Summary
Background
High-volume prescribing of antibiotics in primary care is a major driver of antibiotic resistance. Education of physicians and patients can lower prescribing levels, but it frequently relies on highly trained staff. We assessed whether internet-based training methods could alter prescribing practices in multiple health-care systems.
Methods
After a baseline audit in October to December, 2010, primary-care practices in six European countries were cluster randomised to usual care, training in the use of a C-reactive protein (CRP) test at point of care, in enhanced communication skills, or in both CRP and enhanced communication. Patients were recruited from February to May, 2011. This trial is registered, number ISRCTN99871214.
Results
The baseline audit, done in 259 practices, provided data for 6771 patients with lower-respiratory-tract infections (3742 [55·3%]) and upper-respiratory-tract infections (1416 [20·9%]), of whom 5355 (79·1%) were prescribed antibiotics. After randomisation, 246 practices were included and 4264 patients were recruited. The antibiotic prescribing rate was lower with CRP training than without (33% vs 48%, adjusted risk ratio 0·54, 95% CI 0·42–0·69) and with enhanced-communication training than without (36% vs 45%, 0·69, 0·54–0·87). The combined intervention was associated with the greatest reduction in prescribing rate (CRP risk ratio 0·53, 95% CI 0·36–0·74, p<0·0001; enhanced communication 0·68, 0·50–0·89, p=0·003; combined 0·38, 0·25–0·55, p<0·0001).
Interpretation
Internet training achieved important reductions in antibiotic prescribing for respiratory-tract infections across language and cultural boundaries.
Funding
European Commission Framework Programme 6, National Institute for Health Research, Research Foundation Flanders.
Introduction
Physicians prescribe antibiotics for many patients with acute uncomplicated lower-respiratory-tract infections, which are among the most common acute presentations in primary care.1–3 Most of these infections are viral, and evidence from systematic reviews4 and other studies5,6 suggest only slight benefit is achieved from the prescription of antibiotics. Thus, rationalisation of antibiotic use in the treatment of lower-respiratory-tract infections in primary care is a priority in the prevention of antibiotic resistance.7
C-reactive protein (CRP) has predictive value for pneumonia.8,9 In the IMPAC3T study,10 training of physicians in CRP testing lowered the rate of antibiotic prescribing by 20%. These findings were supported in a later study.11 The usefulness of training in consultation skills requires clarification10 because there is limited evidence for effects on symptom control10,12,13 and whether a particular approach to training can be used in different settings.
Interactive workshops for health-care professionals and education of patients are likely to lower the rate of antibiotic prescribing.12,14,15 The IMPAC3T study10 showed that the training of physicians in advanced communication skills by seminar role-playing and peer feedback on consultation transcripts reduced antibiotic prescribing rates by 20%. The STAR programme involves five stages of web-based training in advanced communication skills that include recording of reactions to scenarios, sharing of accounts of clinical experience, and expert-led face-to-face seminars. This approach led to a 4% reduction in global antibiotic use over 1 year in practices across Wales.16 Nevertheless, because such outreach interventions are generally performed by small groups of highly trained staff based at research centres of excellence, the generalisability of delivery and the potential effects on real-world practice are questionable. Novel techniques are, therefore, needed to lead to changes at national and international levels. Internet training has the advantage that it can be disseminated widely at low cost and does not require highly trained outreach facilitators to be on site. In one study of internet training for general practitioners, the use of an interactive booklet for consultations with children attending for acute respiratory-tract infections was effective, but the sample and the setting had limitations, and no outcomes in patients were documented.13
The Genomics to combat Resistance against Antibiotics in Community-acquired LRTI in Europe (GRACE) consortium developed an internet-based training tool for lower-respiratory-tract infections that was found to be acceptable and applicable by physicians in several European countries.17 We report the effects of training by this method on antibiotic prescribing and symptom control among adult patients.
Methods
Study design
This study was a multinational, cluster, randomised, factorial, controlled trial. We used a randomised cluster design to keep to a minimum contamination (influence on participants' behaviour when another participant or physician alters his or her behaviour) within practices, as more than one physician per practice could participate, and because a practice-based meeting was part of the intervention. The factorial design permitted assessment of two training interventions—CRP testing and communication—in isolation and in combination. A baseline audit was undertaken in participating practices from October to December, 2010, to characterise patients and the routine prescribing behaviour of physicians. The interventions were predominantly aimed at patients with lower-respiratory-tract infections but were broadly based. Thus, we included patients with upper-respiratory-tract infections to assess wider effects of the interventions.
Ethics approval for the UK and Europe was granted by Southampton and South West Hampshire Local Research Ethics Committee. Research sites outside the UK also obtained ethics approval from their local organisations. Patients who fulfilled the inclusion criteria were given written and verbal information about the study and were asked to provide written informed consent.
Practices
All general practices in the localities of study centres were approached and all clinicians (and nurse prescribers in the UK) in eligible practices who prescribed antibiotics for respiratory-tract infections were invited to participate. Eligible practices were those that had not previously used any interventions to reduce rates of antibiotic prescribing and could include more than ten patients in the baseline audit. Networks of at least two practices were selected separately in Antwerp (Belgium), Barcelona (Spain), Cardiff (Wales), Łódź (Poland), Southampton (UK), Szczecin (Poland), Utrecht (Netherlands), and the Spanish Society of Family Medicine (Spain) to ensure a range of cultures, languages, and regions of Europe (north, south, and east) were represented.
Patients
Up to the first 30 patients with lower-respiratory-tract infections and up to the first five with upper-respiratory-tract infections who presented at each practice were recruited from February to May, 2011. Inclusion criteria were age older than 18 years; first consultation for acute cough of up to 28 days' duration or what the clinician believed to be an acute lower-respiratory-tract infection as the main diagnosis, despite cough not being the most prominent symptom; and diagnosis judged by the physician to be an acute upper-respiratory-tract infection (eg, sore throat, otitis media, sinusitis, influenza, and coryzal illness). Exclusion criteria were a working diagnosis of a non-infective disorder (eg, pulmonary embolus, heart failure, oesophageal reflux, or allergy); use of antibiotics in the previous month; inability to provide informed consent (eg, because of dementia, psychosis, or severe depression); pregnancy; and immunological deficiencies. Pneumonia was not an exclusion criterion.
Randomisation and masking
Randomisation of practices was done by KH and MK, was achieved by computer generation of random numbers, and was stratified by network. Minimisation was applied, on the basis of the proportion of patients prescribed antibiotics from the baseline audit, the number of participating physicians per practice, and the number of patients recruited. Practices were assigned to four trial arms: usual care; internet-based training to use a point-of-care CRP test; internet-based training in enhanced communication skills; or combined training in CRP testing and enhanced communication skills.
If only ten patients were recruited in a practice, the network average was used to avoid the unbalancing of randomisation by poorly estimated antibiotic prescribing proportions for that practice. Physicians and patients were unaware of initial group allocation but masking of physicians or patients to the intervention itself was not possible.
Interventions
Randomisation was followed by a period of internet training and a repeat audit of antibiotic prescribing (February to May, 2011, the end of the season for respiratory-tract infections). The interventions were targeted at physicians rather than patients, and were developed iteratively to be sensitive to cultural and national differences (appendix p 1).17 The usual-care group assessed and managed patients according to the practice's normal procedures.
The CRP group received internet training on how to target testing (ie, in cases of clinical uncertainty, such as in patients with abnormal auscultation, dyspnoea, and fever18) and how to negotiate with the patient about management decisions (appendix p 2). Tests were done with QuikRead CRP kits (Orion Diagnostica, Espoo, Finland) after on-site training by the manufacturer. During a run-in period of several weeks before data collection began, physicians practised using the device.
Training in enhanced communication skills focused on the gathering of information on patients' concerns and expectations, exchange of information on symptoms, natural disease course, and treatments, agreement of a management plan, summing up, and providing guidance about when to reconsult. Physicians were also provided with an interactive booklet to use during consultations that included information on symptoms, use of antibiotics and antibiotic resistance, self-help measures, and when to re-consult (appendix p 3). The training was supported by video demonstrations of consultation techniques. The internet modules and materials were translated into the relevant national language and mainly addressed lower-respiratory-tract infections, although many of the issues were relevant to all respiratory-tract infections.
Group practices were asked to appoint a lead physician to organise a structured meeting on prescribing issues. A survey was sent to all physicians after the study about approach to training (eg, completed alone or in a group) and the format of any group meetings (eg, within the practice or across multiple practices).
Case-report forms
During the index consultation physicians documented the duration of illness, severity of cough and other symptoms (rated 0, not problematic, to 4, severely problematic), severity of illness (assigned by physicians; 0, well to 10, very unwell), and use of antibiotics and tests on case-report forms created specifically for the study, and data were uploaded centrally by network facilitators. After randomisation a more detailed case-report form was used in follow-up consultations that included the same details as the index form plus medical history, current medications, smoking status, findings of structured examination, whether CRP was tested, and whether the booklet was used.6 The study did not include an independent data safety monitoring board because it was deemed to be low risk. This approach is in line with that seen in low-risk trials that have been funded by the Medical Research Council.
Statistical analysis
The primary outcome was antibiotic use, as documented on the case-report forms. Limited availability of prescription monitoring prevented the use of pharmacy dispensing data. Several secondary outcomes were assessed. New or worsening symptoms were defined as re-consultation for new or worsening symptoms within 4 weeks, new signs, or hospital admission, assessed by review of medical notes (practice staff, the local study team, or both used a standard form to report these data6). Symptom severity and duration was defined as the severity of symptoms in the 2–4 days after seeing the physician.19 The duration of symptoms rated moderately bad or worse was also recorded.6 Symptoms were rated daily as 0 (no problem) to 6 (as bad as it could be) until they resolved and the information was reported by patients in self-completed diaries.20 For patients who did not return diaries, a short form that asked for details of duration and severity of symptoms and whether antibiotics or the booklet were used was sent by post for completion. If patients did not respond, researchers contacted them by telephone and asked the questions on the form.
To calculate the sample sizes we used α=0·025 (to allow for two interventions) and β=0·2, and assumed that up to 30 patients per practice would be recruited and that antibiotic prescribing would decrease by at least 10%, from 50% to 40%, in either one of the two intervention groups. We also assumed an intracluster coefficient for antibiotic prescribing within practices of up to 0·16 (the mean of values in three previous studies10,21,22). We estimated that a sample of 940 (470×2) patients would be needed. Allowance for an inflation factor of 5·64 due to clustering, which was calculated as 1+([30–1]×0·16), and rounding of numbers for the four subgroups gave an overall sample size of 5302. If a more usual intracluster coefficient of up to 0·06 was assumed23 and controlled for patients' and practices' characteristics, a sample size of 2600 would be required. Thus we aimed to recruit a minimum of 2600 and a maximum of 5400 patients. The above assumptions were probably a conservative estimate of antibiotic reduction, on the basis of data from previous trials.10,13
Analyses were done by intention to treat and used multilevel logistic regression modelling for a factorial study to assess the main outcome (antibiotic use), controlled for baseline antibiotic prescribing rate and with allowance for clustering by physician and practice. The effects of various potential confounders related to clinical severity (age, smoking, sex, major cardiovascular or respiratory comorbidity, baseline symptoms, crepitations, wheeze, pulse higher than 100 beats per min, temperature higher 37·8°C, respiratory rate, blood pressure, physician's rating of severity, and duration of cough) were explored because of the potential for selection bias in an open trial. If interactions between interventions were not significant, the results are presented as the main effects of each intervention (ie, factorial groups with estimates controlling mutually for each intervention). A secondary analysis was done for individual groups because the study was not specifically powered for interactions. The modelled odds ratios were converted to risk ratios according to the method described by Zhang and colleagues.24 We did no interim analyses. This trial is registered, number ISRCTN99871214.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Of 446 practices approached, 259 agreed to participate (figure). They contributed baseline data for 6771 patients (5355 [79·1%] with lower-respiratory-tract infections and 1416 [20·9%] with upper-respiratory-tract infections), of whom 3742 (55·3%) were prescribed antibiotics. 13 practices included fewer than ten patients in the baseline audit and did not progress to randomisation. 372 participating physicians in 228 (92·7%) of 246 randomised practices contributed 4264 patients at follow-up (figure) of whom 3398 (79·7%) of 4264 had lower-respiratory-tract infections and the remainder had other respiratory-tract infections.
Figure.
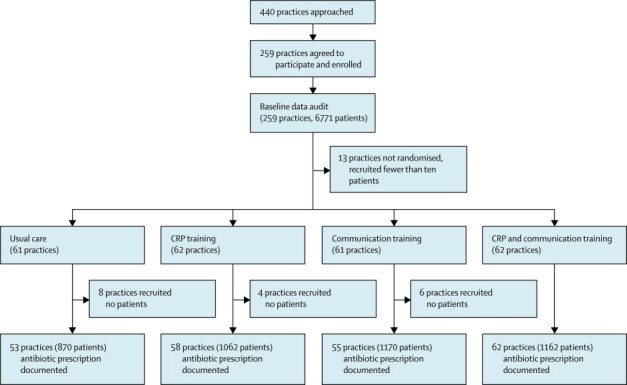
Trial profile
CRP=C-reactive protein.
All groups were well balanced (table 1). Adherence to training was good in all intervention groups, with 99 (87·6%) 113 of practices completing the CRP training and 94 (87·0%) of 108 completing the communication training (appendix p 4).
Table 1.
Clinical characteristics of factorial groups at baseline and during follow-up
| Baseline (n=6771) | No CRP training (n=2040) | CRP training (n=2224) | No communication training (n=1932) | Communication training (n=2332) | |
|---|---|---|---|---|---|
| Gender (male/female) | 2553 (38%)/4218 (62%) | 729 (36%)/1311 (64%) | 801 (36%)/1423 (64%) | 709 (37%)/1223 (63%) | 821 (35%)/1511 (65%) |
| Age (years) | 49·6 (18·6) | 50·9 (17·3) | 51·0 (17·5) | 50·8 (17·6) | 51·1 (17·2) |
| Non-smoker (past or current) | N/A | 1067 (52%) | 1147 (52%) | 1041 (54%) | 1173 (50%) |
| Illness duration before index consultation (days) | 7·8 (7·2) | 7·6 (6·0) | 7·8 (7·1) | 8·0 (7·1) | 7·5 (6·2) |
| Respiratory rate (breaths per min) | N/A | 17·0 (5·3) | 17·0 (5·7) | 17·3 (5·7) | 16·8 (5·2) |
| Temperature (°C) | N/A | 36·7 (0·9) | 36·8 (0·9) | 36·7 (0·8) | 36·8 (0·9) |
| Lung disease (COPD or asthma) | N/A | 341/1992 (17%) | 422/2195 (19%) | 333/1881 (18%) | 430/2306 (19%) |
| Severity score (all symptoms)* | 1·8 (0·5) | 1·9 (0·5) | 2·0 (0·5) | 2·0 (0·6) | 1·9 (0·5) |
| Severity of cough* | 3·0 (0·8) | 3·1 (0·8) | 3·1 (0·8) | 3·0 (0·9) | 3·1 (0·8) |
| Sputum production | 5355/6771 (79%) | 1628/1996 (82%) | 1830/2263 (81%) | 1572/1967 (80%) | 1886/2292 (82%) |
Data are number (%) or mean (SD). CRP=C-reactive protein. COPD=chronic obstructive pulmonary disease.
Severity of symptoms rated as 1=no problem, 2=mild problem, 3=moderate problem, and 4=severe problem.
All the modelled results allowed for clustering by practice but not network, as little variance was seen due to networks (appendix p 4). In the usual-care group 508 (58·4%) of 870 patients were prescribed antibiotics, which was similar to the rate at baseline. Analysis of the factorial groups showed that in the CRP group antibiotic prescribing was reduced by 15% compared with usual care and in the enhanced communication group it was reduced by 9% (table 2). In patients managed by physicians who had received CRP training, the median time to resolution of symptoms rated as moderately bad or worse was the same as that in the usual-care group (5 days), whereas in those treated by physicians in the enhanced-communication group the time was 6 days (table 3). For both interventions symptom severities in the 2–4 days after seeing the doctor were similar to those in the usual-care group, and the rates of new or worsening symptoms did not differ significantly (table 3). In a full model with all potential confounders, the interaction term between CRP and enhanced-communication training for the primary outcome was 1·33 and was not significant (p=0·414). In the individual group results for clinical characteristics and outcomes (appendix pp 5–6) antibiotic prescribing was decreased the most in the combination intervention group compared with usual care (CRP group risk ratios 0·53, 95% CI 0·36–0·74, p<0·0001; communication group 0·68, 0·50–0·89, p=0·003; combined group 0·38, 0·25–0·55, p<0·0001). For lower-respiratory-tract infections versus upper-respiratory-tract infections, results were similar to those for the whole cohort, but in the enhanced-communication group, symptom control for upper-respiratory-tract infections was possibly a little less effective than that for lower-respiratory-tract infections (appendix pp 7–10).
Table 2.
Effectiveness of CRP and enhanced-communication training in reducing antibiotic prescribing rates
| No CRP training | CRP training | No communication training | Communication training | |
|---|---|---|---|---|
| Crude percentage | 48% (984/2040) | 33% (734/2224) | 45% (876/1932) | 36% (842/2332) |
| Basic risk ratio (95% CI)* | 1·00 | 0·58 (0·48–0·70, p<0·0001) | 1·00 | 0·76 (0·63–0·89, p<0·0001) |
| Adjusted risk ratio† | 1·00 | 0·54 (0·42–0·69, p<0·0001) | 1·00 | 0·69 (0·54–0·87, p<0·0001) |
CRP=C-reactive protein.
The basic model adjusted for baseline prescribing and clustering by physician and practice.
The adjusted model additionally controlled for age, smoking, sex, major cardiovascular or respiratory comorbidity, baseline symptoms, crepitations, wheeze, pulse higher than 100 beats per min, temperature higher than 37·8°C, respiratory rate, blood pressure, physician's rating of severity, and duration of cough.
Table 3.
Effectiveness of CRP training and enhanced-communication training on symptom control
| No CRP training | CRP training | No communication training | Communication training | |
|---|---|---|---|---|
| New or worse symptoms | ||||
| Crude percentage of patients (%) | 18% (361/1962) | 19% (399/2159) | 16% (309/1879) | 20% (451/2242) |
| Basic risk ratio* | 1·00 | 1·06 (0·80 to 1·40, p=0·67) | 1·00 | 1·27 (0·96 to 1·67, p=0·10) |
| Adjusted risk ratio† | 1·00 | 1·05 (0·78 to 1·39, p=0·76) | 1·00 | 1·33 (0·99 to 1·74, p=0·055) |
| Symptom severity score days 2–4 after index consultation | ||||
| Crude mean (SD) score | 1·79 (0·99) | 1·79 (1·01) | 1·73 (0·98) | 1·84 (1·02) |
| Basic mean difference* | .. | −0·01 (−0·11 to 0·10, p=0·85) | .. | 0·09 (−0·02 to 0·20, p=0·10) |
| Adjusted mean difference† | .. | 0 (−0·09 to 0·09, p=0·99) | .. | 0·07 (−0·03 to 0·16, p=0·16) |
| Resolution of symptoms rated moderately bad or worse | ||||
| Crude median (IQR) time (days) | 5 (3 to 9) | 5 (3 to 9) | 5 (3 to 7) | 6 (3 to 10) |
| Basic hazard ratio* | 1·00 | 0·99 (0·89 to 1·12, p=0·91) | 1·00 | 0·87 (0·77 to 0·97, p=0·015) |
| Adjusted hazard ratio† | 1·00 | 0·93 (0·83 to 1·04, p=0·21) | 1·00 | 0·83 (0·74 to 0·93, p=0·002) |
CRP=C-reactive protein.
The basic model adjusted for baseline prescribing and clustering by physician and practice.
The adjusted model additionally controlled for age, smoking, sex, major cardiovascular or respiratory comorbidity, baseline symptoms, crepitations, wheeze, pulse higher than 100 beats per min, temperature higher than 37·8°C, respiratory rate, blood pressure, physician's rating of severity, and duration of cough.
30 patients were reported as being admitted to hospital (two in the usual-care group, ten in the CRP group, six in the enhanced-communication group, and 12 in the combined group). The reasons (noted in 15 patients) were mostly cardiorespiratory problems or systemic upset (cardiac n=2; respiratory n=8; generally unwell or pyrexial n=2; gastrointestinal symptoms n=2; sinusitis n=1). The difference in hospital-admission rates between the CRP group and the non-CRP group (22 vs eight) was significant when controlled for clustering (odds ratio 2·61, 95% CI 1·07–6·35, p=0·034), but of borderline significance when controlled for all potential confounders (2·91, 0·96–8·85, p=0·060). No patients died.
Discussion
This study of the effectiveness of internet training to modify antibiotic prescribing for respiratory-tract infections followed a period of careful intervention development across major language, cultural, and health-system boundaries. Our findings suggest that these interventions are transferable between very different primary-care settings.
The practices involved in the study had previously shown no interest in their levels of antibiotic prescribing and many were research naive. The prescribing rate at baseline (55·3%) was similar to that in a previous observational study,5 where most patients were given antibiotics. Although recruitment was not complex, some practices did not include the minimum number of patients in the baseline audit, which supports the everyday nature of those practices. The baseline prescribing rate in the usual-care group was similar to those in the other groups, which suggests minimum attrition bias. Control for patients' characteristics only slightly altered estimated prescribing rates and remained similar in all intervention groups, which indicates minimum confounding. The limited change in prescribing rate in the usual-care group, and the slightly worse symptomatic outcomes in the intervention groups, which were in line with evidence from placebo-controlled trials,4,6 suggest that the reductions in antibiotic prescribing in intervention groups were genuine. We assessed adherence to training but did not observe consultations to avoid introducing bias to clinicians' behaviour. The long-term effects of our study interventions on behaviour are unknown, although similar interventions have had lasting effects.10,16,25
The efficacy of the multifactorial enhanced-communication intervention supports evidence that interactive methods, rather than simply providing educational information, are most effective.12,14 In the STAR trial,16 a 4% reduction in global antibiotic use was noted, but that trial was much more intensive than ours (five online training phases vs one in this study, plus expert-led outreach seminars). Our intervention caused slightly less reduction in prescribing rates than that used in the IMPAC3T study. The risk ratio for antibiotic prescribing was 0·49 for communication training in IMPAC3T, compared with 0·68 in our study,10 but that intervention was also more intensive than ours (outreach visits plus peer review of consultations). Despite our interventions predominantly addressing lower-respiratory-tract infections, prescribing rates fell for upper-respiratory-tract infections, which suggests further modifications of the booklet and training to better address upper-respiratory-tract infections could improve efficacy. Although the diagnostic value of CRP testing has been questioned in systematic reviews,8,26 our findings are similar to those in intensive Dutch trials10,11 and suggest that internet-based training on CRP testing promotes non-antibiotic management strategies when the findings of qualitative work support the quantitative findings.17 In view of the additional resources needed for CRP testing (training, equipment, and time for testing and discussion of results) the cost-effectiveness of this intervention remains to be shown.
No intervention affected symptom severity in the first few days, but the median time to resolution of moderately severe or worse symptoms was slightly longer in the enhanced-communication group than in the usual-care group (by 1 day). The risk of new or worsening symptoms was also raised in the combined group (appendix pp 6–7), which lessens the likelihood that training in CRP training protects against symptom progression. These findings might have been due to the 12% rate of symptom reporting in the usual-care group. However, this rate is lower than that in the placebo group of a previous large trial6 and requires confirmation. Symptom control might be worse in patients with upper-respiratory-tract infections than in those with lower-respiratory-tract infections, but this finding should be interpreted cautiously as it was a secondary outcome and the interaction term was not significant. The IMPAC3T trial10 provided little evidence of poor symptom control, although the duration of severe symptoms and the rates of new or worsening symptoms were not reported. Symptom control was also not reported in a previous trial of internet-based communication training with an accompanying booklet.13 The perception of worse symptoms could have been due to expectations about the effectiveness of antibiotics in an open trial, although similar open trials have not reported such effects.10,27,28 The use of the interactive booklet might have led to increased re-consultation rates by emphasis of the long natural history of respiratory-tract infections and the raising of awareness of symptoms for which re-consultation is recommended. Likewise, although an increased rate of hospital admission could have indicated a negative effect with the CRP intervention, it might have reflected appropriate management. The slightly worse symptom control in the enhanced-communication group highlights the paucity of high-quality evidence for effective treatments.29 Improved symptom control should become a research priority. The risk of potential intervention-related harm (possibility of worse symptom control or increased rate of hospital admissions) must be balanced by the potential benefits (reduced antibiotic prescribing, demedicalisation of self-limiting illness,6,19 reduced risk of antibiotic-associated side-effects, and reduced risk of antibiotic resistance; panel).
Panel. Research in context.
Arnold and Straus12 searched the Cochrane Effective Practice and Organisation of Care Group (EPOC) specialised register (supplemented by the bibliographies of studies found in the register and the Science Citation Index), and identified 39 randomised studies and quasirandomised studies. They found that multifaceted interventions were most successful in reducing the rate of antibiotic prescribing. We searched Medline, Embase, and The Cochrane Library for articles published from January, 1990, to July, 2009, with several combinations of the following keywords: “antibiotic”, “primary care”, “intervention”, “respiratory tract infection”, and specific MeSH terms for respiratory-tract-infection diagnoses. We manually screened reference lists to identify further articles of interest. Inclusion criteria for articles were an intervention primarily targeted at physicians in a primary care setting aiming to improve antibiotic prescription for respiratory-tract infections, studies done in high-income countries, presentation of a standardised outcome of first-choice prescription measured in defined daily dosage, prescriptions or rates, and publication in the English language. Relevance of studies was screened by assessments of titles, keywords, abstracts, and full texts, independently by two reviewers. Disagreements were resolved by consensus or arbitration by a third person. The main reasons for exclusion were a lack of standardised outcomes or a clear description of intervention features. We identified 58 studies describing 87 interventions in primary care related to antibiotic prescribing for respiratory-tract infections, and confirmed that multiple interventions containing at least educational material for physicians were most frequently effective. This finding underscored promising evidence for communication-skills training and near-patient testing.15 Patients' outcomes, however, were rarely reported, the participants and settings were generally restricted, and outreach interventions used highly trained staff at research centres of excellence and, therefore, the generalisability of interventions seemed poor. Only one trial had used the internet for intervention delivery. In a study that involved 246 primary-care practices in six European countries with different languages, cultures, and health systems, we aimed to assess the effect of internet-based training in the use of a C-reactive protein point-of-care test and in enhanced communication skills, supported by an interactive booklet for patients.
Interpretation
Both types of training were associated with notable reductions in antibiotic prescribing rates, but the two training methods combined had the greatest effect (C-reactive-protein intervention risk ratio 0·53, 95% CI 0·36–0·74; enhanced-communication intervention 0·68, 0·50–0·89; combined intervention 0·38, 0·25–0·55). We found evidence of slightly lengthened duration (1 day) of symptoms rated moderately bad or worse with the enhanced-communication training, and increased hospital admissions with C-reactive-protein training (22 vs eight with usual care), but these outcomes may be balanced against the potential benefits (reduced antibiotic prescribing, demedicalisation of self-limiting illness,6,19 reduced risk of antibiotic-associated side-effects, and reduced risk of antibiotic resistance). The easily accessible format of internet-based intervention delivery and the success of the interventions across national language and cultural boundaries suggest that these interventions could be implemented widely in many health systems.
Internet-based training in how to use a CRP point-of-care test or in enhanced communication skills plus use of an interactive information booklet achieved important reductions in antibiotic prescribing for respiratory-tract infections in several countries. These interventions, therefore, can be disseminated widely and maintain efficacy. Effective symptom-control strategies are needed to support reductions in antibiotic prescribing.
Acknowledgments
Acknowledgments
This study was supported by the European Commission Framework 6 Programme (grant 518226). The work in UK was also supported by the National Institute for Health Research and the Research Foundation Flanders (grant G.0274.08N). We thank Ian Williamson for support during the study and help with the protocol development. Members of the GRACE consortium trial team whose hard work has made this possible are Niels Adriaenssens, Juande Alcántara, Javier Arranz, Zuzana Bielicka, Francesco Blasi, Pascale Bruno, Curt Brugman, Jo Coast, Josep Maria Cots, Patricia Fernandez, Guillermo García, Iris Hering, Helena Hupkova, Greet Ieven, Anna Kowalczyk, Jaroslaw Krawczyk, Christine Lammens, Christina Lannering, Marieke Lemiengre, Frank Leus, Katherine Loens, Ana Moragas, Nuria Sanchez-Ruano, Matteu Serra Prat, Richard Smith, Tom Schaberg, Ann de Sutter, Igor Svab, Jackie Swain, Pia Touboul, Robert Veen, and Tricia Worby. We thank all the clinicians and patients who consented to be involved in the study. We also thank the independent GRACE trial steering committee—Patrick Bindels, Gordon Taylor, and Mark Woodhead—for their help and suggestions and supervision of the safety features of the trial in the absence of an independent data safety monitoring board.
Contributors
The study was conceived by PL, SC, CB, HG, and TV. All authors contributed to the development of the protocol, and to the management of the study. GO'R led the day-to-day management of the study supervised by PL and TV. LY led and supervised the design and development of the web-based intervention. PL, NF, CB, and LY led the development of the communication intervention. PL, JWLC, and HM led the development of the intervention for C-reactive-protein testing. HG led the funding application and provided overall coordination of the GRACE consortium. KH coordinated randomisation and PL, BS, MK, and MMu analysed the data. All authors contributed to the interpretation of the data and the writing of the paper. PL, BS, and MMu had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest
We declare that we have no conflicts of interest.
Supplementary Material
References
- 1.Akkerman E, Van der Wouden J, Kuyvenhoven M, Dieleman J, Verheij T. Antibiotic prescribing for respiratory tract infections in Dutch primary care in relation to patient age and clinical entities. J Antimicrob Chemother. 2004;54:1116–1121. doi: 10.1093/jac/dkh480. [DOI] [PubMed] [Google Scholar]
- 2.Petersen I, Johnson A, Islam A, Duckworth G, Livermore D, Hayward A. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ. 2007;335:982. doi: 10.1136/bmj.39345.405243.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroening-Roche J, Soroudi A, Castillo E, Vilke G. Antibiotic and bronchodilator prescribing for acute bronchitis in the emergency department. J Emerg Med. 2012;43:221–227. doi: 10.1016/j.jemermed.2011.06.143. [DOI] [PubMed] [Google Scholar]
- 4.Smith SM, Fahey T, Smucny J, Becker L. Antibiotics for acute bronchitis. Cochrane Database Syst Rev. 2004;4:CD000245. doi: 10.1002/14651858.CD000245.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Butler C, Hood K, Verheij T. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: prospective study in 13 countries. BMJ. 2009;338:b2242. doi: 10.1136/bmj.b2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Little P, Stuart B, Moore M. Amoxicillin for acute lower-respiratory-tract infection in primary care when pneumonia is not suspected: a 12-country, randomised, placebo-controlled trial. Lancet Infect Dis. 2013;13:123–129. doi: 10.1016/S1473-3099(12)70300-6. [DOI] [PubMed] [Google Scholar]
- 7.Goossens H, Ferech M, Vander Stichele R, Elseviers M; ESAC project group Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 8.van der Meer V, Neven AK, van den Broek PJ, Assendelft WJJ. Diagnostic value of C reactive protein in infections of the lower respiratory tract: systematic review. BMJ. 2005;331:26. doi: 10.1136/bmj.38483.478183.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falk G, Fahey T. C-reactive protein and community-acquired pneumonia in ambulatory care: systematic review of diagnostic accuracy studies. Fam Pract. 2009;26:10–21. doi: 10.1093/fampra/cmn095. [DOI] [PubMed] [Google Scholar]
- 10.Cals J, Butler C, Hopstaken R, Hood K, Dinant G. Effect of point of care testing for C reactive protein and training in communication skills on antibiotic use in lower respiratory tract infections: cluster randomised trial. BMJ. 2009;338:b1374. doi: 10.1136/bmj.b1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cals J, Schot M, de Jong S, Dinant G, Hopstaken R. Point-of-care C-reactive protein testing and antibiotic prescribing for respiratory tract infections: a randomized controlled trial. Ann Fam Med. 2010;8:124–133. doi: 10.1370/afm.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold S, Straus S. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev. 2005;4:CD003539. doi: 10.1002/14651858.CD003539.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis N, Butler C, Hood K, Simpson S, Wood F, Nuttall J. Effect of using an interactive booklet about childhood respiratory tract infections in primary care consultations on reconsulting and antibiotic prescribing: a cluster randomised controlled trial. BMJ. 2009;339:b2885. doi: 10.1136/bmj.b2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Brien M, Rogers S, Jamtvendt G. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2007;4:CD000409. doi: 10.1002/14651858.CD000409.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Velden A, Pijpers E, Kuyvenhoven M, Tonkin-Crine S, Little P, Verheij T. Effectiveness of physician-targeted interventions to improve antibiotic use for respiratory tract infections. Br J Gen Pract. 2012;62:e801–e807. doi: 10.3399/bjgp12X659268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler C, Simpson S, Dunstan F. Effectiveness of multifaceted educational programme to reduce antibiotic dispensing in primary care: practice based randomised controlled trial. BMJ. 2012;344:d8173. doi: 10.1136/bmj.d8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anthierens S, Tonkin-Crine S, Douglas E. General practitioners' views on the acceptability and applicability of a web-based intervention to reduce antibiotic prescribing for acute cough in multiple European countries: a qualitative study prior to a randomised trial. BMC Fam Pract. 2012;13:101. doi: 10.1186/1471-2296-13-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coenen S, Michiels B, Renard D, Denekens J. Antibiotic prescribing for acute cough: the effect of perceived patient demand. Br J Gen Pract. 2006;56:183–190. [PMC free article] [PubMed] [Google Scholar]
- 19.Little P, Rumsby K, Kelly J. Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection: a randomised controlled trial. JAMA. 2005;293:3029–3035. doi: 10.1001/jama.293.24.3029. [DOI] [PubMed] [Google Scholar]
- 20.Watson L, Little P, Williamson I, Moore M, Warner G. Validation study of a diary for use in acute lower respiratory tract infection. Fam Pract. 2001;18:553–554. doi: 10.1093/fampra/18.5.553. [DOI] [PubMed] [Google Scholar]
- 21.Coenen S, Van Royen P, Michiels B, Denekens J. Optimizing antibiotic prescribing for acute cough in general practice: a cluster-randomized controlled trial. J Antimicrob Chemother. 2004;54:661–672. doi: 10.1093/jac/dkh374. [DOI] [PubMed] [Google Scholar]
- 22.Welschen I, Kuyvenhoven M, Hoes A, Verheij T. Effectiveness of a multiple intervention to reduce antibiotic prescribing for respiratory tract symptoms in primary care: randomised controlled trial. BMJ. 2004;329:431. doi: 10.1136/bmj.38182.591238.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams G, Gulliford M, Ukoumunne O, Eldridge S, Chinn S, Campbell M. Patterns of intra-cluster correlation from primary care research to inform study design and analysis. J Clin Epidemiol. 2004;57:785–794. doi: 10.1016/j.jclinepi.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Yu K. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 25.Cals J, De Bock L, Beckers P-J. Enhanced communication skills and C-reactive protein point-of-care testing for respiratory tract infection: 3·5-year follow-up of a cluster randomized trial. Ann Fam Med. 2013;11:157–164. doi: 10.1370/afm.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falk G, Fahey T. C-reactive protein and community-acquired pneumonia in ambulatory care: systematic review of diagnostic accuracy studies. Fam Pract. 2009;26:10–21. doi: 10.1093/fampra/cmn095. [DOI] [PubMed] [Google Scholar]
- 27.Little P, Rumsby K, Kelly J. Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection: a randomised controlled trial. JAMA. 2005;293:3029–3035. doi: 10.1001/jama.293.24.3029. [DOI] [PubMed] [Google Scholar]
- 28.Little PS, Williamson I, Warner G, Gould C, Gantley M, Kinmonth AL. An open randomised trial of prescribing strategies for sore throat. BMJ. 1997;314:722–727. doi: 10.1136/bmj.314.7082.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith S, Schroeder K, Fahey T. Over-the-counter (OTC) medications for acute cough in children and adults in ambulatory settings. Cochrane Database Syst Rev. 2007;8 doi: 10.1002/14651858.CD001831.pub4. CD001831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


