Abstract
Von Willebrand disease (VWD) is a common bleeding disorder with prevalence in the United States of 0.01% to 1% and a prevalence in the region around Milwaukee, Wisconsin of at least 0.025%. Care of local patients with VWD primarily occurs through our comprehensive treatment center, although some patients are managed solely by their primary care physician or community hematologist. Type 1 VWD is the most common subtype, with more females carrying this diagnosis than males. Diagnosis and treatment in general follows guidelines outlined by the National Institutes of Health. An ongoing study, the Zimmerman Program for the Molecular and Clinical Biology of VWD, is currently enrolling patients with all VWD subtypes across the US in order to better delineate the extent of VWD and correlate bleeding symptoms with laboratory findings and VWF (von Willebrand factor) sequence variations. Results so far have shown that VWF gene polymorphisms are common, particularly in African Americans, and may affect laboratory assays of VWF function.
INTRODUCTION
von Willebrand disease (VWD) is a common inherited bleeding disorder. The prevalence of VWD has been estimated at anywhere from 0.01% to 1% depending on the population studied and the criteria used for diagnosis.1,2 The prevalence of type 3 VWD in North America has been given as 1.38 per million.3 The International Society on Thrombosis and Haemostasis has recommended that in order to diagnose VWD, the patient should have a personal history of bleeding, family history of VWD or bleeding symptoms, and laboratory results suggestive of VWD.4
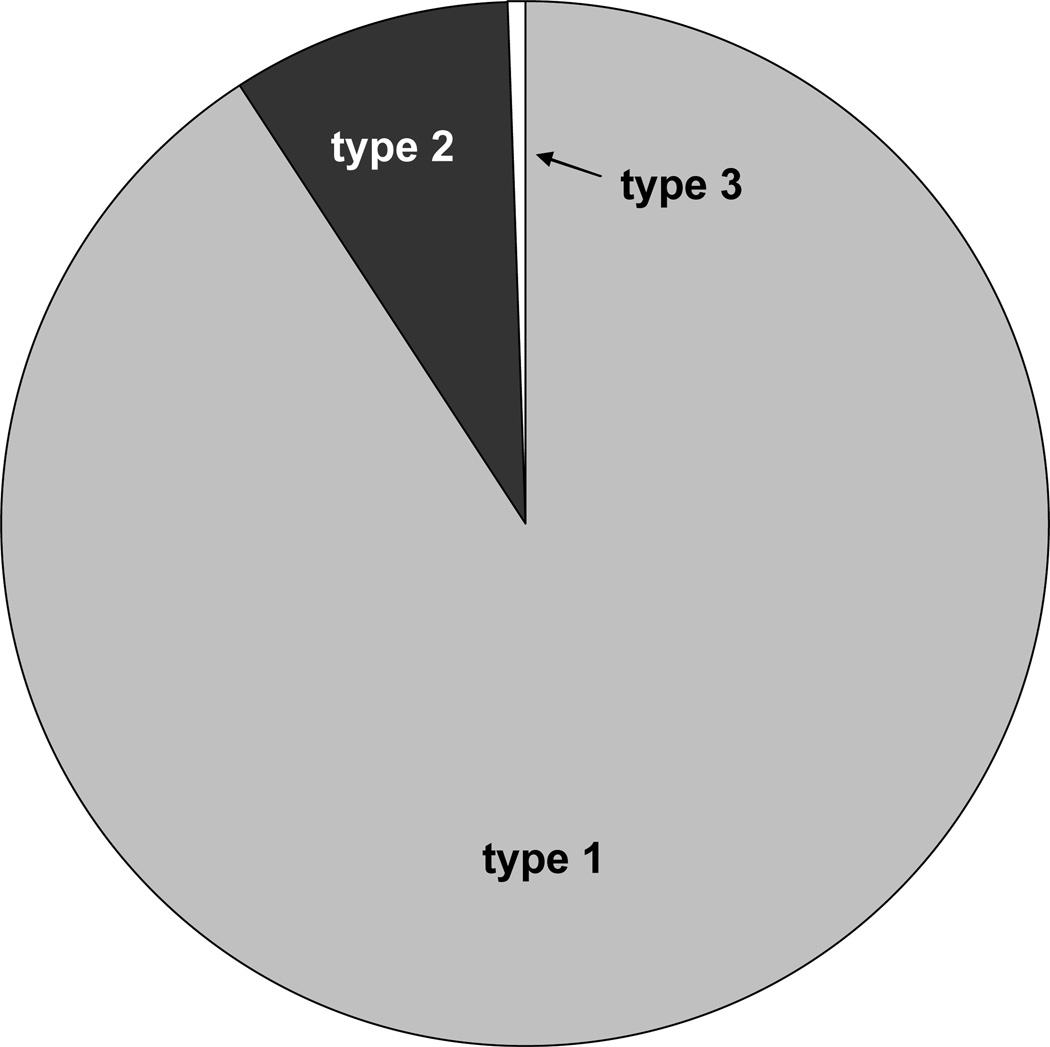
In our clinic, we follow over 1000 VWD patients. Of those patients, 865 are type 1, 82 type 2, and 6 type 3 with a small number of patients with as of yet undefined VWD (table 1). Figure 1 shows the distribution of VWD patients in our local population. These patients come from a referral population which consists of the Milwaukee area, plus parts of northern Wisconsin, northern Illinois, and the upper peninsula of Michigan. The population of this region is slightly over 4 million, yielding a VWD prevalence of 0.025%. It is likely, however, that there are many patients with VWD not cared for at our center, as referral to a hematologist does not always result in the patient being referred to the Comprehensive Center for Bleeding Disorders.
Table 1.
VWD demographics for patients referred to the Comprehensive Center for Bleeding Disorders in Milwaukee, Wisconsin
| Number (%) | ||
|---|---|---|
| VWD type | 1 | 865 (90.8) 1 |
| 2 | 82 (8.6) | |
| 2A | 39 | |
| 2B | 9 | |
| 2M | 23 | |
| 2N | 11 | |
| 3 | 6 (0.6) | |
| Undefined/Unclassified | 782 | |
| Sex | Male | 424 (44.5) |
| Female | 529 (55.5) | |
| Age | <5 | 57 (6.0) |
| 5–20 | 436 (45.8) | |
| >20 | 460 (48.3) |
Includes one patient with type 1 NY and 3 patients with type 1C (clearance).
Primarily patients with low VWF but not meeting specific diagnostic criteria, not included in total. If included, would represent 7.6% of total VWD patients.
Figure 1.
VWD distribution by subtype in the Milwaukee area. Type 1 subjects (90.8%) are shown in grey, type 2 (8.6%) in black, and type 3 (0.6%) in white. Type 1 VWD comprises the majority of patients in our practice.
Additional information on the distribution of VWD in the US is provided by the Centers for Disease control. Hemophilia treatment centers (HTCs) are encouraged to enroll VWD patients in the Universal Data Collection program, which has yielded useful information regarding diagnosis and treatment of those patients with congenital bleeding disorders cared for in HTCs.5 As of December 2010, there were 4912 type 1, 628 type 2, and 332 type 3 VWD patients.6 Of these patients, the male:female ratio was 1:1.6 for the type 1 patients, but closer to equivalent for types 2 and 3 with ratios of 1:1.2 and 1:0.8 respectively. The increased number of females with type 1 VWD likely reflects the common presentation with menorrhagia and subsequent diagnosis of a bleeding disorder. It is not clear whether there is truly an increased prevalence in women or merely an increase in the diagnosis of type 1 VWD in this population. Type 1 patients represented 84%, type 2 patients 11%, and type 3 patients 6% of those with defined VWD.6 The majority (75%) were Caucasian. This population may be more heavily weighted towards those with more severe disease than observed in Milwaukee, reflecting the referral patterns in the US and the fact that many centers do not enroll patients with milder forms of VWD in the Universal Data Collection project.
THE ZIMMERMAN PROGRAM FOR THE MOLECULAR AND CLINICAL BIOLOGY OF VWD
In order to better explore the diagnosis of VWD in the United States, a study has been initiated similar to ones undertaken in Canada and Europe.7,8 The Zimmerman Program for the Molecular and Clinical Biology of VWD is a multicenter study enrolling both healthy controls and VWD patients. Eight primary centers, in Milwaukee, Atlanta, Detroit, Houston, Indianapolis, Iowa City, New Orleans, and Pittsburgh, provided the healthy controls, representing all ethnicities, as well as the bulk of enrolled VWD subjects. Numerous secondary centers (21 to date) also contributed smaller numbers. To date, 246 normal controls have been recruited, along with 502 index cases, primarily carrying a diagnosis of type 1 VWD but also including type 2 and type 3 subjects.
Analysis of the VWD patients is ongoing, but the healthy controls have already yielded some data of interest. The Zimmerman Program is unique by design in that a large number of African American controls were enrolled. For the purposes of this study, self-identification of race was provided by the subject at the time of enrollment. The racial distribution of controls enrolled to date is 28% African American and 65% Caucasian (with the remainder Middle East, Far East, and Native American). 13% of the subjects identified as Hispanic. African American subjects were over-represented compared to the distribution of races within the US, where African Americans comprise 6.2% of the Wisconsin population and 12.9% of the US population.
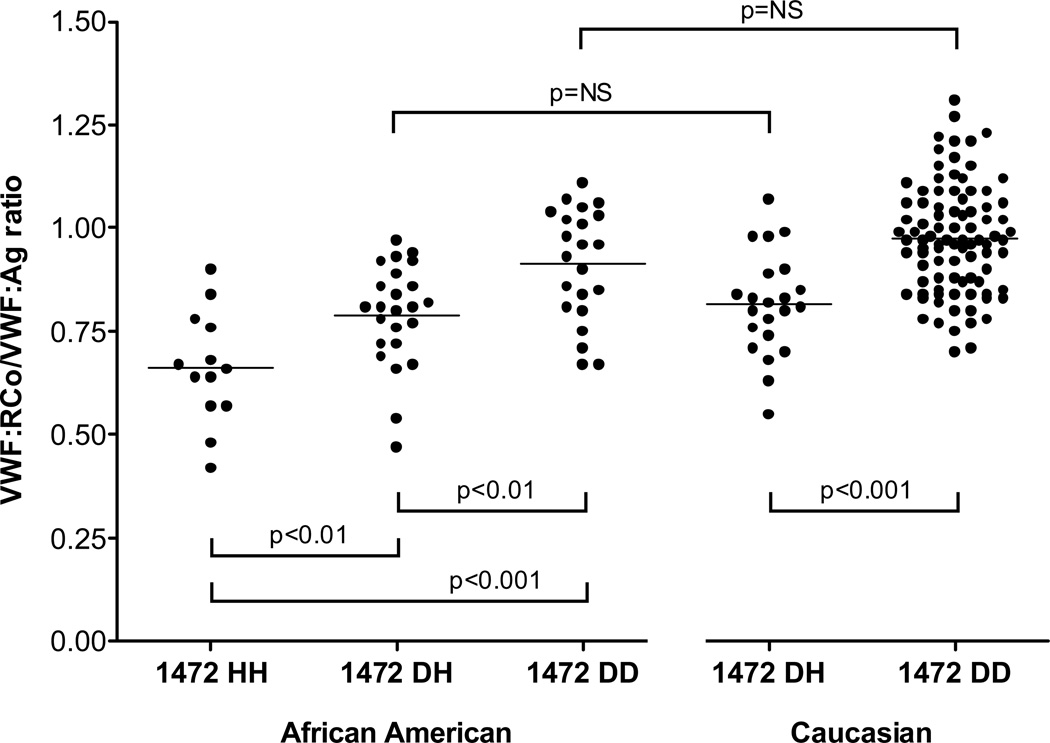
The African American subjects display a wide range of polymorphisms. One in particular, D1472H, was associated with a decrease in the VWF ristocetin cofactor to VWF antigen (VWF:RCo/VWF:Ag) ratio.9 These ratios were low enough in some cases to qualify for a diagnosis of type 2M VWD had the subject been under investigation for a bleeding disorder. However, none of the healthy controls with this polymorphism had an elevated bleeding score. Subjects homozygous for the D1472H polymorphism had a VWF:RCo/VWF:Ag ratio that was lower than those heterozygous for the polymorphism, while both were decreased compared to subjects without this polymorphism (figure 2). The effect was independent of race, although the frequency of the 1472H allele was much higher in African Americans (approximately 63%) as compared to Caucasians (approximately 17%).
Figure 2.
VWF:RCo/VWF:Ag ratio varies depending on allele status of D1472H in both African American and Caucasian healthy controls enrolled in the Zimmerman Program for the Molecular and Clinical Biology of VWD. All subjects had VWF:RCo and VWF:Ag performed at a central laboratory. No subject had a pre-existing diagnosis of a bleeding disorder or an abnormal bleeding score.
In addition to the 1472H polymorphism, an exon 18 mutation, H817Q, that has been previously reported in type 2N VWD also occurs with increased frequency in African American controls.10 The presence of the mutation was associated with decreased VWF-FVIII binding, albeit with levels that are only slightly below the lower end of the normal reference interval. The 817Q allele was present with a frequency of approximately 0.1, predicting that one of 1000 African Americans should be homozygous for this allele. Given the rarity of type 2N VWD in the African American population, it is possible that homozygosity for the 817Q allele alone is insufficient to cause type 2N VWD but further investigation is required to understand the clinical significance of this genetic variant.
DIAGNOSIS OF VWD IN THE US
Recently, the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health issued guidelines for the diagnosis, evaluation, and management of VWD.11 These guidelines are meant for all medical practitioners involved in the diagnosis and care of VWD patients, and are the result of expert consensus. The NHLBI guidelines have also been synthesized into recommendations for the obstetrics and gynecology literature.12 Variability in diagnosis and treatment of VWD exists, in part due to regional practice and in part due to the variety of clinicians who care for these patients.
Diagnosis of VWD is primarily based on VWF:Ag and on the VWF:RCo activity assay. Many laboratories perform these assays, while relatively few are able to perform VWF multimer analysis. Specialty laboratories may offer a more complete panel of tests to distinguish VWD variants. The laboratory at the BloodCenter of Wisconsin, for example, provides factor VIII activity (FVIII:C), VWF:Ag, VWF:RCo, and VWF multimers together as a “VWD profile”. Blood type is also sometimes obtained, as VWF levels do vary by blood type.13 Our protocol is outlined in table 2. Some US reference laboratories provide VWF collagen binding (VWF:CB) as part of their routine von Willebrand panel. Most clinicians do not routinely perform collagen binding, but the ratio of VWF:CB/VWF:Ag may be a more sensitive screen for type 2A and 2B VWD than the VWF:RCo/VWF:Ag ratio.14,15 The VWF:CB/VWF:Ag ratio alone, however, may miss some forms of type 2M VWD, although there have been reports of VWD mutations that do specifically affect VWF-collagen binding without a concomitant decrease in VWF:RCo.16–18
Table 2.
Laboratory studies performed in diagnosis of VWD
| Initial workup | VWF:Ag, VWF:RCo, FVIII:C, multimers1 |
| If ↓ VWF:RCo/VWF:Ag, abnormal multimers | LD-RIPA, VWF:PB, consider sequencing |
| If ↓ VWF:RCo/VWF:Ag, normal multimers | Consider sequencing |
| If ↓ FVIII:C/VWF:Ag | VWF:FVIIIB |
| If ↓↓ VWF:RCo and VWF:Ag | VWFpp, survival studies |
Abbreviations: VWF:Ag=VWF antigen; VWF:RCo=VWF ristocetin cofactor activity; FVIII:C=factor VIII activity; multimers=multimer distribution analysis; LD-RIPA=low dose ristocetin-induced platelet aggregation; VWF:PB=VWF-platelet binding; VWF:FVIIIB=VWF-FVIII binding; VWFpp=VWF propeptide.
Repeat VWF:Ag and VWF:RCo to confirm low levels
Confirmatory testing is not generally available in most hospitals but some specialty laboratories do offer further testing. At the BloodCenter of Wisconsin, low-dose ristocetin-induced platelet binding is available to confirm a diagnosis of type 2B VWD and factor VIII binding (VWF:FVIIIB) is available to confirm a diagnosis of type 2N VWD. Ristocetin-induced platelet aggregation may be helpful in suspected 2B or platelet-type VWD. DNA sequencing may be useful in select cases of VWD. Reports of mutation analysis by other groups suggest that in type 1 VWD, chances of finding a mutation are greater in those patients with low VWF levels, in particular with VWF:Ag <30 IU/dL.19,20 Our center currently offers VWD subtype-specific sequencing for type 2A, 2B, 2M, and type 1C (clearance) VWD, as well as platelet-type VWD. Type 2B and platelet-type VWD are both “gain-of-function” defects, with similar clinical presentations. Making a distinction between these entities is important because treatment recommendations differ. While laboratory diagnostic protocols for distinguishing these syndromes have been published, distinction using phenotypic assays is difficult for most clinical laboratories. Gene sequencing should greatly improve diagnostic accuracy.
Our local clinical experience is consistent with the report of Favaloro, suggesting that the majority of patients with “gain-of-function” defects have type 2B VWD.21 We currently have 9 patients with type 2B VWD and 1 with platelet-type VWD, or approximately 10% platelet-type and 90% type 2B VWD. The sequencing experience of our Hemostasis Reference Laboratory, which receives samples from across the US, is similar, with 59 cases of type 2B VWD in contrast to 4 cases of platelet-type VWD (6.3%). Whole gene sequencing is not performed routinely, since this is not yet of clinical utility due to the massive number of polymorphisms also contained within the VWF gene.22,23 There are US laboratories offering full length VWF sequencing, but in general this is not performed outside of research applications.
Assessment of the diagnosis and treatment of VWD in the US is complicated by the fact that not all patients are seen by hematologists, or by hemophilia treatment centers (HTCs). Referral patterns vary greatly among practitioners, such that some patients are sent on to see a specialist and some are diagnosed and managed in the primary care setting without referral to a hematologist. The majority of pediatric patients are referred to a pediatric hematologist, many of them affiliated with HTCs. In some areas, however, the only pediatric hematologist available may be someone whose primary practice is in oncology. For adults, the decision to refer to a specialist is in the primary physician’s hands. Adult hematology in the US is often a practice that combines “benign hematology” with management of leukemia and lymphoma. Thus, often adult hematologists do not focus on bleeding disorders. This means that many patients with VWD, especially mild VWD, are not followed by HTCs such as the Comprehensive Center for Bleeding Disorders. Of course some patients, in particular those with the most severe forms of VWD, are referred to HTCs, but this is not the case for the majority of adults.
In our practice, a complete von Willebrand disease workup consists of FVIII:C, VWF:Ag, VWF:RCo, and VWF multimer distribution (table 2). A complete blood count is usually performed to evaluate the platelet count, which is typically (but not always) decreased in type 2B and platelet-type VWD.24 Most patients have had preliminary screening tests performed prior to arrival in our clinic, including CBC (complete blood count), PT (prothrombin time), and APTT (activated partial thromboplastin time). If these have not been performed, we include them with our workup, along with a thrombin time for evaluation of fibrinogen. Additional testing is performed as needed to clarify the diagnosis, particularly for type 2 variants (with diagnostic criteria outlined in table 3). At this time, we do not routinely perform VWF:CB in all patients, only those enrolled in a research protocol such as the Zimmerman Program. We expect that VWF:CB may play a larger role in the future. Patients are diagnosed with type 3 VWD if there is undetectable VWF:Ag and VWF:RCo. Frequently the VWF:Ag and VWF:RCo are rechecked to confirm low levels before a definitive diagnosis is made, often in conjunction with a trial of desmopressin for type 1 patients. Although a trial of desmopressin is not typically performed as part of the diagnostic workup, it may be important in distinguishing those patients with a clearance defect (type 1C) who have an initial response to desmopressin but a significantly shortened half-life.25,26 Type 1 VWD is diagnosed if the VWF:Ag and VWF:RCo are <30 (some use a cutoff of 35–40) IU/dL, while those with VWF:Ag and VWF:RCo between 30 (or 35–40) and 50 IU/dL are labeled as “deficiency of VWF”; a cutoff of 30 is consistent with the NHBLI guidelines. Many of the patients with “deficiency of VWF”, however, are treated perioperatively in a similar fashion to those with lower VWF levels, particularly if they have a past history of bleeding symptoms.
Table 3.
VWD diagnosis by type
| VWD diagnosis | Laboratory findings |
| Type 1 | ↓ (but proportional) VWF:Ag and VWF:RCo with normal multimers |
| Type 1C | ↓ VWF:Ag with ↑ VWFpp/VWF:Ag ratio |
| Type 2A | ↓ VWF:RCo/VWF:Ag ratio with abnormal multimers |
| Type 2B | ↓ VWF:RCo/VWF:Ag ratio with abnormal multimers and ↑ LD-RIPA, abnormal VWF:PB, possible thrombocytopenia |
| Type 2M | ↓ VWF:RCo/VWF:Ag ratio with normal multimers |
| Type 2N | ↓ FVIII:C/VWF:Ag and abnormal VWF:FVIIIB |
| Type 3 | Absent VWF:Ag and VWF:RCo |
Abbreviations: VWF:Ag=VWF antigen; VWF:RCo=VWF ristocetin cofactor activity; FVIII:C=factor VIII activity; multimers=multimer distribution analysis; LD-RIPA=low dose ristocetin-induced platelet aggregation; VWF:PB=VWF-platelet binding; VWF:FVIIIB=VWF-FVIII binding; VWFpp=VWF propeptide.
TREATMENT OF VWD IN THE US
Treatment of VWD in the US varies by subtype. Most patients with type 1 VWD receive desmopressin as first line therapy. This may be administered intravenously or via intranasal spray. Some clinicians have used subcutaneous administration but this is not an FDA-approved route of administration in the United States. Several intranasal desmopressin preparations are at present available, such that clinicians must be careful to specify the preparation with a sufficiently high desmopressin concentration (Stimate®, 150 mcg/spray, CSL Behring) in order to achieve a therapeutic effect. The exception is those patients with clearance defects (type 1C) who may experience a rise in VWF:Ag and VWF:RCo in response to desmopressin but who rapidly clear their endogenous VWF.25 In these patients, VWF-containing concentrates are chosen to achieve sustained therapeutic VWF levels. Some type 2 patients may also receive desmopressin for minor bleeds such as epistaxis, in particular our type 2A patients, who may have a clinical response despite a lack of durable rise in VWF levels. Surgical treatment or major bleeds, however, are treated with VWF-containing concentrates. Use of desmopressin for type 2B VWD has been thought to be relatively contraindicated, but not all patients will have significant thrombocytopenia with its use. If a desmopressin trial is performed in type 2B VWD patients, platelet counts are monitored carefully. The NHBLI guidelines support the potential use of desmopressin in these patients.11
The intravenous form of desmopressin is generally used before surgical procedures, while the intranasal form may be administered at home to treat minor bleeding episodes such as prolonged epistaxis or menorrhagia. A test dose of desmopressin is usually administered in order to document responsiveness. VWF response is examined at 1 hour post administration as well as 2 and/or 4 hours post administration to evaluate for a potential clearance defect. One concern with the use of desmopressin is the risk of hyponatremia and subsequent seizures. Patients are instructed to monitor fluid intake and restrict fluids to maintenance levels for 24 hours following desmopressin administration.27 However, patients who neglect to follow these instructions may present with seizures due to the hyponatremia. Some organizations recommend checking sodium levels after desmopressin administration, although this is not performed in all cases and may be unnecessary in low-risk settings (such as older children and adults receiving a single dose of desmopressin). Hyponatremic seizures following use of desmopressin have been reported for both adults and children.28,29
Antifibrinolytic drugs are used for mucosal bleeding, particularly following tonsillectomy or tooth extraction or for women with menorrhagia. In the US, aminocaproic acid has been the only option for many years, but oral tranexemic acid has recently been approved by the US Food and Drug Administration (FDA). Oral administration is used for menorrhagia and post-tonsillectomy surgical prophylaxis, while topical administration is used for prevention or treatment of bleeding following tooth extraction. For minor dental procedures, treatment is given for 5–7 days and up to 14 days for multiple molar extractions; for tonsillectomy, antifibrinolytic therapy is generally continued for up to 14 days or until the eschar is shed.
VWF-containing concentrates are the primary therapy for patients with type 2 and type 3 VWD, as well as for the more severely affected type 1 patient who does not mount a hemostatic response to desmopressin or for those with accelerated VWF clearance. At this time, plasma derived concentrates are used exclusively, although it is hoped that a recombinant VWF concentrate will be available soon. Most patients are treated on demand, but some type 2 and type 3 VWD patients with significant bleeding symptoms require prophylaxis due to a history of chronic significant bleeding.30 Indications for prophylaxis in our VWD cohort have included recurrent epistaxis with anemia, prevention of ovarian hemorrhage after interruption of oral contraception in support of family planning, and secondary prophylaxis of target joint bleeding. In the US, Humate-P® (CSL Behring) is the predominant factor used for VWD, with VWF activity approximately 2.4 times that of the FVIII activity per dose. Wilate® (Octapharma) was recently approved by the FDA for use in VWD. Its formulation, however, provides VWF activity approximately equal to the FVIII activity rather than the comparative increase in VWF activity over FVIII provided by Humate-P. There are theoretical risks associated with elevated FVIII levels in the setting of other thrombotic risk factors, potentially leading to thrombosis, although this has not been a major issue in VWD treatment to date. Alphanate® (Grifols) has also been licensed in the US for VWF replacement therapy; the ratio of FVIII to VWF has been debated in the literature but the labeling suggests that the ratio of VWF to FVIII may be near 1:1 in this product.31
CONCLUSIONS
The prevalence of VWD in the US is approximately 0.01–1% based on various approaches, with the local population in and around Milwaukee, Wisconsin demonstrating a prevalence of 0.025% based on our referral data, but this is probably greatly underestimated due to the fact that many patients, particularly those with mild disease, continue to obtain care through community-based practices. Diagnosis utilizes consensus guidelines put forth by the NHLBI consensus panel, although it should be kept in mind that many of the guidelines are not evidence-based and may not be followed by all community practitioners or even by treating hematologists. VWD diagnosis and management is best performed through hematology specialists familiar with the complexities of this condition, such as those in a hemophilia treatment center. Not all patients in the US, however, have ready access to this expertise. Improving the diagnosis of VWD is one of the goals of the Zimmerman Program, but much work remains to be done regarding treatment and quality of life for affected patients.
Acknowledgements
This work was supported by grant funding from the National Institutes of Health (HL102260 to VF and HL33721, HL044612, and program project grant HL081588 to RRM). The authors would also like to acknowledge the support of their colleagues at the BloodCenter of Wisconsin and Medical College of Milwaukee, including the Hemostasis Reference Laboratory and Comprehensive Center for Bleeding Disorders nurses and staff.
REFERENCES
- 1.Werner EJ, Broxson EH, Tucker EL, Giroux DS, Shults J, Abshire TC. Prevalence of von Willebrand disease in children: a multiethnic study. J Pediatr. 1993;123(6):893–898. doi: 10.1016/s0022-3476(05)80384-1. [DOI] [PubMed] [Google Scholar]
- 2.Sadler JE, Mannucci PM, Berntorp E, et al. Impact, diagnosis and treatment of von Willebrand disease. Thromb Haemost. 2000;84(2):160–174. [PubMed] [Google Scholar]
- 3.Weiss HJ, Ball AP, Mannucci PM. Incidence of severe von Willebrand's disease. N Engl J Med. 1982;307(2):127. doi: 10.1056/NEJM198207083070222. [DOI] [PubMed] [Google Scholar]
- 4.Sadler JE, Budde U, Eikenboom JC, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4(10):2103–2114. doi: 10.1111/j.1538-7836.2006.02146.x. [DOI] [PubMed] [Google Scholar]
- 5.Soucie JM, Cianfrini C, Janco RL, et al. Joint range-of-motion limitations among young males with hemophilia: prevalence and risk factors. Blood. 2004;103(7):2467–2473. doi: 10.1182/blood-2003-05-1457. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control. Universal Data Collection Project. [Accessed December 9 2010]; [Google Scholar]
- 7.O'Brien LA, James PD, Othman M, et al. Founder von Willebrand factor haplotype associated with type 1 von Willebrand disease. Blood. 2003;102(2):549–557. doi: 10.1182/blood-2002-12-3693. [DOI] [PubMed] [Google Scholar]
- 8.Tosetto A, Rodeghiero F, Castaman G, et al. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: results from a multicenter European study (MCMDM-1 VWD) J Thromb Haemost. 2006;4(4):766–773. doi: 10.1111/j.1538-7836.2006.01847.x. [DOI] [PubMed] [Google Scholar]
- 9.Flood VH, Gill JC, Morateck PA, et al. Common VWF exon 28 polymorphisms in African Americans affecting the VWF activity assay by ristocetin cofactor. Blood. 2010;116(2):280–286. doi: 10.1182/blood-2009-10-249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman KD, Bellissimo DB, Christopherson PA, et al. Fourteen Percent of Healthy African Americans participating in the Zimmerman Program for the Molecular and Clinical Biology of VWD (ZPMCB-VWD) are heterozygous for the VWF gene mutation H817Q associated with type 2N von Willebrand Disease. Blood. 2010;116(21):239. [Google Scholar]
- 11.Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA) Haemophilia. 2008;14(2):171–232. doi: 10.1111/j.1365-2516.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 12.James AH, Manco-Johnson MJ, Yawn BP, Dietrich JE, Nichols WL. Von Willebrand disease: key points from the 2008 National Heart, Lung, and Blood Institute guidelines. Obstet Gynecol. 2009;114(3):674–678. doi: 10.1097/AOG.0b013e3181b191ea. [DOI] [PubMed] [Google Scholar]
- 13.Gill JC, Endres-Brooks J, Bauer PJ, Marks WJ, Jr, Montgomery RR. The effect of ABO blood group on the diagnosis of von Willebrand disease. Blood. 1987;69(6):1691–1695. [PubMed] [Google Scholar]
- 14.Adcock DM, Bethel M, Valcour A. Diagnosing von Willebrand disease: a large reference laboratory's perspective. Semin Thromb Hemost. 2006;32(5):472–479. doi: 10.1055/s-2006-947860. [DOI] [PubMed] [Google Scholar]
- 15.Favaloro EJ. An update on the von Willebrand factor collagen binding assay 21 years of age and beyond adolescence but not yet a mature adult. Semin Thromb Hemost. 2007;33(8):727–744. doi: 10.1055/s-2007-1000364. [DOI] [PubMed] [Google Scholar]
- 16.Ribba AS, Loisel I, Lavergne JM, et al. Ser968Thr mutation within the A3 domain of von Willebrand factor (VWF) in two related patients leads to a defective binding of VWF to collagen. Thromb Haemost. 2001;86(3):848–854. [PubMed] [Google Scholar]
- 17.Riddell AF, Gomez K, Millar CM, et al. Characterization of W1745C and S1783A 2 novel mutations causing defective collagen binding in the A3 domain of von Willebrand factor. Blood. 2009;114(16):3489–3496. doi: 10.1182/blood-2008-10-184317. [DOI] [PubMed] [Google Scholar]
- 18.Flood VH, Lederman CA, Wren JS, et al. Absent collagen binding in a VWF A3 domain mutant: utility of the VWF:CB in diagnosis of VWD. J Thromb Haemost. 2010;8(6):1431–1433. doi: 10.1111/j.1538-7836.2010.03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodeve A, Eikenboom J, Castaman G, et al. Phenotype and genotype of a cohort of families historically diagnosed with type 1 von Willebrand disease in the European study, Molecular and Clinical Markers for the Diagnosis and Management of Type 1 von Willebrand Disease (MCMDM-1VWD) Blood. 2007;109(1):112–121. doi: 10.1182/blood-2006-05-020784. [DOI] [PubMed] [Google Scholar]
- 20.James PD, Notley C, Hegadorn C, et al. The mutational spectrum of type 1 von Willebrand disease: Results from a Canadian cohort study. Blood. 2007;109(1):145–154. doi: 10.1182/blood-2006-05-021105.. [DOI] [PubMed] [Google Scholar]
- 21.Favaloro EJ. Phenotypic identification of platelet-type von Willebrand disease and its discrimination from type 2B von Willebrand disease: a question of 2B or not 2B? A story of nonidentical twins? Or two sides of a multidenominational or multifaceted primary-hemostasis coin? Semin Thromb Hemost. 2008;34(1):113–127. doi: 10.1055/s-2008-1066019. [DOI] [PubMed] [Google Scholar]
- 22.Sadler JE, Ginsburg D. A database of polymorphisms in the von Willebrand factor gene and pseudogene. For the Consortium on von Willebrand Factor Mutations and Polymorphisms and the Subcommittee on von Willebrand Factor of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 1993;69(2):185–191. [PubMed] [Google Scholar]
- 23.International Society on Thrombosis and Haemostasis Scientific and Standardization Committee. [Accessed December 21, 2010];ISTH-SSC VWF Online Database. http://www.vwf.group.shef.ac.uk. [Google Scholar]
- 24.Federici AB, Mannucci PM, Castaman G, et al. Clinical and molecular predictors of thrombocytopenia and risk of bleeding in patients with von Willebrand disease type 2B: A cohort study of 67 patients. Blood. 2009;113(3):526–534. doi: 10.1182/blood-2008-04-152280. [DOI] [PubMed] [Google Scholar]
- 25.Haberichter SL, Balistreri M, Christopherson P, et al. Assay of the von Willebrand factor (VWF) propeptide to identify patients with type 1 von Willebrand disease with decreased VWF survival. Blood. 2006;108(10):3344–3351. doi: 10.1182/blood-2006-04-015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haberichter SL, Castaman G, Budde U, et al. Identification of type 1 von Willebrand disease patients with reduced von Willebrand factor survival by assay of the VWF propeptide in the European study: molecular and clinical markers for the diagnosis and management of type 1 VWD (MCMDM-1VWD) Blood. 2008;111(10):4979–4985. doi: 10.1182/blood-2007-09-110940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey AG, McNaull PP, Jooste E, Tuchman JB. Perioperative crystalloid and colloid fluid management in children: where are we and how did we get here? Anesth Analg. 2010;110(2):375–390. doi: 10.1213/ANE.0b013e3181b6b3b5. [DOI] [PubMed] [Google Scholar]
- 28.Jimenez-Yuste V, Prim MP, De Diego JI, et al. Otolaryngologic surgery in children with von Willebrand disease. Arch Otolaryngol Head Neck Surg. 2002;128(12):1365–1368. doi: 10.1001/archotol.128.12.1365. [DOI] [PubMed] [Google Scholar]
- 29.Olowokure O, Fishman M, Cromwell C, Aledort L. DDAVP for von Willebrand menorrhagia--severe hyponatraemia, haemolysis, seizure, coma.!! Caution. Haemophilia. 2009;15(3):837. doi: 10.1111/j.1365-2516.2009.01997.x. [DOI] [PubMed] [Google Scholar]
- 30.Berntorp E, Abshire T, Federici AB. Regular Replacement Therapy as Prophylaxis In Severe Forms of Von Willebrand Disease: Initial Results From the Von Willebrand Disease Prophylaxis Network (VWD PN) Study Group. Blood. 2010;116(21):236. [Google Scholar]
- 31.Rivard GE, Aledort L. Alphanate Surgical Investigators. Efficacy of factor VIII/von Willebrand factor concentrate Alphanate in preventing excessive bleeding during surgery in subjects with von Willebrand disease. Haemophilia. 2008;14(2):271–275. doi: 10.1111/j.1365-2516.2007.01616.x. [DOI] [PubMed] [Google Scholar]