Abstract
Sexual dysfunction is common with selective serotonin reuptake inhibitor use for major depressive disorder. Studies have shown associations between genetic variation in the adenosine triphosphate (ATP)-binding cassette, subfamily B, member 1 gene (ABCB1), which encodes the drug efflux transporter P-glycoprotein (PGP), and selective serotonin reuptake inhibitor response. This study measured functionally implicated ABCB1 variants (rs2235015, rs1128503, rs2032582, and rs1045642) and sexual dysfunction using the Changes in Sexual Functioning Questionnaire. This study included outpatients (18–40 years of age) treated for major depressive disorder with a selective serotonin reuptake inhibitor for 6 weeks. Changes in Sexual Functioning Questionnaire outcomes were stratified by ABCB1 genotype and PGP substrate status. The authors recruited 82 individuals (22 men and 57 women). Women receiving a PGP substrate with a rs1128503 TT genotype had a significantly lower Changes in Sexual Functioning Questionnaire total score (37.2 ± 5.4), indicating greater sexual dysfunction, than did those with the CT (42.9 ± 6.3) or CC genotypes (46.6 ± 5.6), F(2) = 6.00, p = .005, p = .02, with multiple testing correction. The results indicate a relationship between genotypes at rs1128503, total sexual dysfunction, and PGP substrates use for women and may explain some of the sexual dysfunction variability seen with selective serotonin reuptake inhibitor treatment. Results need to be confirmed with a larger sample size that includes men.
Sexual dysfunction is a common side effect patients often experience during the treatment of major depressive disorder with selective serotonin reuptake inhibitors (SSRI). However, the severity of sexual dysfunction varies and does not occur in everyone. Previously our group reported a pharmacogenetic relation between the serotonin 2A receptor (HTR2A) and G-protein beta3 subunit (GNB3) genes and sexual dysfunction with SSRI use (Bishop, Moline, Ellingrod, Schultz, & Clayton, 2006). Others have reported a relation between genetic variation within the cytochrome P4502D6 gene and sexual dysfunction from paroxetine use (Zourkova, Ceskova, Hadasova, & Ravcukova, 2007). No sexual dysfunction studies have considered a pharmacogenetic target such as the ATP-binding cassette, subfamily B, member 1 gene (ABCB1), which encodes for the drug efflux transporter p-glycoprotein (PGP) located on the blood brain barrier and may influence the central nervous system activity of some antidepressant agents.
The ABCB1 gene encodes a transmembrane protein expressed in the liver, kidney, intestine, brain, and other tissues, that is an efflux pump with broad substrate specificity (He, Li, Kanwar, & Zhou, 2011; Urquhart & Kim, 2009). Recently, several antidepressants including citalopram, paroxetine, and sertraline have been reported to be ABCB1 substrates. Furthermore, a relation between genetic variation within ABCB1 and SSRI response has been reported (Kato et al., 2008; Nikisch, Eap, & Baumann, 2008; Uhr et al., 2008)
Given PGP’s importance in the central nervous system penetration of SSRIs such as citalopram, paroxetine, and sertraline the purpose of this study was to understand the relation between the potentially functional ABCB1 variants (C3435T (rs1045642), G2677T (rs2032582), C1236T (rs1128503), and rs2235015) and sexual dysfunction during SSRI therapy as measured in a population of 18–40-year-old women at low risk for other causes of sexual dysfunction. We hypothesized that genetic variation known to influence the function of PGP would increase the risk of sexual dysfunction from SSRI use because of putatively higher antidepressant concentrations in the central nervous system.
METHOD
Outpatient participants with depression were recruited through local advertisements while being treated with citalopram, escitalopram, fluoxetine, paroxetine, or sertraline for depression. Many of those included in this study were also part of a previously published pharmacogenomics point-prevalence study of SSRI use in men and women with major depressive disorder (Bishop et al., 2006).
Participants were included if they had been treated with an SSRI for at least 6 weeks, were between ages 18 and 40 years, and did not report any problems with sexual desire or functioning before starting the medication. Potential participants were excluded if they were taking another medication for depression or any other medications known to affect sexual functioning (positively or negatively), significant residual depression symptoms as measured by Hamilton Rating Scale for Depression 21-item version scores >10, had any other documented primary Axis I diagnosis, cardiovascular disease, neurological disorder, diabetes mellitus (Type I or II), genitourinary disease, or reported frequent urinary tract infections. Chart reviews were conducted to confirm that the participants met the aforementioned inclusion criteria and carried a diagnosis of depression.
Assessments
Study assessments were completed as described previously (Bishop et al., 2006). Participants were briefly assessed in person at the University of Iowa General Clinical Research Center where they gave informed consented using a document approved by the University of Iowa Institutional Review Board. The general assessment included vital signs, height, weight, and sociodemographic variables previously associated with sexual well-being (i.e., age, marital status, number of children, years of education, alcohol consumption, smoking status, or illicit drug use). Other assessments included the Hamilton Rating Scale for Depression, Hamilton Rating Scale for Anxiety, specific SSRI used, dose and duration of use.
We assessed sexual dysfunction with the self-administered Changes in Sexual Function Questionnaire (CSFQ), which has been validated in healthy and depressed clinic populations and used in longitudinal and cross-sectional studies of well-being (Clayton, McGarvey, & Clavet, 1997). The CSFQ consists of separate assessments for men and women and includes questions to assess a variety of different causes of change in sexual functioning or desire (i.e., relationship changes, stress level, illness, medications). Sexual dysfunction is determined by falling below sex-specific thresholds on the total score (<41 for women), where overall lower scores are indicative of decreased sexual desire or functioning. The CSFQ also consists of subscales designed to assess specific aspects of sexual well-being; pleasure (scored 1 to 5), desire/interest (scored 3 to15), desire/frequency (scored 2 to 10), arousal (scored 3 to 15), and orgasm (scored 3 to 15). CSFQ total score was determined a priori to be our primary outcome variable for these analyses with subscale measures assessed as secondary outcomes.
Genomic DNA was isolated from buccal cells collected with cheek brushes (Cyto-pak, Medical Packaging Corp., Camarillo, CA) using a previously described protocol (Richards et al., 1993). After amplification by polymerase chain reaction, products were visualized by electrophoresis on 1.8% agarose gels stained with ethidium bromide. The four ABCB1 variants (rs2235015, rs2032582, rs1128503, and rs1045642) were genotyped using Pyrosequencing Technology (Ronaghi, 2003). Additional assay specifics may be obtained through author request. These variants had been previously identified in relation to SSRI response and central nervous system penetration (Hoffmeyer et al., 2000; Kato et al., 2008; Nikisch et al., 2008; Uhr et al., 2008). Genotype calls were made blinded to participant assessments. Ambiguous calls were repeated with a consensus assessment of genotypes. Call rates were 99% for these assays.
Statistical Analysis
We assessed sociodemographic and clinical variable differences between medication groups (PGP substrate and non-PGP substrate) and genotype groups using chi square and analysis of variance (or an appropriate nonparametric alternative if applicable) for categorical and continuous data, respectively. Pearson’s correlation coefficients were used to identify any significant relations among age, treatment duration, Hamilton Rating Scale for Depression scores, Hamilton Rating Scale for Anxiety scores and CSFQ total scores in all participants, regardless of medication status. Those using citalopram, escitalopram, paroxetine or sertraline were placed PGP substrate group (n = 43), while those receiving fluoxetine were placed in the nonsubstrate group (n = 14) on the basis of data presented by Uhr (Uhr et al., 2008).
The primary genetic analyses consisted of conducting a genotype association analysis of the relation between each ABCB1 variant and CSFQ scores. For each variant (rs1045642, rs2032582, rs1128503, and rs2235015), analysis of variance was done using the CSFQ scores as a dependent variable, genotypes as independent variables, stratified by PGP substrate status.. CSFQ total score and subscale measures (i.e., pleasure, frequency, interest, arousal, and orgasm) were assessed as continuous variables. We used a two-tailed alpha value of p ≤.05 as the threshold for statistical significance. The p values were Bonferroni-corrected to account for multiple comparisons of four ABCB1 variants with p < .05 considered significant. Statistics packages were SAS version 9.0, SAS JMP version 9.0.2.
RESULTS
We recruited 82 individuals (22 men and 57 women) with Hamilton Rating Scale for Depression scores ≤10. Because of the small number of male subjects (n = 22), and the even smaller number that were receiving non-PGP substrate SSRIs (n = 4), we only examined women in this pharmacogenetic analysis. Tables 1 and 2 summarize the demographics and characteristics of the participants included in this study as a whole and stratified by PGP substrate status. There were no significant correlations between CSFQ total scores and age, Hamilton Rating Scale for Depression, Hamilton Rating Scale for Anxiety, or treatment duration. The prevalence of SSRI-associated sexual dysfunction, as defined by CSFQ total score threshold of <41 was 42%. Dysfunction did not differ by medication groups with a rate of 42% in PGP substrate group and 43% in the non-PGP substrate group (p = .95). All ABCB1 genotype were normally distributed and did not deviate from Hardy Weinberg Equilibrium (p > .63).
TABLE 1.
Participant Demographics and Characteristics Stratified by PGP Substrate Status
| Category | All (N = 57) | PGP substrate (n = 43) | Non-PGP substrate (n = 14) |
|---|---|---|---|
| Measure | |||
| Age, years (M ± SD) | 25.6 ± 5.3 | 24.9 ± 5.0 | 27.8 ± 5.9 |
| HAM-D (M ± SD) | 5.5 ± 2.6 | 5.1 ± 2.7 | 6.5 ± 2.0 |
| HAM-A (M ± SD) | 5.6 ± 2.7 | 5.5 ± 3.0 | 5.9 ± 1.6 |
| Race | |||
| Caucasian | 52 (91%) | 39 (91%) | 13 (93%) |
| African American | 2 (4%) | 2 (5%) | 0 (0%) |
| Other | 3 (5%) | 2 (4%) | 1 (7%) |
| Marital status | |||
| Single | 42 (74%) | 33 (77%) | 10 (71%) |
| Married | 15 (26%) | 10 (23%) | 4 (29%) |
| Children | |||
| 0 | 49 (86%) | 39 (91%) | 11 (79%) |
| ≥1 | 8 (14%) | 4 (9%) | 3 (21%) |
| Dose category | |||
| Low | 39 (68%) | 29 (67%) | 10 (71%) |
| High | 18 (32%) | 14 (33%) | 4 (29%) |
| Medication | |||
| Citalopram | 3 (5%) | 3 (7%) | 0 (0%) |
| Escitalopram | 19 (33%) | 19 (44%) | 0 (0%) |
| Fluoxetine | 14 (25%) | 0 (0%) | 14 (100%) |
| Paroxetine | 5 (9%) | 5 (12%) | 0 (0%) |
| Sertraline | 16 (28%) | 16 (37%) | 0 (0%) |
PGP = P-glycoprotein; HAM-D = Hamilton Depression Rating Scale; HAM-A = Hamilton Anxiety Rating Scale; SD = Standard Deviation; m = measure.
TABLE 2.
CSFQ Scores Stratified by Genotype for rs1128503 for Women Using a PGP Substrate
| CC genotype (n = 14) | CT genotype (n = 21) | TT genotype (n = 8) | |
|---|---|---|---|
| % total CSFQ score <41 | 14 | 33 | 75 |
| Measure | M ± SD | M ± SD | M ± SD |
| Total score* | 46.6 ± 5.6 | 42.9 ± 6.3 | 37.2 ± 5.4 |
| Pleasure score | 3.0 ± 1.2 | 2.7 ± 1.0 | 2.4 ± 0.9 |
| Frequency score | 6.1 ± 1.8 | 5.4 ± 1.4 | 5.3 ± 1.8 |
| Interest score | 8.4 ± 2.8 | 8.3 ± 2.8 | 7.4 ± 2.6 |
| Arousal score | 9.5 ± 3.0 | 9.3 ± 2.1 | 8.3 ± 2.2 |
| Orgasm score | 9.4 ± 3.1 | 8.9 ± 3.6 | 6.9 ± 3.0 |
The corrected p value for total score was .022. The p values are corrected for multiple testing.
Corrected p value of <.05.
CSFQ = Changes in Sexual Functioning Questionnaire; m = measure; SD = standard deviation.
We found a significant relation between the ABCB1 rs1128503 variant and SSRI-associated sexual dysfunction as measured by CSFQ total scores for the PGP substrate group. Overall, we observed an allelic dose effect with women with the CC genotype (46.6 ± 5.6) having the highest CSFQ scores, followed by those with the CT genotype (42.9 ± 6.3) and the lowest scores observed in the TT genotype group (37.2 ± 5.4), F(2) = 6.00, p = .005. After Bonferroni correction to account for multiple comparisons (dividing by four to reflect the number of genetic variants assessed), these results remained significant (p = .02). In women not receiving a PGP substrate, there was no evidence for a significant association observed, F(2) 1.034, p = .39. This relation for women receiving a PGP substrate is shown in Figure 1. In secondary analyses, there was no evidence for genotype associations and CSFQ subscale scores in either PGP substrate status.
FIGURE 1.
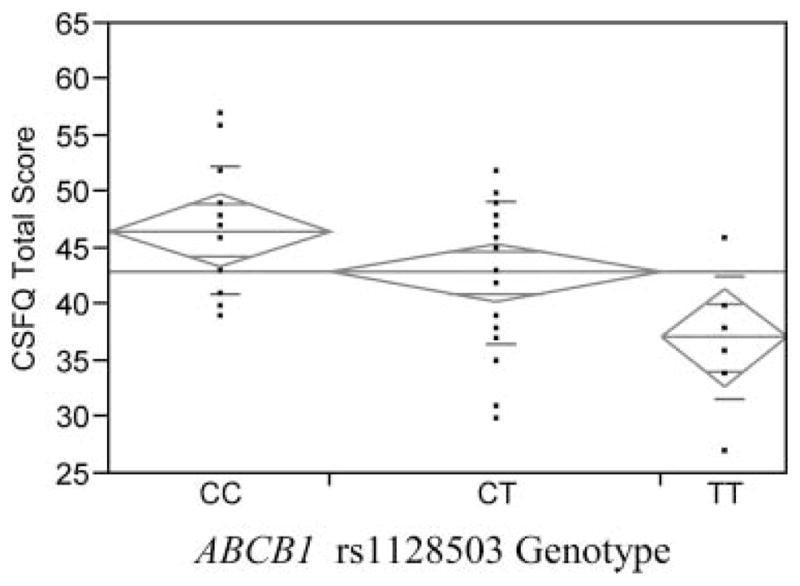
One-way analysis of Total Changes in Sexual Function Questionnaire Sexual Score by rs1128503 for women taking an SSRI that is an ABCB1 substrate is shown. Analysis of variance means are shown.
DISCUSSION
The primary result of this study is that ABCB1 genetic variation may be associated with SSRI linked sexual dysfunction, which to our knowledge is the first examination of this relationship. In particular, we found a significant association between ABCB1 rs1128503 and CSFQ total scores in women receiving a PGP substrate SSRI. These results were statistically and clinically meaningful. There appears to be an allelic dose effect. Women taking a PGP substrate SSRI with the rs1128503 TT genotype had mean CSFQ total scores below the threshold score for sexual dysfunction. Women with the CT and CC genotypes had threshold total scores above the sexual dysfunction threshold. If these results are confirmed, individuals who have the rs1128503 CC genotype may have a lower likelihood of sexual side effects if they take an SSRI that is not a PGP substrate.
The functional significance of the rs1128503 variant is unknown. It is a synonymous variant within exon 12 of the gene, occurring at position 1236(CIT) and amino acid position 412 (Gly), which may be in linkage disequilibrium with other functional variants. The ABCB1 family of drug transporters has been relatively understudied in relation to drug response in depression and much of the literature is somewhat conflicting. For example, Nikisch and colleagues measured citalopram plasma and CSF concentrations related to ABCB1 variants along with treatment response to determine pharmacogenetic associations between the two (Nikisch et al., 2008). They reported that the rs2032582 G2677T/A homozygous variant genotype (TT) was associated with increased plasma and CSF concentrations of citalopram, and treatment response for those with this genotype was worse than the wild type. This contradicts previous finding that the G allele was associated with a poor treatment response (Kato et al., 2008). All of this contradictory evidence suggests the need for more carefully designed experiments with standardized methodology to determine the significance of these pharmacogenetic associations in relation to treatment response and side effects.
The primary limitations of our study are the sample size and the need to restrict this analysis to women. In addition, the majority of the sample reported being part of the Caucasian population, which may have also biased our results because ABCB1 does exhibit some racial differences (Ameyaw et al., 2001). Our results did not differ when we excluded the few non-White participants enrolled in this study. The small sample size precludes our ability to reliably infer ABCB1 haplotypes and other genetic variants which may also be related to SSRI sexual dysfunction. Another limitation of this investigation is that participants were recruited after they had been treated for at least 6 weeks and a structured assessment of sexual functioning was unable to be done before treatment was started, however within our exclusion criteria we did not consent participants who endorsed having sexual functioning issues before starting SSRIs. While this was done to minimize the possibility that the participant’s sexual dysfunction was not the result of inadequately treated depression, this process may have eliminated those who discontinued medication before 6 weeks of treatment because of adverse side effects such as sexual dysfunction or lack of response. Given that 42% of men and 15% of women discontinue antidepressant treatment over sexual adverse effects, it is possible that we may have missed those who experienced the worst sexual dysfunction (Rosenberg, Bleiberg, Koscis, & Gross, 2003). Thus, we feel the relation between the rs1128503 TT genotype and CSFQ scores may be stronger than reported because of this same phenomenon within our study design. It is known that switching within medication classes (e.g., SSRI to SSRI) is beneficial and results in improved tolerability and/or response, even though in efficacy and effectiveness studies, the side effect and response profiles for SSRIs are similar. The idea that genetic variation related to the transport of these medications across the blood brain barrier may contribute to the disposition to these drugs and that this had such an observable effect in this small sample is pretty meaningful in this regard.
CONCLUSIONS
In conclusion, genetic variation within ABCB1 (specifically the rs1128503 variant) appear to be related to sexual dysfunction for individuals receiving a PGP substrate SSRI for treatment of major depressive disorder. Although more detailed investigations into the relations between genetic variations within ABCB1 as well as a better understanding of which SSRIs are glycoprotein substrates are needed, the results of this study shed some light onto the pharmacogenetics of SSRI associated sexual dysfunction. Thus, for women with the ABCB1 rs1128503 TT genotype, use of a non-PGP substrate such as fluoxetine may help to reduce the occurrence of sexual dysfunction from medication use. For those who are T allele carriers, additional monitoring for the occurrence of sexual dysfunction may help to identify this issue early on and decrease the potential for pre–mature medication discontinuation before a depressive response can be seen. Although these results help in our quest to clinically incorporate personalized medicine within the treatment of depression, they should be taken cautiously until they are replicated in a larger sample that includes male subjects.
Acknowledgments
The authors acknowledge Dr. Anita H. Clayton for her permission to use the Changes in Sexual Function Questionnaire.
This research was supported by NIH-NCRR 1UL1RR024979, 1KL2RR024980, and 1TL1RR024981 from the University of Iowa; UL1RR024986 from the University of Michigan; and K08MH083888 (NIMH).
Contributor Information
MICHAEL J. BLY, College of Pharmacy, University of Michigan, Ann Arbor, Michigan, USA
JEFFREY R. BISHOP, College of Pharmacy and Medicine, Department of Psychiatry, University of Illinois at Chicago, Chicago, Illinois, USA
KELAN L. H. THOMAS, Pharmacy, University of Southern California, Los Angeles, California, USA
VICKI L. ELLINGROD, College of Pharmacy and School of Medicine, Department of Psychiatry, University of Michigan, Ann Arbor, Michigan, USA
References
- Ameyaw MM, Regateiro F, Li T, Liu X, Tariq M, Mobarek A, McLeod HL. MDR1 pharmacogenetics: Frequency of the C3435T mutation in exon 26 is significantly influenced by ethnicity. Pharmacogenetics. 2001;11:217–221. doi: 10.1097/00008571-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Bishop JR, Moline J, Ellingrod VL, Schultz SK, Clayton AH. Serotonin 2A -1438 G/A and G-protein Beta3 subunit C825T polymorphisms in patients with depression and SSRI-associated sexual side-effects. Neuropsychopharmacology. 2006;31:2281–2288. doi: 10.1038/sj.npp.1301090. [DOI] [PubMed] [Google Scholar]
- Clayton AH, McGarvey EL, Clavet GJ. The Changes in Sexual Functioning Questionnaire (CSFQ): Development, reliability, and validity. Psychopharmacology Bulletin. 1997;33:731–745. [PubMed] [Google Scholar]
- He SM, Li R, Kanwar JR, Zhou SF. Structural and functional properties of human multidrug resistance protein 1 (MRP1/ABCC1) Current Medicinal Chemistry. 2011;18:439–481. doi: 10.2174/092986711794839197. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, Brinkmann U. Functional polymorphisms of the human multidrug-resistance gene: Multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Fukuda T, Serretti A, Wakeno M, Okugawa G, Ikenaga Y, Kinoshita T. ABCB1 (MDR1) gene polymorphisms are associated with the clinical response to paroxetine in patients with major depressive disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32:398–404. doi: 10.1016/j.pnpbp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Nikisch G, Eap CB, Baumann P. Citalopram enantiomers in plasma and cerebrospinal fluid of ABCB1 genotyped depressive patients and clinical response: A pilot study. Pharmacological Research. 2008;58:344–347. doi: 10.1016/j.phrs.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Richards B, Skoletsky J, Shuber AP, Balfour R, Stern RC, Dorkin HL, Klinger KW. Multiplex PCR amplification from the CFTR gene using DNA prepared from buccal brushes/swabs. Human Molecular Genetics. 1993;2:159–163. doi: 10.1093/hmg/2.2.159. [DOI] [PubMed] [Google Scholar]
- Ronaghi M. Pyrosequencing for SNP genotyping. Methods in Molecular Biology. 2003;212:189–195. doi: 10.1385/1-59259-327-5:189. [DOI] [PubMed] [Google Scholar]
- Rosenberg KP, Bleiberg KL, Koscis J, Gross C. A survey of sexual side effects among severely mentally ill patients taking psychotropic medications: Impact on compliance. Journal of Sex & Marital Therapy. 2003;29:289–296. doi: 10.1080/00926230390195524. [DOI] [PubMed] [Google Scholar]
- Uhr M, Tontsch A, Namendorf C, Ripke S, Lucae S, Ising M, Holsboer F. Polymorphisms in the drug transporter gene ABCB1 predict antidepressant treatment response in depression. Neuron. 2008;57:203–209. doi: 10.1016/j.neuron.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Urquhart BL, Kim RB. Blood-brain barrier transporters and response to CNS-active drugs. European Journal of Clinical Pharmacology. 2009;65:1063–1070. doi: 10.1007/s00228-009-0714-8. [DOI] [PubMed] [Google Scholar]
- Zourkova A, Ceskova E, Hadasova E, Ravcukova B. Links among paroxetine-induced sexual dysfunctions, gender, and CYP2D6 activity. Journal of Sex & Marital Therapy. 2007;33:343–355. doi: 10.1080/00926230701385589. [DOI] [PubMed] [Google Scholar]


