Abstract
Studies have demonstrated ERP abnormalities related to concentration difficulties in post-traumatic stress disorder (PTSD). We used an identical-twin, case-control design to investigate whether these abnormalities reflect pre-trauma vulnerability or the acquired consequence of PTSD. Vietnam combat veterans and their non-combat-exposed, identical twins completed a three-tone oddball task. Veterans with PTSD had delayed target N2 latencies compared to veterans without PTSD. In a small non-medicated, non-smoking subsample, veterans with PTSD also had significantly diminished target P3b amplitudes. A mixed-model, random-effects analysis on the non-medicated, non-smoking subsample that included the combat-unexposed co-twins showed a significant Diagnosis × combat Exposure interaction for target P3b amplitude. Results replicate increased N2 latency and diminished P3b amplitude in PTSD and suggest that diminished P3b amplitude is an acquired condition in PTSD.
Discriptors: Twin studies, Posttraumatic stress disorder, Event-related potentials, P3b
Over the last decade, a number of electrophysiologic studies have found that individuals with posttraumatic stress disorder (PTSD) show abnormal brain processing of neutral stimuli that are unrelated to traumatic events (for a review see Karl, Malta, & Maercker, 2006). In an initial study, McFarlane, Weber, and Clark (1993) measured event-related potentials (ERPs) during a three-tone auditory “oddball” task (Pfefferbaum et al., 1990) and reported that PTSD patients demonstrated delayed N2 and diminished P3b components to target stimuli compared to healthy, non-traumatized control participants. These ERP components are thought to reflect brain activity associated with the voluntary engagement of attention toward task-relevant events. Specifically, delayed N2 latency to target stimuli suggests a slowing in the neural systems concerned with the detection and redirection of attention towards environmental change. Reduced P3b amplitude purportedly reflects a reduction in the attentional resources allocated towards stimulus processing (Polich, 2003), which may be related to motivational (Ford, 1999; Friedman, 1990) in addition to cognitive factors. The presence of these abnormalities suggests that PTSD is associated with a general information processing abnormality that extends beyond the processing of traumatic memories and threatening environmental stimuli (Grossman, Buchsbaum, & Yehuda, 2002). These abnormalities provide electrophysiological support for the DSM-IV (American Psychiatric Association, 1994) PTSD symptom of disturbed concentration (D.3).
Although delayed N2 latency has not been replicated in PTSD, most (Charles et al., 1995; Metzger, Orr, Lasko, Berry, & Pitman, 1997a; Metzger, Orr, Lasko, & Pitman, 1997b; Araki et al., 2005), but not all (Kimble, Kaloupek, Kaufman, & Deldin, 2000; Neylan et al., 2003; Metzger et al., 2002) subsequent studies have found further evidence for diminished auditory P3b amplitude in individuals with PTSD. In meta-analytic review, Karl and colleagues (Karl et al., 2006) summarized study findings of parietal P3b amplitude in PTSD by calculating the overall mean weighted effect size r for two homogenous study subsamples, i.e., those comparing PTSD patients with trauma-exposed vs. non-trauma-exposed comparison groups. Meta-analysis of three studies (N = 56) using a trauma-exposed, and two studies (N = 71) using a non-trauma-exposed, control group each yielded medium effect sizes (r = −.40 and −.31, respectively), with support for overall significantly smaller P3b amplitude in PTSD patients. Only a single study of female Vietnam nurse veterans has reported significantly larger, rather than smaller, P3b amplitudes in individuals with PTSD (Metzger et al., 2002). The authors speculate that these anomalous findings might reflect an effort-related overcompensation in this unique sample of highly functioning and motivated PTSD patients. It has also been suggested that heightened P3b amplitude may be a trait marker for anxiety as individuals with anxiety disorders have been found to exhibit abnormally increased P3b amplitudes (Enoch, White, Harris, Rohrbaugh, & Goldman, 2001). Finally, there is some evidence that psychotropic medication normalizes the P3b component in PTSD (Metzger et al., 1997b), as reported for other clinical disorders (Sanz, Molina, Martin-Loeches, Calcedo, & Rubia, 2001).
N2 latency and P3b amplitude abnormalities are by no means specific to PTSD. Delayed N2 latencies have been observed in both psychiatric (e.g. depression, alcoholism, and schizophrenia [Sandman, Gerner, O’Halloran, & Isenhart, 1987]) and neurological (e.g., Huntington’s [Hömberg et al., 1986], Parkinson’s and Alzheimer’s [Goodman, & Aminoff, 1985; Holt et al., 1995]) disorders. Similarly, reduced P3b amplitudes have been found in depressive (Bruder et al., 1995; Diner, Holcomb, & Dykman, 1985), obsessive-compulsive (Beech, Ciesielski, & Gordon, 1983; Ciesielski, Beech, & Gordon, 1981), attention-deficit(Klorman, Brumaghim, Borgsted, & Salzman, 1991), learning (Lubar, Gros, Shively, & Mann, 1990; Dainer et al., 1981) and reading (Holcomb, Ackerman, & Dykman, 1985) disorders, schizophrenia (Ford, 1999), alcoholism (Carlson, Ianoco, & McGue, 2002), and head injury (Clark, O’Hanlon, Wright, & Geffen, 1992). Smaller P3b amplitudes have also been found in tobacco smokers (Anokhin et al., 2000). Although the clinical nonspecificity of P3b abnormalities limits its diagnostic utility, the strength of this ERP component may be as an index for measuring and tracking changes in general cognitive efficiency associated with psychopathology (Polich & Herbst, 2000).
Studies comparing ERP responses in monozygotic versus dizygotic twin pairs indicate that P3b amplitude is under partial genetic control (Katsanis, Ianoco, McGue, & Carlson, 1997). Importantly, both P3b and N2 ERP abnormalities might serve as vulnerability markers for neurological and/or psychiatric disorders. Studies have found evidence of delayed N2 latency in first-degree relatives of persons with Huntington’s disease (Hömberg et al., 1986) and reduced P3b amplitude in first-degree relatives of persons with alcoholism (Carlson et al., 2002) and schizophrenia (Kidogami, Yoneda, Asaba, & Sakai, 1991; Saitoh et al., 1984). One study of adolescent twins concordant or discordant for alcohol use disorders yielded results consistent with P3b amplitude as a vulnerability marker for alcoholism (Carlson et al., 2002).
PTSD is among the few mental disorders with a formally recognized etiology, in this case a psychologically traumatic event. At issue is whether abnormalities found to be associated with PTSD represent pre-trauma vulnerability factors for psychiatric symptoms following traumatic exposure, or acquired signs that develop after traumatic exposure. Although a longitudinal research design would be the choice methodology for investigating these competing interpretations, such studies are difficult because they require pre- and post-trauma assessments on individuals who may or may not come to be exposed to trauma, may or may not go on to develop PTSD, and may or may not be lost to follow-up. A second strategy to elucidate the pre vs. post-trauma origin of PTSD abnormalities is to use an identical twin, case-control design in which a non-trauma-exposed identical twin serves as a surrogate for what the trauma-exposed person would be like in absence of the traumatic experience (e.g., pre-trauma). Specifically, the non-trauma-exposed twin surrogate shares the genetic makeup of the trauma-exposed twin and much of the early developmental environment, but not the effects of trauma. The present study was conducted as part of a larger scale twin study of Vietnam combat veterans and their non-combat-exposed, identical twins (Orr et al., 2003). Here we investigated whether predicted ERP abnormalities found in combat veterans with PTSD would also be present in their identical co-twins who had not served in combat.
Methods
Participants
The participants were drawn from a pool of individuals who participated in a previously described study of heart rate responses to loud tones (Orr et al., 2003). A full description of the recruitment sources and strategy, and characteristics of the participant population, has already appeared in that report. That study’s sample consisted of 103 male monozygotic twin pairs, in which one twin served in combat in Vietnam and his co-twin did not. Exclusion criteria for twin pairs included the following in one or both members: a.) past but not current Vietnam-related PTSD; b.) current non-Vietnam related PTSD; c.) past non-Vietnam related PTSD for participants who never met criteria for Vietnam-related PTSD; and d.) current or past schizophrenic, paranoid, bipolar I, or other psychotic disorder. Single or both members of a twin pair were also excluded in subsidiary analyses if they used psychotropic or other potentially confounding medications or substances, or smoking tobacco, during the month prior to testing. It was necessary to include non-medicated singletons in order to maintain statistical power in this rare and unique sample. The study design included four groups of participants allocated within two factors. Twin pairs were classified according to the combat-exposed twin’s PTSD Diagnosis, viz., current, combat-related PTSD (P+) or non- (i.e., never had) combat-related PTSD (P−). Each pair contained two Exposure levels: combat-exposed (Ex) and non-combat exposed (Ux). Thus, there were four participant groups: combat-exposed twin with PTSD (ExP+), combat-exposed twin without PTSD (ExP−), (high-risk) combat-unexposed co-twin of twin with PTSD (UxP+), and (low-risk) combat-unexposed co-twin of twin without PTSD (UxP−). Sample and subsample sizes are listed in the Table. For tests of the origin of ERP abnormalities, a statistical approach was used that is capable of handling missing data in one member of a twin pair (see below).
Table.
Group mean demographic, self-report, and dependent measures for the combat-exposed and combat-unexposed veterans
| Full Sample | Non-medicated, non-Smoking Subsample | |||||||
|---|---|---|---|---|---|---|---|---|
| ExP+ (n=37) | ExP− (n=47) | UxP+ (n=37) | UxP− (n=48) | ExP+ (n=8) | ExP− (n=21) | UxP+ (n=13) | UxP− (n=21) | |
| Measure | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M(SD) | M (SD) | M (SD) |
| Age* | 49.6 (2.7) | 49.0 (2.2) | 49.9 (4.0) | 49.0 (2.2) | 48.5 (2.5) | 49.7 (2.2) | 51.5 (5.5) | 49.3 (2.2) |
| Combat Severity | 7.4 (2.6) | 3.8 (2.5) | __ | __ | 7.3 (3.3) | 3.3 (2.2) | __ | __ |
| Months in Vietnam | 12.3 (5.9) | 11.9 (3.9) | __ | __ | 12.3 (1.8) | 11.1 (2.6) | __ | __ |
| Mississippi Scale† | 121.8 (23.9) | 72.1 (17.8) | __ | __ | 113.5 (26.3) | 67.0 (17.3) | __ | __ |
| CAPS Total | 70.1 (17.7) | 7.6 (8.2) | __ | __ | 64.1 (18.8) | 6.3 (8.0) | __ | __ |
| Target P3b Amp | 11.0 (4.4) | 11.7 (3.8) | 11.5 (6.2) | 10.9 (4.5) | 9.4 (4.5) | 13.2 (3.9) | 12.9 (5.1) | 11.2 (4.1) |
| Target N2 Latency‡ | 232.8 (19.0) | 225.0 (16.3) | 228.6 (17.0) | 225.4 (15.8) | __ | __ | __ | __ |
| Mean Reaction Time§ | 437.9 (110.8) | 432.0 (97.9) | 419.9 (81.7) | 421.7 (86.4) | 459.5 (126.5) | 419.5 (75.9) | 381.3 (49.7) | 429.1 (102.7) |
Abbreviations: ExP+=combat exposed twin with PTSD; UxP+=combat unexposed co-twin of twin with PTSD; ExP−=combat-exposed twin without PTSD; UxP−=combat-unexposed co-twin of twin without PTSD; PTSD=posttraumatic stress disorder; CAPS Total=Clinician-Administered PTSD total scale score.
Age as of October 1, 1997.
Mississippi Scale for Combat–Related PTSD
The following number of participants had unscorable N2 components: ExP+=11; UxP+=14; ExP−=11; UxP−=12.
To the target stimuli, in ms.
All participants completed the three-tone auditory oddball task used in previous studies (e.g., McFarlane et al., 1993; Metzger et al., 1997a, 1997b, 2002). The task parameters, data acquisition and scoring were identical to that used in the study of female Vietnam nurse veterans (Metzger et al., 2002; Psychophysiology). The research protocol was approved by the institutional review board of the Manchester, NH VA Medical Center. Written informed consent was obtained from each participant after the procedures had been fully explained.
Psychodiagnostics and Self-reports
The Clinician Administered PTSD Scale: Current and Lifetime Diagnosis Version (CAPS-DX) (Blake et al., 1995; Weathers, Keane, & Davidson, 2001) was administered to determine the presence or absence of combat-related PTSD in the combat-exposed twins. Self-reports included the 35-item Mississippi Scale for Combat-Related PTSD (Keane, Caddell, & Taylor, 1988) and an 18-item Combat Severity Scale (Janes, Goldberg, Eisen, & True, 1991). Each participant completed a Stressful Life Events Checklist (unpublished, available upon request) designed to quantify the number of lifetime (non-combat) experiences that potentially met the DSM-IV A.1 (stressor) and A.2 (response) criteria.
Procedure
Each participant completed a three-tone target detection task adapted from Pfefferbaum and colleagues (Pfefferbaum, Wenegrat, Ford, Roth, & Kopell, 1984) that required the identification of infrequently presented target tones (2000-Hz) embedded in a series of equally infrequent distractor (500-Hz) and frequent common tones (1000-Hz). There were 285 stimulus presentations involving 40 target, 40 distractor, and 205 common tones. Tones were presented in a pseudorandom order such that no two infrequent tones occurred consecutively. The inter-stimulus interval ranged randomly between 1950 to 2050 ms. Auditory stimuli were generated by STIM software (Neuro Scan, Inc) and were of 70 dB intensity with 10-msec rise and fall times and 70 msec duration. Tones were presented binaurally over E-A-RTONE (Aearo Company) insert earphones.
The testing took place in a sound-attenuated room connected via wires to an adjoining portion of the laboratory in which the experimental apparatus was located. Participants were seated upright for the testing procedure in a comfortable armchair and fitted with earphones. They were instructed as follows: “In this session you will hear a series of tones. The series is made-up of three distinct tones, that is, a low-, medium-, and high-pitch tone. They will not occur in any particular order. Please press the black button as quickly as you can, without compromising accuracy, whenever you hear the high-pitch tone. It is important that you remain as quiet and as relaxed as possible with your eyes closed throughout the entire procedure.”
Dependent measures included central N2 latency and parietal P3b amplitude to target stimuli. We selected the parietal site for P3b measurement because this is where it is maximal and most heritable (Ramachandran, Porjesz, Begleiter, & Litke, 1996). The central site was selected for N2 latency because our previous work suggests that N2 has a frontocentral distribution and is larger for targets than distractors at Cz (McFarlane et al., 1993). Electroencephalogram (EEG) activity was recorded from midline sites (Fz, Cz, and Pz; 10–20 System (Jasper, 1958) using tin electrodes embedded in a nylon cap (Electro-Cap International), referenced to linked earlobes, and grounded at the forehead. Electrooculogram (EOG) activity was recorded at the outer canthus and infraorbitally to the left eye. Impedances were kept below 5 KOhms and approximately equal. Signals were amplified (Coulbourn High Gain Bioamplifiers), filtered (0.1–150 Hz), and digitally sampled at 1000 Hz (Neuro Scan, Inc), with a resultant signal sensitivity of 0.049 µV/bit. The EEG was epoched from 100 ms pre-stimulus to 900 ms post-stimulus, averaged at each site according to stimulus type, and digitally bandpass-filtered between 0.1 and 14 Hz (12 dB/Oc). Trials with excessive eye-movement artefact (EOG range ±85 µV) or associated with incorrect behavioral responses were excluded from the averaging process.
Scoring of ERP Components and Statistical Analysis
Peak amplitude measures for P3b were determined at the Pz site from each participant's averaged target waveform. P3b was defined as the most positive point between 300–500 ms post-stimulus onset. By convention (e.g., McFarlane et al., 1993; Metzger et al., 1997), N2 latency to targets was derived from difference waveforms obtained by subtracting the ERP waveform for common tones from the ERP waveform for target tones. N2 was defined as the most negative point between 200–325 ms preceding the P3b component at the Cz site.
To test whether results obtained in the combat veterans replicated earlier findings, independent Student’s t-tests were performed to determine the significance of differences between ExP+ and ExP− groups. Analyses of N2 latency were performed on the full sample of participants. However, based upon our previous results in combat-exposed Vietnam veteran singletons (Metzger et al., 1997b), analyses of P3b amplitude was performed a priori on a subsample of participants who were non-medicated and who had a negative urine screen for substances of abuse. For reasons explained below, subsidiary analyses of P3b amplitude were also limited to non-tobacco smoking participants.
Second, to examine the origin of any group differences in the ExP+ vs. ExP-participants, the data from all four groups (ExP+, UxP+, ExP−, UxP−) were analyzed by means of a mixed model that treated Diagnosis as a between-pairs fixed effect, Exposure as a within-pairs fixed effect(repeated measure), and pairs as a random effect (Little, Miliken, Stroup, & Wolfinger, 1996). The model is capable of handling missing data in one member of a twin pair. It yields a t statistic for each main effect and the interaction. If a dependent variable represents a vulnerability factor for PTSD, the model predicts a significant Diagnosis main effect. If, on the other hand, a dependent variable represents an acquired PTSD sign, the model predicts a significant Diagnosis by Exposure interaction. Because of the clear directionality of the ERP hypotheses (N2 latency greater, and P3b amplitude less, in PTSD vs. non-PTSD pairs, and in combat-exposed vs. –unexposed twins), p values from these analyses are one-tailed.
Results
Demographic, Self-report, and Reaction Time Data: Full Sample
Group means and standard deviations (SDs) for demographic, self-report, and dependent measures for the full sample of combat-exposed and -unexposed co-twins are presented in the Table. Student’s t comparisons between the ExP+ and ExP− groups in the full sample indicated no significant differences between the combat-exposed groups in age (t(82) = 1.0, p = .33), months spent in Vietnam (t(77) = 0.4, p = .69), or reaction times to the target stimuli (t(78) = 0.3, p = .80). However, participants in the ExP+ group reported significantly greater combat severity (t(82) = 6.4, p < .001) and had significantly more severe PTSD symptoms as measured by the CAPS (t(80) = 21.1, p < .001) and the Mississippi Scale (t(82) = 10.9, p < .001) than the ExP− group.
Demographic, Self-report, and Reaction Time Data: Non-Medicated, Non-Smoking Subsample
Group means and standard deviations (SDs) for demographic, self-report, and dependent measures for the non-medicated, non-smoking subsample of combat-exposed and -unexposed co-twins are presented in the Table. Student’s t comparisons between the ExP+ and ExP− subsamples indicated no significant differences between the combatexposed groups in age (t(27) = −1.3, p = .21) or months spent in Vietnam (t(23) = 1.1, p = .28). However, participants in the ExP+ group reported significantly greater combat severity (t(27) = 3.7, p = .01) and had significantly more severe PTSD symptoms as measured by the CAPS (t(27) = 11.8, p < .001) and the Mississippi Scale (t(27) = 5.6, p < .001) than the ExP− group.
Examination of reaction times to target stimuli for the non-medicated, non-smoking subsamples yielded the following results. An ExP+ versus ExP− contrast produced Student’s t(25) = 1.0, p = 0.31 (effect size, Cohen’s d = .44; weighted by sample size). The mixed model yielded: for Diagnosis t = 0.5, p = 0.62; for Exposure t = 0.8, p = 0.45; for Diagnosis × Exposure t = 1.8, p = 0.08. A comparison between the ExP+ versus UxP+ subsamples indicated a near-significant trend for a longer mean reaction time in the ExP+ group: t = 1.9, p = 0.07 (effect size, Cohen’s d = 1.0; weighted by subsample size).
Target N2 Latency: Full Sample
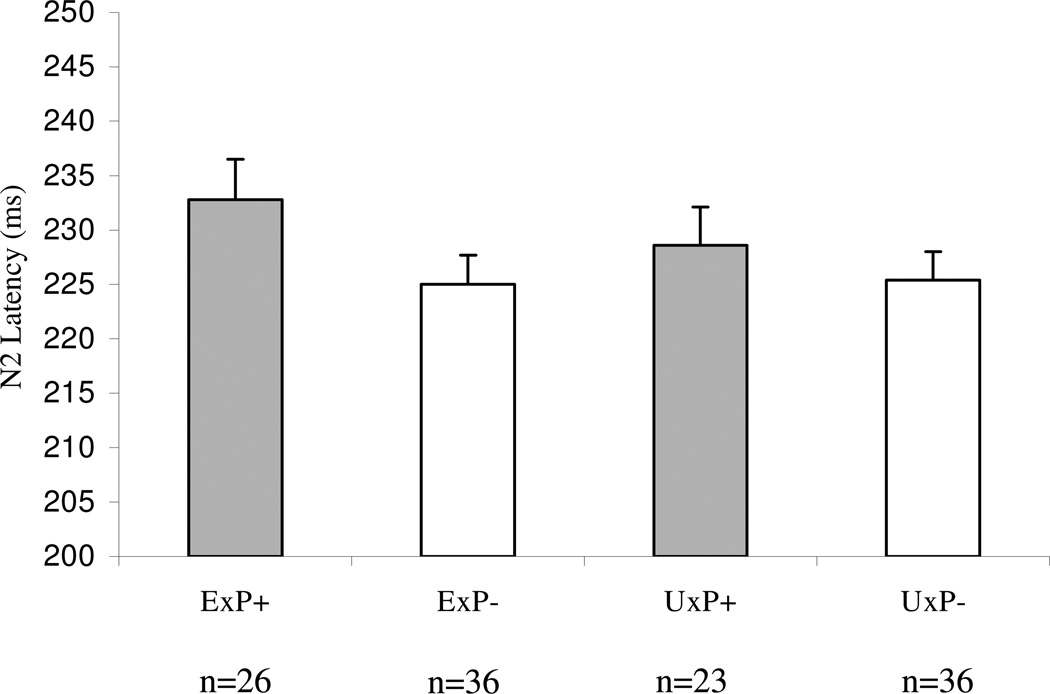
Replicating previous findings, Student’s t comparisons within the full sample of participants with scorable N2 latency data (ExP+, n = 26; ExP−, n = 36; UxP+, n = 23 and UxP−, n = 36) indicated significantly longer target N2 latency in the ExP+ compared to ExP− group (t(60) = 1.7, p = .04). Calculation of Cohen’s d, weighted by sample size, yielded a medium effect size of .45. In the mixed model analysis, for Diagnosis, t = 1.5, p = 0.14; for Exposure, t = 0.5, p = 0.63 for Diagnosis × Exposure, t = 0.9, p = 0.37. None of the following variables were found to be associated with N2 latency in the full sample at screening p < 0.20: age; number of potentially traumatic, lifetime, non-combat events; presence of an affective or an anxiety disorder; combat severity (in the exposed twin); and number of cigarettes smoked, or number of alcoholic or caffeinated beverages consumed in the 24 hours and past 30 days preceding testing. Hence these variables were not considered to have potentially confounded the N2 latency results.
Target P3b Amplitude: Full sample
There were no significant findings or trends for P3b amplitude to targets in the full (non-medicated and medicated combined) sample (see Table).
Target P3b Amplitude: Non-medicated Subsample
There was a near-significant difference between the non-medicated ExP+ and ExP− subsample target P3b amplitudes (Student’s t(41) = 1.6, p = 0.06). In the mixed model analysis, for Diagnosis t = 0.5, p = 0.60; for Exposure t = 0.1, p = 0.94, Diagnosis × Exposure t = 1.6, p = 0.14. The following variables were not found to be correlated with P3b amplitude in the non-medicated sample at screening p < .20: age; number of potentially traumatic, lifetime, non-combat events; presence of an affective or an anxiety disorder; combat severity (in the exposed twin); and number of alcoholic beverages consumed. However, number of caffeinated beverages consumed in the past 24 hours (r = −.17, p = 0.11), and number of cigarettes smoked in the past 24 hours (r = −.17, p = 0.11) and past 30 days (r = −.19, p = 0.06) preceding testing were negatively correlated with P3b amplitude. In addition, among the non-medicated subjects (n = 98), P3b amplitudes were significantly smaller in participants who smoked tobacco in the previous 30 days (n = 34; M = 10.1; SD = 4.0) than in those who did not (n = 64; M = 11.9; SD = 4.4; t(96) = 2.0, p = .047; two-tailed). Hence these variables potentially confounded the P3b amplitude results. Unfortunately, there were too few non-caffeine consumers to perform analyses in this subsample. However, adjusting the P3b amplitude analyses for number of caffeinated beverages consumed by means of analysis of covariance did not substantially change the above results (data not shown).
Target P3b Amplitude: Non-medicated, non-smoking Subsample
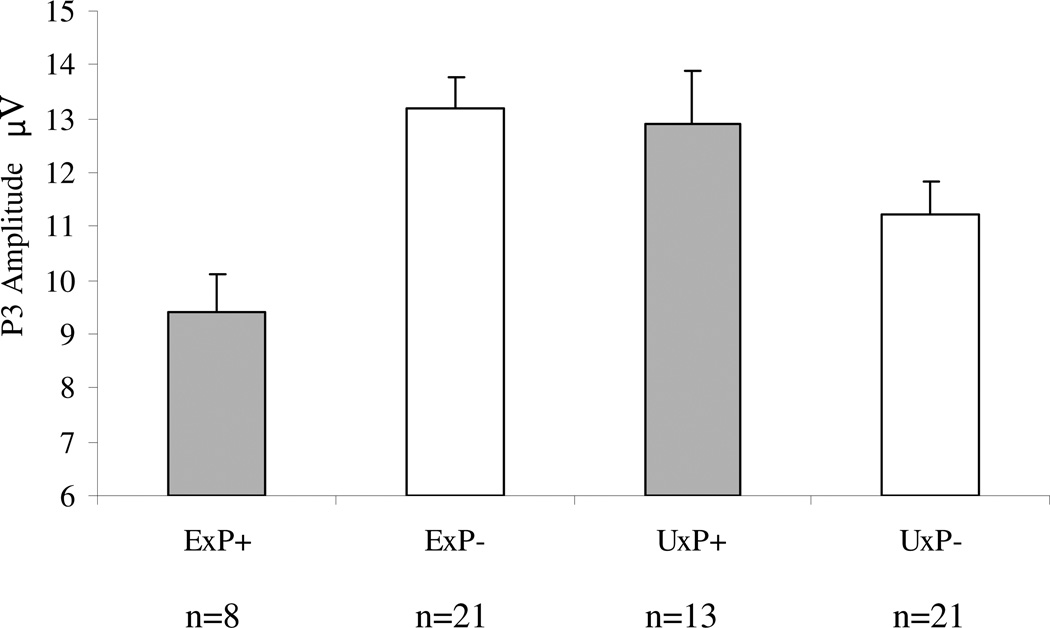
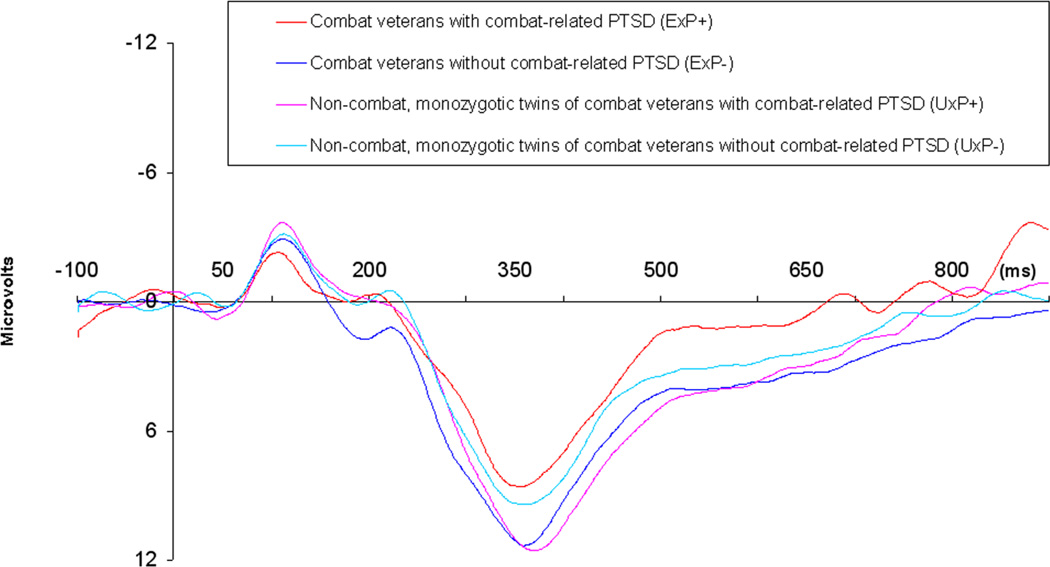
P3b amplitude analyses were repeated on the non-medicated subsample after removing the 34 tobacco-smoking participants. The subsample sizes for these analyses were: ExP+, n = 8; ExP−, n = 21; UxP+, n = 13 and UxP−, n = 21. An ExP+ versus ExP− contrast yielded Student’s t(27) = 2.2, p = 0.02 (effect size, Cohen’s d = .93; weighted by sample size). The mixed model yielded: for Diagnosis t = 0.4, p = 0.67; for Exposure t = 0.01, p = 0.99; for Diagnosis × Exposure t = 2.8, p = 0.009. A comparison between the ExP+ versus UxP+ subsamples indicated a tendency towards a smaller mean P3b amplitude in the ExP+ group: t = 1.6, p = 0.13 (effect size, Cohen’s d = .71; weighted by subsample size). Group means (SEs) for P3b amplitude to targets in the non-smoking, non-medicated subsample are presented in Figure 2. Group grand average ERP waveforms for the non-smoking, non-medicated subsample are shown in Figure 3.
Figure 2. Group mean P3b amplitudes to target stimuli at the Pz site in the non-medicated, non-smoking subsample.
ExP+: combat veterans with combat-related PTSD; ExP−: combat veterans without combat-related PTSD; UxP+: non-combat, monozygotic twins of combat veterans with combat-related PTSD; UxP−: non-combat, monozygotic twins of combat veterans without combat-related PTSD. Error bars indicate standard errors.
Figure 3. Group grand average event-related potential (ERP) waveforms at the Pz site to Target stimuli in the non-medicated, non-smoking sample.
The large downward deflections represent the P3b component. Scale: Tics on the vertical axis represent 6 µV units; Tics on the horizontal axis represent 100 msec units relative to stimulus onset.
Discussion
With reference to the non-medicated, non-smoking subsample, the results obtained in combat veterans with current PTSD vs. combat veterans who never had combat-related PTSD replicate previously reported findings of diminished auditory P3b amplitude to target tones in this disorder. Where differences exist between participants with vs. without PTSD, data from their non-combat exposed co-twins offer the possibility of resolving their origin. With regard to decreased P3b amplitude, the mixed model results revealed a significant pair Diagnosis × Combat Exposure interaction. This is illustrated by the pattern in Figure 2, in which the combat-exposed PTSD veteran group shows lower P3b amplitudes than the non-combat-exposed (high risk) PTSD co-twin group, as well as than the combat-exposed, non-PTSD veteran group and the (low-risk) non-combat exposed non-PTSD co-twin group. Although the direct comparison between the combat-exposed PTSD group (ExP+) and the non-combat-exposed (high risk) PTSD co-twin group (UxP+) did not yield a significant difference, the effect size associated with this comparison was relatively large. This suggests that inadequate statistical power, likely due to the small subsample sizes, was responsible for the failure to reach a conventional level of statistical significance. Overall, the pattern of findings supports the conclusion that diminished P3b is an acquired sign of combat-related PTSD, and not a pre-existing vulnerability factor. Similarities in the patterns of P3b amplitude and reaction time findings within the subsample of non-medicated, non-smoking veterans suggests that a behavioural manifestation of reduced P3b in PTSD may be slower cognitive processing and/or responses to task-relevant cues.
A conceptual limitation of this study is that the design cannot rule out the possibility that unique environmental difference(s) between the combat-exposed twins with PTSD and their non-combat-exposed co-twins other than the trauma of combat are responsible for diminished P3b amplitude in the former. However, because the most salient, common difference between these twins was the presence of combat-related PTSD in one but not the other, it is reasonable to attribute the diminished P3b amplitudes to the presence of combat-related PTSD. The design employed here also cannot rule out the possibility that the non-combat-exposed co- twins of combat veterans with PTSD share latent inherited phenotypes predisposing to diminished P3b responses that require traumatic exposure in order to be activated (Kendler, 2001). A methodological limitation is the resulting small and unequal samples utilized in P3b analyses when participants with factors known to influence P3b amplitude were excluded. This limitation, however, may be difficult to overcome given 1) the generally high incidence of these factors in study populations of this age and 2) the limited availability of the unique and rare population under study. Finally, the elapsed time between combat exposure and the performance of this study means that the lifetime history of disorder is subject to the limitations of lengthy retrospective recall, and the data only apply to a small window of observation in these individuals’ chronic PTSD.
The finding that tobacco use (smoking) was an important covariate in the present study is worthy of comment. In addition to the reported negative correlation between the number of cigarettes smoked and P3b amplitude, we also found smaller P3b amplitude in smokers compared to non-smokers, collapsing across non-medicated study groups (see Anokhin et al., 2000) These findings suggest that the effects of smoking may potentially obscure the pattern of P3b results and should be addressed in future clinical ERP studies, especially in study populations where groups might differ in smoking status.
We previously reported in the same participant sample that decreased heart rate responses to loud tones also conformed to the pattern of an acquired PTSD sign (Orr et al., 2003). As in the auditory oddball task employed here, that loud tone task employed neutral (i.e., trauma-irrelevant) stimuli. Those results, along with the present results, support the conclusion that the range of acquired abnormalities found in combat-related PTSD extends beyond responses to reminders of the trauma that induced them. It remains for future studies to examine whether this is also true in non-combat-related PTSD.
The present results in the PTSD vs. non-PTSD combat veterans also replicate previously reported findings in this disorder of delayed N2 latency to target tones in the same task. Unfortunately, the lack of statistically significant findings from the mixed model analysis precludes inferences regarding the origin of increased N2 latency in PTSD.
Figure 1. Group mean N2 latencies to target stimuli (target – common) at the Cz site for the full sample.
ExP+: combat veterans with combat-related PTSD; ExP−: combat veterans without combat-related PTSD; UxP+: non-combat, monozygotic twins of combat veterans with combat-related PTSD; UxP−: non-combat, monozygotic twins of combat veterans without combat-related PTSD. Error bars indicate standard errors. The reduced sample sizes are due to the exclusion of participants with unusable N2 data.
Acknowledgements
Our thanks are offered to Darren Weber, Heike Croteau, and Dr. Mark Lasko for technical assistance. Additional Harvard/VA PTSD Twin Study Investigators include Seth A. Eisen, M.D., Mark W. Gilbertson, Ph.D., Gregory M. Gillette, M.D., Jack Goldberg, Ph.D., Tamara V. Gurvits, M.D., Ph.D., William G. Henderson, Ph.D., Terence M. Keane, Ph.D., Michael J. Lyons, Ph.D., Arieh Y. Shalev, M.D., William R. True, Ph.D., Ming T. Tsuang, M.D., Ph.D., Frank W. Weathers, Ph.D., and Rachel Yehuda, Ph.D. This study was supported by USPHS grant #MH54636. The study was also supported in part by NH&MRC Program Grant No. 300403. The United States Department of Veterans Affairs also provided financial support for the development and maintenance of the Vietnam Era Twin (VE) Registry. Through their support of the VET Registry, numerous organizations provided invaluable assistance, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; Internal Revenue Service; National Institutes of Health; National Opinion Research Center; National Research Council, National Academy of Sciences; and Institute for Survey Research, Temple University. The authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible.
Footnotes
Financial Disclosures. Ms. Veltmeyer and Drs. Metzger, Clark, McFarlane, Lasko, Orr, and Pitman declare no possible conflict of interest, financial or otherwise, related directly or indirectly to the submitted work.
This article is dedicated to the memory of one of its authors, Dr. Stephen Paige.
References
- Anokhin AP, Vedeniapin AB, Sirevaag EJ, Bauer LO, O’Connor SJ, Kuperman S, Porjesz B, et al. The P300 brain potential is reduced in smokers. Psychopharmacology. 2000;149:409–413. doi: 10.1007/s002130000387. [DOI] [PubMed] [Google Scholar]
- Araki T, Kasai K, Yamasue H, Kato N, Kudo N, Ohtani T, Nakagome K, Kirihar K, Yamada H, Abe O, Iwanami A. Association between lower P300 amplitude and smaller anterior cingulate cortex volume in patients with posttraumatic stress disorder, a study of victims of Tokyo subway sarin attack. Neuroimage. 2005;25:43–50. doi: 10.1016/j.neuroimage.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Beech HR, Ciesielski KT, Gordon PK. Further observations of evoked potentials in obsessional patients. British Journal of Psychiatry. 1983;142:605–609. doi: 10.1192/bjp.142.6.605. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Stewart JW, Towey JP, Leite P, Voglmaier, Quitkin FM. Brain event-related potentials to complex tones in depressed patients, relations to perceptual asymmetry and clinical features. Psychophysiology. 1995;32(4):373–381. doi: 10.1111/j.1469-8986.1995.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG, McGue M. P300 amplitude in adolescent twins discordant and concordant for alcohol use disorders. Biological Psychology. 2002;61:2003–2227. doi: 10.1016/s0301-0511(02)00059-5. [DOI] [PubMed] [Google Scholar]
- Charles G, Hansenne M, Ansseau M, Pitchot W, Machowski R, Schittecatte M, Wilmotte J. P300 in posttraumatic stress disorder. Neuropsychobiology. 1995;32(2):72–74. doi: 10.1159/000119216. [DOI] [PubMed] [Google Scholar]
- Ciesielski KT, Beech HR, Gordon PK. Some electrophysiological observations in obsessional states. British Journal of Psychiatry. 1981;138:479–484. doi: 10.1192/bjp.138.6.479. [DOI] [PubMed] [Google Scholar]
- Clark CR, O'Hanlon AP, Wright MJ, Geffen GM. Event-related potential measurement of deficits in information processing following moderate to severe closed head injury. Brain Injury. 1992;6(6):509–520. doi: 10.3109/02699059209008148. [DOI] [PubMed] [Google Scholar]
- Dainer KB, Klorman R, Salzman LF, Hess DW, Davidson PW, Michael RL. Learning-disordered children's evoked potentials during sustained attention. Journal of Abnormal Child Psychology. 1981;9(1):79–94. doi: 10.1007/BF00917859. [DOI] [PubMed] [Google Scholar]
- Diner BC, Holcomb PJ, Dykman RA. P300 in major depressive disorder. Psychiatry Research. 1985;15(3):175–184. doi: 10.1016/0165-1781(85)90074-5. [DOI] [PubMed] [Google Scholar]
- Enoch MA, White KV, Harris CR, Rohrbaugh JW, Goldman D. Alcohol use disorders and anxiety disorder: Relation to the P300 event-related potential. Aloholism: Clinical and Experimental Research. 2001;25:1293–1300. [PubMed] [Google Scholar]
- Karl A, Malta LS, Maercker A. Meta-analytic review of event-related potential studies in post-traumatic stress disorder. Biological Psychology. 2006;71:123–147. doi: 10.1016/j.biopsycho.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Felmingham KL, Bryant RA, Kendall C, Gordon E. Event-related potential dysfunction in posttraumatic stress disorder, The role of numbing. Psychiatry Research. 2002;109:171–179. doi: 10.1016/s0165-1781(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Ford JM. Schizophrenia, The broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- Friedman D. Event-related potentials in populations at genetic risk, A methodological review. In: Rohrbaugh JW, Parasuraman R, Johnson R, editors. Event-related Brain Potentials, Basic Issues and Applications. New York: Oxford University Press; 1990. pp. 310–332. [Google Scholar]
- Goodman DS, Aminoff MJ. Electrophysiological differences between subtypes of dementia. Brain. 1986;109:1103–1113. doi: 10.1093/brain/109.6.1103. [DOI] [PubMed] [Google Scholar]
- Grossman R, Buchsbaum MS, Yehuda R. Neuroimaging studies in posttraumatic stress disorder. Psychiatry Clinics of North America. 2002;25(2):317–340. doi: 10.1016/s0193-953x(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ, Ackerman PT, Dykman RA. Cognitive event-related brain potentials in children with attention and reading deficits. Psychophysiology. 1985;22(6):656–667. doi: 10.1111/j.1469-8986.1985.tb01663.x. [DOI] [PubMed] [Google Scholar]
- Holt LE, Raine A, Pa G, Schneider LS, Henderson VW, Pollock VE. P300 topography in Alzheimer's disease. Psychophysiology. 1995;32(3):257–265. doi: 10.1111/j.1469-8986.1995.tb02954.x. [DOI] [PubMed] [Google Scholar]
- Hömberg V, Hefter H, Granseyer G, Strauss W, Lange H, Hennerici M. Event-related potentials in patients with Huntington's disease and relatives at risk in relation to detailed psychometry. Electroencephalography and Clinical Neurophysiology. 1986;56:552–569. doi: 10.1016/0013-4694(86)90143-4. [DOI] [PubMed] [Google Scholar]
- Janes GR, Goldberg J, Eisen SA, True WR. Reliability and validity of a combat exposure index for Vietnam era veterans. Journal of Clinical Psychology. 1991;47(1):80–86. doi: 10.1002/1097-4679(199101)47:1<80::aid-jclp2270470112>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, McGue MK, Carlson SR. P300 event-related potential heritability in monozygotic and dizygotic twins. Psychophysiology. 1997;34:47–58. doi: 10.1111/j.1469-8986.1997.tb02415.x. [DOI] [PubMed] [Google Scholar]
- Keane TM, Caddell JM, Taylor KL. Mississippi Scale for Combat-Related Posttraumatic Stress Disorder, three studies in reliability and validity. Journal of Consuling and Clinical Psychology. 1988;56(1):85–90. doi: 10.1037//0022-006x.56.1.85. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Twin studies of psychiatric illness, an update. Archives of General Psychiatry. 2001;58:1014. doi: 10.1001/archpsyc.58.11.1005. [DOI] [PubMed] [Google Scholar]
- Kidogami Y, Yoneda H, Asaba H, Sakai T. P300 in first degree relatives of schizophrenics. Schizophrenia Reearch. 1991;6(1):9–13. doi: 10.1016/0920-9964(91)90015-j. [DOI] [PubMed] [Google Scholar]
- Kimble M, Kaloupek D, Kaufman M, Deldin P. Stimulus novelty differentially affects attentional allocation in PTSD. Biological Psychiatry. 2000;47(10):880–890. doi: 10.1016/s0006-3223(99)00258-9. [DOI] [PubMed] [Google Scholar]
- Klorman R, Brumaghim J, Borgsted A, Salzman LF. How event-related potentials help to understand the effects of stimulants on attention deficit hyperactivity disorder. Advances in Psychophysiology. 1991;4:107–153. [Google Scholar]
- Little RC, Miliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. Cary, NC: SAS Institute Inc; 1996. [Google Scholar]
- Lubar J, Gross D, Shively M, Mann C. Differences between normal, learning disabled and gifted children based upon an auditory evoked potential task. Journal of Psychophysiology. 1990;4:249–260. [Google Scholar]
- McFarlane AC, Weber DL, Clark CR. Abnormal stimulus processing in posttraumatic stress disorder. Biological Psychiatry. 1993;34(5):311–320. doi: 10.1016/0006-3223(93)90088-u. [DOI] [PubMed] [Google Scholar]
- Metzger LJ, Carson MA, Paulus LA, Lasko NB, Paige SR, Pitman RK, Orr SP. Event-related potentials to auditory stimuli in female Vietnam nurse veterans with posttraumatic stress disorder. Psychophysiology. 2002;39:49–63. doi: 10.1017/S0048577202001002. [DOI] [PubMed] [Google Scholar]
- Metzger LJ, Orr SP, Lasko NB, Berry NJ, Pitman RK. Evidence for diminished P3b amplitudes in PTSD. Annals of the New York Academy of Sciences. 1997a;821:499–503. doi: 10.1111/j.1749-6632.1997.tb48315.x. [DOI] [PubMed] [Google Scholar]
- Metzger LJ, Orr SP, Lasko NB, Pitman RK. Auditory event-related potentials to tone stimuli in combat-related posttraumatic stress disorder. Biological Psychiatry. 1997b;42(11):1006–1015. doi: 10.1016/s0006-3223(97)00138-8. [DOI] [PubMed] [Google Scholar]
- Neylan TC, Jasiukaitis PA, Lenoci M, Scott JC, Metzler TJ, Weiss DS, Schoenfeld FB, Marmar CR. Temporal instability of auditory and visual event-related potentials in posttraumatic stress disorder. Biological Psychiatry. 2003;53:216–225. doi: 10.1016/s0006-3223(02)01450-6. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Hu FB, Shalev AY, Pitman RK. Physiologic responses to sudden, loud tones in monozygotic twins discordant for combat exposure, association with posttraumatic stress disorder. Archives of General Psychiatry. 2003;60(3):283–288. doi: 10.1001/archpsyc.60.3.283. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Wenegrat BG, Ford JM, Roth WT, Kopell BS. Clinical application of the P3b component of event-related potentials. II. Dementia, depression and schizophrenia. Electroencephalography and Clinical Neurophysiology. 1984;59(2):104–124. doi: 10.1016/0168-5597(84)90027-3. [DOI] [PubMed] [Google Scholar]
- Polich J. Theoretical overview of P3a and P3b. In: Polich J, editor. Detection of change, event-related potential and fMRI findings. Boston, MA: Kluwer Academic Publishers; 2003. pp. 83–98. [Google Scholar]
- Polich J, Herbst KL. P300 as a clinical assay, rationale, evaluation, and findings. International Journal of Psychophysiology. 2000;38:3–19. doi: 10.1016/s0167-8760(00)00127-6. [DOI] [PubMed] [Google Scholar]
- Ramachandran G, Porjesz B, Begleiter H, Litke A. A simple auditory oddball task in young adult males at high risk for alcoholism. Alcoholis: Clinical and Experimental Research. 1996;20:9–15. doi: 10.1111/j.1530-0277.1996.tb01035.x. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Niwa S, Hiramatsu K, Kameyama T, Rymar K, Itoh K. Abnormalities in late positive components of event-related potentials may reflect a genetic predisposition to schizophrenia. Biological Psychiatry. 1984;19(3):293–303. [PubMed] [Google Scholar]
- Sandman CA, Gerner R, O'Halloran JP, Isenhart R. Event-related potentials and item recognition in depressed, schizophrenic and alcoholic patients. International Journal of Psychophysiology. 1987;5:215–225. doi: 10.1016/0167-8760(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Sanz M, Molina V, Martin-Loeches M, Calcedo A, Rubia FJ. Auditory P300 event related potential and serotonin reuptake inhibitor treatment in obsessive-compulsive disorder patients. Psychiatry Research. 2001;101:75–81. doi: 10.1016/s0165-1781(00)00250-x. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale, a review of the first ten years of research. Depression and Anxiety. 2001;13(3):132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]