Abstract
Human induced pluripotent stem cells (hiPSCs) are generated through the reprogramming of somatic cells into an embryonic stem cell-like state, such that vascular cells differentiated from hiPSCs might be a suitable autologous cell source for vascular regeneration. The goal of this study was to assess whether cotransplantation of endothelial cells (ECs) and smooth muscle cells (SMCs) differentiated from hiPSCs could promote neovascularization and tissue repair in a murine dermal wound model. hiPSCs were differentiated into ECs and SMCs; the differentiated cells displayed cell-specific surface markers. Compared to primary somatic cells, ECs and SMCs, which were differentiated from hiPSCs, strongly cooperated to enhance in vitro tubular network formation. In vivo gel assays in athymic nude mice showed that the coimplantation of differentiated ECs and SMCs significantly increased vascularization, unlike that observed in the case of implantation of differentiated ECs alone. In a murine full-thickness wound model, when compared with the transplantation of primary somatic cells or phosphate-buffered saline, cotransplantation of differentiated ECs and SMCs markedly enhanced neovascularization in injured tissues and accelerated wound healing. These results demonstrate that cotransplantation of hiPSC-derived ECs and SMCs may be feasible as a new autologous cell therapy for neovascularization and tissue repair.
Introduction
Over the past decade, diverse adult stem cells have been found to contribute to neovascularization and tissue repair processes in ischemic tissues. Their therapeutic potentials have been illustrated in animal models of ischemic disease, including myocardial infarction, diabetic wounds, and peripheral arterial disease. However, it has been difficult to obtain sufficient numbers of adult stem cells with great vascular regeneration ability for their clinical application, which has led to an increased interest in developing other sources of autologous stem cells. In this regard, human induced pluripotent stem cells (hiPSCs) may represent a suitable source of autologous pluripotent stem cells without the limitations of adult stem cells, and ethical concerns associated with human embryonic stem cells (hESCs).1 Several previous studies have demonstrated the therapeutic promise of hiPSC-derived endothelial cells (ECs) and endothelial progenitors in animal models of peripheral arterial diseases.2,3 Thus, the development of hiPSCs and their differentiation into vascular cells offer an opportunity to obtain a high yield of autologous cells required for vascular regeneration.
During neovascularization process, various cell types and growth factors are involved in regulating vessel development and remodeling. In particular, a close interaction between ECs and smooth muscle cells (SMCs) is required for the formation of arterial vascular network. The importance of arteriogenesis has been appreciated in the dermal wound-healing process. Previous reports revealed that the decrease of arteriolar blood flow in murine cutaneous wounds significantly reduced the density of functional capillaries, thus impairing vascular perfusion and cell survival in the skin.4 Other studies also demonstrated that increased arteriogenesis substantially accelerated dermal wound repair as much as it did in the peripheral arterial disease models.5 These results suggest that regeneration of arterioles might be more efficacious in the recovery of dermal blood perfusion and tissue repair compared with capillaries. In this regard, treatments composed of two cell types, such as ECs and SMCs, would be more beneficial than those using a single cell type alone in terms of regenerating the arterial vasculature in the wound-healing process. Therefore, the present study aimed to assess whether the coadministration of hiPSC-derived ECs and SMCs could enhance neovascularization and tissue repair in a murine full-thickness dermal wound model.
Materials and Methods
Cell culture
An expanded Supplementary Materials and Methods section (Supplementary Data are available online at www.liebertpub.com/tea) is available that includes detailed methods for cell culture, vascular differentiation of hESCs and hiPSCs, characterization of differentiated cells, in vitro assay, angiogenesis protein array, wound analysis, and immunohistochemistry.
In vivo gel assay
An aliquot (3×104 cells) of EC alone or of a mixture of ECs+SMCs (at a ratio of 60:40 ECs:SMCs) was suspended in a 0.2 mL solution containing Matrigel (BD Biosciences, Bedford, MA) and rat-tail type 1 neutralized collagen (1.5 mg/mL; BD Biosciences) at a 1:1 ratio in the endothelial growth medium (EGM)-2 (Lonza, Walkersville, MD). The cell suspension was subcutaneously injected into 7-week-old male Balb/c athymic nude mice (Charles River Laboratories, Yokohama, Japan). After 2 weeks, the injected Matrigel/collagen mixture was harvested with neighboring tissues and processed for further histological analysis. All animal procedures, which were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health, were approved by the Institutional Animal Care and Use Committee of Ajou University. For surgical procedures, mice were anesthetized with an intraperitoneal injection of ketamine–xylazine (79.5 and 9.1 mg/kg, respectively). The adequacy of anesthesia was assessed by monitoring the pedal withdrawal reflex response.
Dermal wound animal model
A full-thickness excisional wound (0.5 cm in diameter) was created on the dorsomedial back of a male athymic nude mouse with a standard skin biopsy punch (Acuderm, Inc., Fort Lauderdale, FL). Immediately after surgery, ECs (1×105 in 50 μL of phosphate-buffered saline [PBS]), ECs+SMCs (6×104 ECs+4×104 SMCs in 50 μL of PBS), or PBS were injected at three different sites into intact dermis near the created wound. On days 0, 3, 7, and 10 after treatment, wounds were documented with a digital camera for the analysis of the open wound area.6 On day 10 after treatment, wound tissues were harvested and longitudinally cut in half through the least-healed portion. They were fixed, embedded, and serially sectioned perpendicular to the wound surface to analyze the granulation tissue area and the immunohistology.
Statistical analysis
All data are presented as mean±SEM. Statistical significance was evaluated by one-way analysis of variance followed by the Bonferroni's post hoc multiple comparison test. A p-value of <0.05 was considered statistically significant. The number of samples examined is indicated by n.
Results
Vascular differentiation of hiPSCs and characterization of hiPSC-derived ECs and SMCs
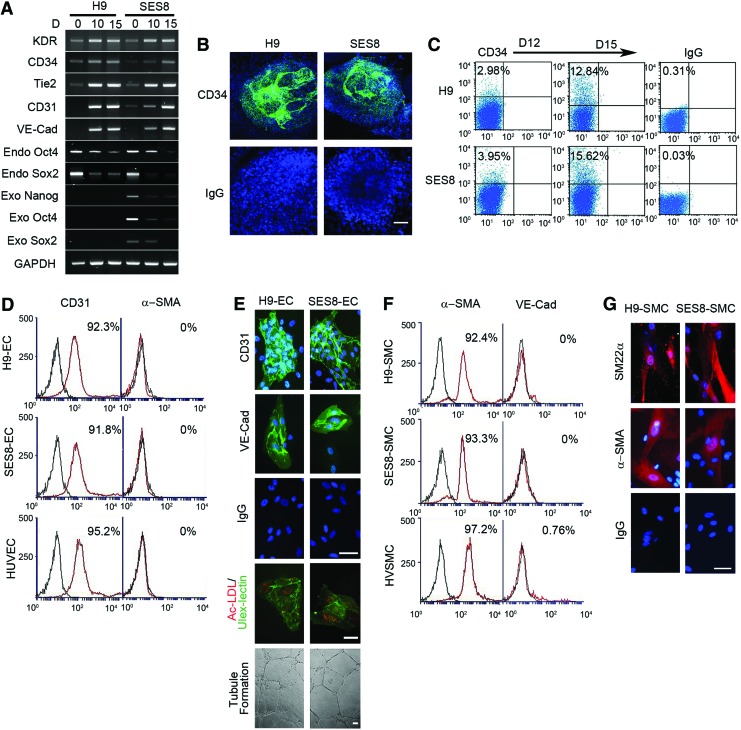
The hiPSC line used in the present study, SES8, was previously established and characterized.7 For vascular differentiation, embryonic bodies (EBs) were formed from hiPSCs (SES8) and hESCs (H9) and cultured in a differentiation medium. During the differentiation process, the expression of mesoderm-specific genes (KDR, CD34, Tie2, CD31, and VE-Cad) was markedly upregulated, whereas that of pluripotency-related genes was downregulated (Fig. 1A). As early as day 12 of differentiation, CD34-positive cells appeared mainly at the center of EBs from SES8 or H9. The number of CD34-positive cells further increased on day 15 of differentiation (Fig. 1B, C). CD34-positive cells purified from EBs were further differentiated into ECs or SMCs by culturing in EC- or SMC-specific differentiation media, respectively. Flow cytometric analysis showed that >90% of differentiated ECs and SMCs expressed CD31 or α-SMA, respectively (Fig. 1D, F). Immunocytochemical analysis confirmed that they were positively stained for EC-specific markers (CD31, VE-Cad, and ulex-lectin) or SMC-specific markers (α-SMA, SM22-α), respectively (Fig. 1E, G). In addition, differentiated ECs manifested endothelial functions such as tubular network formation on Matrigel and uptake of acetylated low-density lipoprotein (Fig. 1E).
FIG. 1.
Vascular differentiation of hiPSCs and characterization of hiPSC-derived ECs and SMCs. (A) The mRNA profile of mesoderm-specific genes and pluripotency-related genes (Endo, endogenous gene; Exo, lentiviral transgene) during vascular differentiation of hESCs (H9) and hiPSCs (SES8). Undifferentiated cells (D 0) and differentiated cells harvested on day 10 (D 10) and day 15 (D 15) were used for RT-PCR analysis. GAPDH was used as a loading control. (B) Representative CD34-stained images of EBs differentiated for 12 days. Scale bar is 200 μm. (C) Flow cytometric analysis of CD34 expression in EBs differentiated for 12 days (D12) and 15 days (D15). (D) Flow cytometric analysis of CD31 expression in ECs from H9 (H9-EC) and SES8 (SES8-EC). Red histograms represent cells stained with specific IgGs. Isotype-matched control IgGs are overlaid as a black line on each histogram. HUVECs were used as a positive control. (E) Characterization of H9-ECs and SES8-ECs. Differentiated ECs were positively stained with anti-CD31and anti-VE-Cad IgGs. Their endothelial phenotype was confirmed by the tubular formation assay on Matrigel and staining with DiI-acLDL (red) and FITC-ulex-lectin (green). (F) Flow cytometric analysis of α-SMA expression in SMCs derived from H9 (H9-SMC) and SES8 (SES8-SMC). HVSMC was used as a positive control. (G) Immunocytochemical analysis of H9-SMCs and SES8-SMCs. Differentiated SMCs were positively stained with anti-SM22α and anti-α-SMA IgGs. All scale bars are 50 μm. hiPSC, human induced pluripotent stem cell; EC, endothelial cell; SMC, smooth muscle cell; hESC, human embryonic stem cell; acLDL, acetylated low-density lipoprotein; HUVEC, human umbilical vein endothelial cell; HVSMC, human vascular SMC. Color images available online at www.liebertpub.com/tea
Cooperation between hiPSC-derived ECs and SMCs promotes tubular network formation
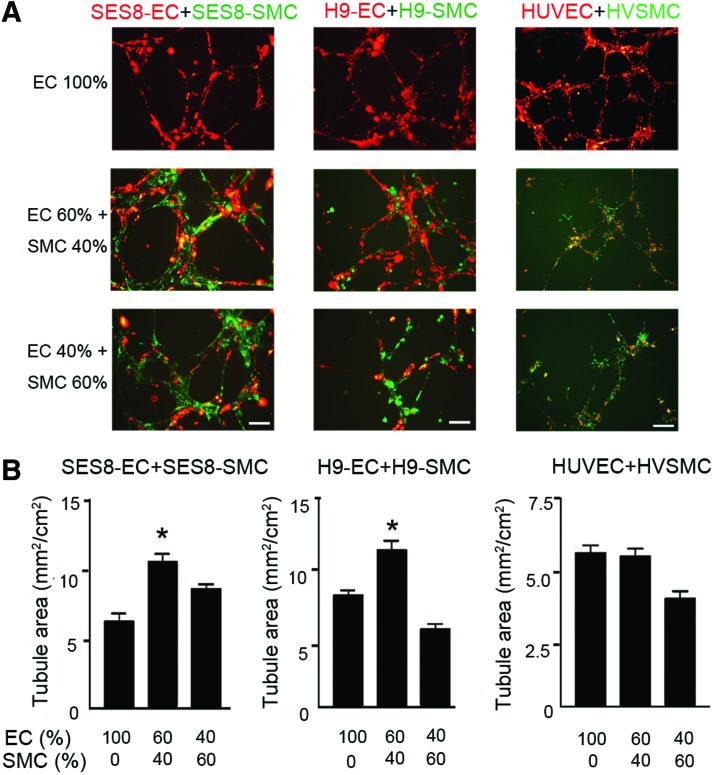
Because VSMCs stabilize and provide physical support to the nascent vasculature, SMCs as well as ECs are required for the formation of functional arterial vasculature. We performed an in vitro tube formation assay with different combinations of differentiated ECs and SMCs. The extent of the tubular network formation was influenced by the ratio of ECs to SMCs (Fig. 2). Compared with SES8-ECs alone (EC 100%), a 60:40 ratio of SES8-ECs:SES8-SMCs significantly promoted tubular network formation on Matrigel. However, further addition of SES8-SMCs to a ratio of 40:60 (ECs:SMCs) decreased tubular network formation. Similar results were observed in the identical experiments performed with H9-ECs and H9-SMCs. However, no such increase was found in an experiment with human umbilical vein endothelial cells (HUVECs) and human vascular SMCs (HVSMCs). These data suggest that, when combined at an appropriate ratio, ECs and SMCs derived from hiPSCs or hESCs cooperate to enhance tubular network formation.
FIG. 2.
Cooperation between hiPSC-derived ECs and SMCs promotes tubular network formation. Representative images (A) and quantitative analysis (B) of tube formation. The same total number of cells was seeded on Matrigel at ratios of 100:0, 60:40, and 40:60 (DiI-labeled ECs:DiO-labeled SMCs). The tubule area was measured after overnight incubation (mean±SEM, *p<0.05 vs. EC 100% group, n=4). Scale bars are 100 μm. Color images available online at www.liebertpub.com/tea
Coimplantation of hiPSC-derived ECs and SMCs enhances in vivo neovascularization
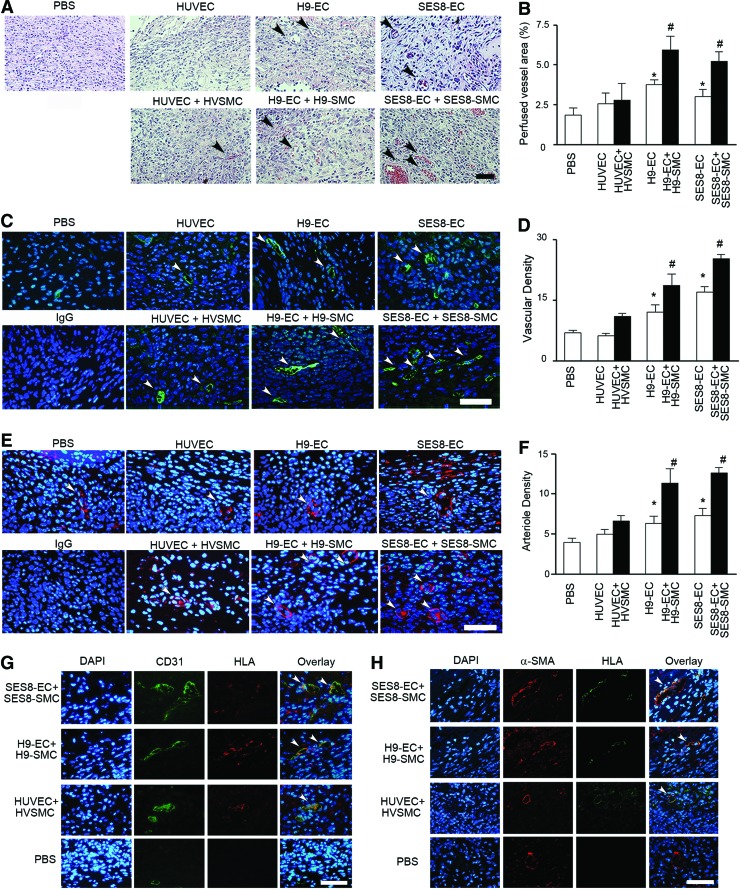
To determine the effect of cotransplantation of hiPSC-derived ECs and SMCs on in vivo neovascularization, SES8-ECs were implanted alone or coimplanted with SES8-SMCs at a ratio of 60:40 (ECs:SMCs) in athymic nude mice as a suspension in Matrigel/collagen. On day 14 post-treatment, implanted gels were harvested. Hematoxylin and eosin (H&E) staining visualized the erythrocyte-filled blood vessels that had anastomosed to the host vascular network (Fig. 3A, B). When primary ECs (HUVECs) or a mixture of primary ECs with primary SMCs (HVSMCs) were implanted, there was no significant increase in the perfused vessel area within the gel as compared with a PBS control. However, mice transplanted with SES8-ECs or H9-ECs exhibited a significantly greater erythrocyte-filled vascular area than those treated with HUVECs or PBS. Notably, mice coimplanted with SES8-ECs+SES8-SMCs or H9-ECs+H9-SMCs showed a further increase in the perfused vascular area compared with mice treated with SES8-ECs or H9-ECs alone. Immunohistological analyses consistently revealed that the transplantation of SES8-ECs or H9-ECs alone significantly enhanced the number of CD31-positive blood vessels compared with HUVEC or PBS controls (Fig. 3C–F). Cotransplantation of ECs with SES8-SMCs or H9-SMCs further increased blood vessel and arteriole densities. We also investigated whether implanted human cells were incorporated into mouse microvasculature using a costaining assay with anti-HLA IgGs. Gels containing ECs and SMCs derived from SES8 or H9 exhibited significantly more neovessels lined with human ECs (HLA/CD31 double-positive) or human SMCs (HLA/α-SMA double-positive) compared with those injected with primary cells or PBS (Fig. 3G, H).
FIG. 3.
Coimplantation of hiPSC-derived ECs and SMCs significantly enhances in vivo neovascularization. (A, B) Representative H&E images and quantitative analysis of perfused blood vessel area in Matrigel/collagen gels containing PBS (vehicle control), ECs alone (white bars), or a mixture of ECs+SMCs (black bars). The area of erythrocyte-filled vessels (arrowheads) on H&E-stained images was expressed as a percentage of the total tissue area (*p<0.05 vs. PBS, #p<0.05 vs. EC+SMC counterpart, n=5). (C, D) Representative images of CD31(green)-stained blood vessels (arrowheads) and quantitative analysis of vascular density in Matrigel/collagen gels (*p<0.05 vs. PBS, #p<0.05 vs. EC+SMC counterpart, n=5). (E, F) Representative images of α-SMA (red)-stained arterioles (arrowheads) and quantitative analysis of the arteriole density in Matrigel/collagen gels (*p<0.05 vs. PBS, #p<0.05 vs. EC+SMC counterpart, n=5). Vascular and arteriole densities were determined as the number of CD31- or α-SMA-positive vessels per hpf, respectively. (G, H) Representative images showing the incorporation of human ECs (G) and human SMCs (H). Arrowheads indicate CD31/HLA double-positive cells (G) and α-SMA/HLA double-positive cells (H). Nuclei were stained with DAPI and scale bars are100 μm. All data are presented as mean±SEM. The number of mice used for each group was five. PBS, phosphate-buffered saline; hpf, high-power field. Color images available online at www.liebertpub.com/tea
Angiogenic secretome profiles of differentiated ECs and SMCs and their paracrine interaction
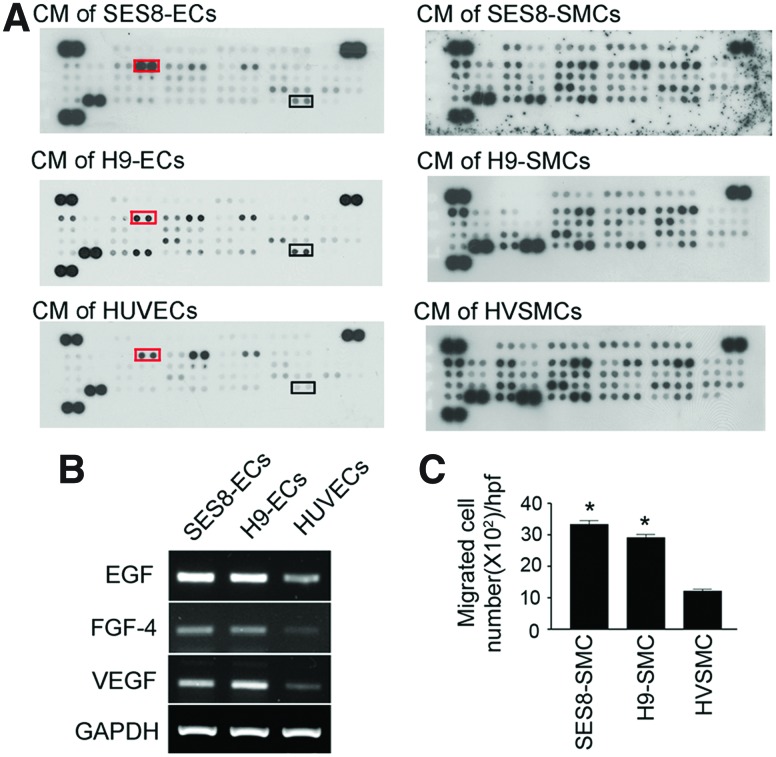
To investigate the possible mechanism by which differentiated cells from H9 and SES8 exhibited a greater neovascularization activity than primary cells, the amount of vascularization-related soluble factors in their conditioned medium (CM) was determined using a human angiogenesis protein array (Fig. 4A). SES8-SMCs and H9-SMCs appeared to have similar growth factor and cytokine expression profiles to HVSMCs. However, SES8-ECs and H9-ECs released greater amounts of vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and fibroblast growth factor (FGF)-4 than HUVECs, which was confirmed by RT-PCR analysis (Fig. 4B). Given that these secreted factors have angiogenic properties, it is conceivable that the greater in vivo neovascularization ability of SES8-ECs or H9-ECs than HUVECs might be at least partially due to their paracrine activity.8,9 In addition, EGF and FGF-4 released from differentiated ECs might contribute to the interaction between differentiated ECs and SMCs. The activation of their receptors has been reported to promote SMC migration and enhance arteriogenesis.8,10 Therefore, transwell migration assay was performed with the CM harvested from ECs to assess the paracrine interaction between differentiated ECs and SMCs. Figure 4C revealed that secreted factors from SES8-ECs and H9-ECs significantly promoted the chemotactic migration ability of SES8-SMCs and H9-SMCs, respectively, whereas those from HUVECs failed to increase the migration of HVSMCs. This result may explain how differentiated SMCs and ECs were coaligned to form vascular network in in vitro tube formation and in vivo gel assays.
FIG. 4.
Angiogenic secretome profiles of differentiated ECs and SMCs, and their paracrine interaction. (A) The angiogenesis proteome profiler array system was used to screen the cytokines and growth factors in the CM harvested from ECs and SMCs. The boxes on membranes marked EGF (right boxes) and VEGF (left boxes). (B) mRNA levels of VEGF, EGF, and FGF-4 in SES8-ECs, H9-ECs, and HUVECs were analyzed using RT-PCR. (C) Quantification of the number of migrated SMCs. SMCs were placed in the upper transwell chamber and the CM harvested from ECs was added to the lower chamber (mean±SEM, *p<0.05 vs. HUVEC, n=4). CM, conditioned medium; EGF, epidermal growth factor; VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor. Color images available online at www.liebertpub.com/tea
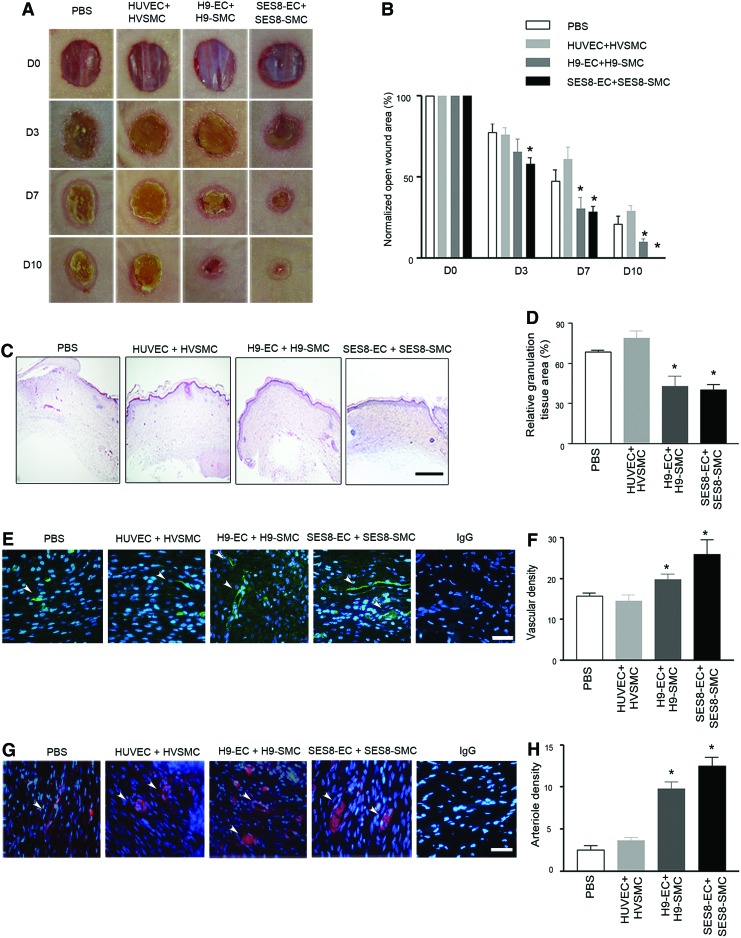
Cotransplantation of hiPSC-derived ECs and SMCs promotes neovascularization and accelerates tissue repair in murine dermal wounds
To evaluate the therapeutic effect of the combined administration of hiPSC-derived ECs and SMCs on neovascularization and tissue repair, cells were transplanted around full-thickness dermal wounds in athymic nude mice. Mice treated with ECs and SMCs derived from SES8 or H9 exhibited significantly smaller open wound areas as early as day 7 after injury compared with mice treated with PBS (vehicle control) or primary cells (Fig. 5A, B). The granulation tissue area was measured in the midwound regions from tissues harvested at day 10 after injury. Dermal wounds treated with SES8-ECs+SES8-SMCs or H9-ECs+H9-SMCs had a substantially reduced cross-sectional granulation tissue area than wounds treated with primary cell controls or PBS (Fig. 5C, D). To investigate whether coadministration of ECs and SMCs derived from SES8 or H9 might increase neovascularization at the wounded tissue, the blood vessel and arteriole densities were measured by counting CD31- or α-SMA-positive neovessels in the granulation tissues of wound sections. The blood vessel and arteriole densities in wounds coimplanted with SES8-ECs+SES8-SMCs or H9-ECs+H9-SMCs were significantly greater than those in wounds implanted with primary somatic cells or PBS (Fig. 5E–H). Although slightly higher in magnitude, the increase in vascular density in wounds treated with SES8-derived cells was not significantly different from that observed in wounds treated with H9-derived cells.
FIG. 5.
Cotransplantation of hiPSC-derived ECs and SMCs promotes neovascularization and accelerates tissue repair in murine dermal wounds. (A) Representative images of dermal wounds in athymic nude mice cotransplanted with ECs and SMCs (HUVEC+HVSMC; H9-EC+H9-SMC; SES8-EC+SES8-SMC) or PBS (vehicle control) on the indicated days (D) after wounding. (B) Open wound area was expressed as a percentage of the initial wound area (*p<0.05 vs. PBS, n=6). (C, D) Representative H&E staining images of granulation tissues at day 10 and quantitative analysis of the relative granulation tissue area (*p<0.05 vs. PBS, n=6). (E, F) Representative images of CD31 (green)-stained blood vessels (arrowheads) and quantitative analysis of the vascular density in granulation tissues on day 10 (*p<0.05 vs. PBS, n=6). (G, H) Representative images of α-SMA (red)-stained arterioles (arrowheads) and quantitative analysis of the arteriole density in granulation tissues at day 10 (*p<0.05 vs. PBS, n=6). Vascular and arteriole densities were determined as the number of CD31- or α-SMA-positive vessels per hpf, respectively. Nuclei were stained with DAPI (blue) and scale bars are 100 μm. All data are presented as mean±SEM. The number of mice used for each group was six. Color images available online at www.liebertpub.com/tea
Discussion
In the present study, we derived ECs and SMCs from hiPSCs using a stepwise vascular differentiation protocol. The hiPSC-derived ECs and SMCs exhibited greater ability to form tubular networks in the matrix than did primary somatic ECs or SMCs. In particular, the cotransplantation of hiPSC-derived ECs and SMCs had a more beneficial effect on vascular and tissue regeneration than transplantation of hiPSC-derived ECs alone. Other studies with adult stem cells have also revealed that the coadministration of endothelial progenitors with SMCs significantly promotes new arteriole formation.11,12 When adult mesenchymal progenitors bearing phenotypic characteristics of pericytes were cotransplanted with endothelial progenitors, they enhanced the development of stable vascular networks in ischemic tissues.13,14 In this regard, the current study aimed to examine the therapeutic benefit brought by the cooperation between hiPSC-derived ECs and SMCs for vascular regeneration in the wound-healing process.
The hiPSC-derived ECs and SMCs exhibited more intercellular association in Matrigel than primary somatic ECs or SMCs. This increased physical association between hiPSC-derived ECs and SMCs may involve their paracrine crosstalk, in that, the CM harvested from hiPSC-derived ECs substantially promoted the migration ability of hiPSC-derived SMCs. Consistent with in vitro data, Matrigel/collagen implants containing hiPSC-ECs and SMCs showed increased density of blood vessels and arterioles compared to gels containing hiPSC-ECs alone. HiPSC-ECs were incorporated into the murine vasculature and hiPSC-SMCs were found in the periendothelial layers of the neovessel. In contrast, injection of primary somatic ECs and SMCs induced only a marginal increase in neovessel formation compared to PBS controls. The observed increase in vascular density might not be simply due to the physical engraftment of hiPSC-derived cells into the vasculature. Transplanted cells are also likely to contribute to neovascularization by secreting bioactive molecules that stimulate murine angiogenesis or by establishing a regenerative environment at the injured tissues. Indeed, the human angiogenesis protein array experiment revealed that SES8-ECs and H9-ECs released greater amounts of VEGF, EGF, FGF-4, and VEGF-C than HUVECs. These data suggest that the enhanced in vivo neovascularization in groups treated with SES8- or H9-derived cells might be at least partially due to the paracrine activity of SES8-ECs or H9-ECs. In a murine full-thickness dermal wound model, combined administration of hiPSC-derived ECs and SMCs had a greater beneficial effect on arteriogenesis and tissue regeneration than transplantation of hiPSC-derived ECs alone.
The present study is the first to our knowledge to show the therapeutic benefits brought by the cooperation between hiPSC-derived ECs and SMCs in the wound-healing process. Transplanted cells not only acted as vascular building blocks during neovascularization, but also might provide paracrine factors for endogenous angiogenesis and tissue repair. Although further studies are required to investigate the immunogenicity of hiPSC-derived cells, the cotransplantation of hiPSC-derived ECs and SMCs could serve as a potential therapeutic alternative for dermal wound healing and other ischemic vascular diseases.15
Supplementary Material
Acknowledgments
This work was supported by the Bio & Medical Technology Development Program (2010-0020275) (2012M3A9C6050368) and the Basic Science Research Program (2012-0003729) through the National Research Foundation funded by the Korean Government.
Disclosure Statement
No competing financial interests exist.
References
- 1.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Park S.W. Jun Koh Y. Jeon J. Cho Y.H. Jang M.J. Kang Y., et al. Efficient differentiation of human pluripotent stem cells into functional CD34+ progenitor cells by combined modulation of the MEK/ERK and BMP4 signaling pathways. Blood. 2010;116:5762. doi: 10.1182/blood-2010-04-280719. [DOI] [PubMed] [Google Scholar]
- 3.Rufaihah A.J. Huang N.F. Jame S. Lee J.C. Nguyen H.N. Byers B., et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2011;31:e72. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harder Y. Amon M. Erni D. Menger M.D. Evolution of ischemic tissue injury in a random pattern flap: a new mouse model using intravital microscopy. J Surg Res. 2004;121:197. doi: 10.1016/j.jss.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 5.Straino S. Germani A. Di Carlo A. Porcelli D. De Mori R. Mangoni A., et al. Enhanced arteriogenesis and wound repair in dystrophin-deficient mdx mice. Circulation. 2004;110:3341. doi: 10.1161/01.CIR.0000147776.50787.74. [DOI] [PubMed] [Google Scholar]
- 6.Kim J.Y. Song S.H. Kim K.L. Ko J.J. Im J.E. Yie S.W., et al. Human cord blood-derived endothelial progenitor cells and their conditioned media exhibit therapeutic equivalence for diabetic wound healing. Cell Transplant. 2010;19:1635. doi: 10.3727/096368910X516637. [DOI] [PubMed] [Google Scholar]
- 7.Lee T.H. Song S.H. Kim K.L. Yi J.Y. Shin G.H. Kim J.Y., et al. Functional recapitulation of smooth muscle cells via induced pluripotent stem cells from human aortic smooth muscle cells. Circ Res. 2010;106:120. doi: 10.1161/CIRCRESAHA.109.207902. [DOI] [PubMed] [Google Scholar]
- 8.van Cruijsen H. Giaccone G. Hoekman K. Epidermal growth factor receptor and angiogenesis: opportunities for combined anticancer strategies. Int J Cancer. 2005;117:883. doi: 10.1002/ijc.21479. [DOI] [PubMed] [Google Scholar]
- 9.Jazwa A. Kucharzewska P. Leja J. Zagorska A. Sierpniowska A. Stepniewski J., et al. Combined vascular endothelial growth factor-A and fibroblast growth factor 4 gene transfer improves wound healing in diabetic mice. Genet Vaccines Ther. 2010;8:6. doi: 10.1186/1479-0556-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poling J. Szibor M. Schimanski S. Ingelmann M.E. Rees W. Gajawada P., et al. Induction of smooth muscle cell migration during arteriogenesis is mediated by Rap2. Arterioscler Thromb Vasc Biol. 2011;31:2297. doi: 10.1161/ATVBAHA.111.232835. [DOI] [PubMed] [Google Scholar]
- 11.Melero-Martin J.M. Khan Z.A. Picard A. Wu X. Paruchuri S. Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 12.Foubert P. Matrone G. Souttou B. Lere-Dean C. Barateau V. Plouet J., et al. Coadministration of endothelial and smooth muscle progenitor cells enhances the efficiency of proangiogenic cell-based therapy. Circ Res. 2008;103:751. doi: 10.1161/CIRCRESAHA.108.175083. [DOI] [PubMed] [Google Scholar]
- 13.Melero-Martin J.M. De Obaldia M.E. Kang S.Y. Khan Z.A. Yuan L. Oettgen P., et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traktuev D.O. Prater D.N. Merfeld-Clauss S. Sanjeevaiah A.R. Saadatzadeh M.R. Murphy M., et al. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res. 2009;104:1410. doi: 10.1161/CIRCRESAHA.108.190926. [DOI] [PubMed] [Google Scholar]
- 15.Zhao T. Zhang Z.N. Rong Z. Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.