholar]
- 29.Rosenthal ES. The utility of EEG, SSEP, and other neurophysiologic tools to guide neurocritical care. Neurotherapeutics. 2012;9:24–36. doi: 10.1007/s13311-011-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bushnik T, Englander J, Wright J, Kolakowsky-Hayner SA. Traumatic brain injury with and without late posttraumatic seizures: what are the impacts in the post-acute phase: a NIDRR Traumatic Brain Injury Model Systems study. The Journal of head trauma rehabilitation. 2012;27:E36–E44. doi: 10.1097/HTR.0b013e318273375c. [DOI] [PubMed] [Google Scholar]
- 31.Dreier JP, Major S, Pannek HW, Woitzik J, Scheel M, Wiesenthal D, Martus P, Winkler MK, Hartings JA, Fabricius M, Speckmann EJ, Gorji A. Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain. 2012;135:259–275. doi: 10.1093/brain/awr303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dohmen C, Sakowitz OW, Fabricius M, Bosche B, Reithmeier T, Ernestus RI, Brinker G, Dreier JP, Woitzik J, Strong AJ, Graf R. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol. 2008;63:720–728. doi: 10.1002/ana.21390. [DOI] [PubMed] [Google Scholar]
- 33.Dreier JP, Woitzik J, Fabricius M, Bhatia R, Major S, Drenckhahn C, Lehmann TN, Sarrafzadeh A, Willumsen L, Hartings JA, Sakowitz OW, Seemann JH, Thieme A, Lauritzen M, Strong AJ. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–3237. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- 34.Hartings JA, Strong AJ, Fabricius M, Manning A, Bhatia R, Dreier JP, Mazzeo AT, Tortella FC, Bullock MR. Spreading depolarizations and late secondary insults after traumatic brain injury. J Neurotrauma. 2009;26:1857–1866. doi: 10.1089/neu.2009.0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartings JA, Watanabe T, Bullock MR, Okonkwo DO, Fabricius M, Woitzik J, Dreier JP, Puccio A, Shutter LA, Pahl C, Strong AJ. Spreading depolarizations have prolonged direct current shifts and are associated with poor outcome in brain trauma. Brain. 2011;134:1529–1540. doi: 10.1093/brain/awr048. [DOI] [PubMed] [Google Scholar]
- 36.Vespa PM. Metabolic penumbra in intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2009;40:1547–1548. doi: 10.1161/STROKEAHA.108.542803. [DOI] [PubMed] [Google Scholar]
- 37.Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke; a journal of cerebral circulation. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- 38.Jordan KG. Emergency EEG and continuous EEG monitoring in acute ischemic stroke. Journal of clinical neurophysiology: official publication of the American Electroencephalographic Society. 2004;21:341–352. [PubMed] [Google Scholar]
- 39.Jordan KG. Continuous EEG monitoring in the neuroscience intensive care unit and emergency department. J Clin Neurophysiol. 1999;16:14–39. doi: 10.1097/00004691-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Datta A, Bikson M, Fregni F. Transcranial direct current stimulation in patients with skull defects and skull plates: high-resolution computational FEM study of factors altering cortical current flow. NeuroImage. 2010;52:1268–1278. doi: 10.1016/j.neuroimage.2010.04.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chafi MS, Karami G, Ziejewski M. Biomechanical assessment of brain dynamic responses due to blast pressure waves. Ann Biomed Eng. 2010;38:490–504. doi: 10.1007/s10439-009-9813-z. [DOI] [PubMed] [Google Scholar]
- 42.Vatta F, Bruno P, Inchingolo P. Improving lesion conductivity estimate by means of EEG source localization sensitivity to model parameter. J Clin Neurophysiol. 2002;19:1–15. doi: 10.1097/00004691-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Ramon C, Schimpf PH, Haueisen J. Influence of head models on EEG simulations and inverse source localizations. Biomed Eng Online. 2006;5:10. doi: 10.1186/1475-925X-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. EEG source imaging. Clin Neurophysiol. 2004;115:2195–2222. doi: 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Zumer JM, Attias HT, Sekihara K, Nagarajan SS. A probabilistic algorithm integrating source localization and noise suppression for MEG and EEG data. NeuroImage. 2007;37:102–115. doi: 10.1016/j.neuroimage.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 46.Lantz G, Grave de Peralta R, Spinelli L, Seeck M, Michel CM. Epileptic source localization with high density EEG: how many electrodes are needed? Clin Neurophysiol. 2003;114:63–69. doi: 10.1016/s1388-2457(02)00337-1. [DOI] [PubMed] [Google Scholar]
- 47.Wolters CH, Anwander A, Tricoche X, Weinstein D, Koch MA, MacLeod RS. Influence of tissue conductivity anisotropy on EEG/MEG field and return current computation in a realistic head model: a simulation and visualization study using high-resolution finite element modeling. NeuroImage. 2006;30:813–826. doi: 10.1016/j.neuroimage.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Haueisen J, Tuch DS, Ramon C, Schimpf PH, Wedeen VJ, George JS, Belliveau JW. The influence of brain tissue anisotropy on human EEG and MEG. NeuroImage. 2002;15:159–166. doi: 10.1006/nimg.2001.0962. [DOI] [PubMed] [Google Scholar]
- 49.Babiloni F, Babiloni C, Carducci F, Romani GL, Rossini PM, Angelone LM, Cincotti F. Multimodal integration of high-resolution EEG and functional magnetic resonance imaging data: a simulation study. NeuroImage. 2003;19:1–15. doi: 10.1016/s1053-8119(03)00052-1. [DOI] [PubMed] [Google Scholar]
- 50.Vespa PM, Nuwer MR, Nenov V, Ronne-Engstrom E, Hovda DA, Bergsneider M, Kelly DF, Martin NA, Becker DP. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg. 1999;91:750–760. doi: 10.3171/jns.1999.91.5.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vespa PM, Miller C, McArthur D, Eliseo M, Etchepare M, Hirt D, Glenn TC, Martin N, Hovda D. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35:2830–2836. [PMC free article] [PubMed] [Google Scholar]
- 52.Vespa PM, Nenov V, Nuwer MR. Continuous EEG monitoring in the intensive care unit: early findings and clinical efficacy. J Clin Neurophysiol. 1999;16:1–13. doi: 10.1097/00004691-199901000-00001. [DOI] [PubMed] [Google Scholar]