Abstract
Hypertension has adverse effects on cognition, can alter cerebral vasculature integrity, and is associated with the pathogenesis of dementia. Using meta-analysis, we correlated blood pressure to multiple cognitive domains among older adults free of clinical stroke and dementia. We identified 230 studies indexed in PubMed and PsycINFO relating blood pressure and cognition. After applying exclusion criteria, we selected n = 12 articles with n = 4,076 participants (age range 43–91 years). Meta-analysis yielded an association between blood pressure and episodic memory (r = −.18, p < .001) and between blood pressure and global cognition (r = −.07, p < .001). When limiting analyses to studies adjusting for vascular covariates (n = 8, n = 2,141), blood pressure was modestly related to global cognition (r = −.11, p < .001), attention (r = .14, p = .002), and episodic memory (r = −.20, p < .001) with a trend for language (r = −.22, p = .07). Findings underscore the need to manage blood pressure as a key prevention method in minimizing abnormal cognitive aging prior to the onset of clinical dementia.
Keywords: Cardiovascular disease, Dementia, Learning and episodic memory, Executive functioning, Meta analysis
Introduction
Dementia is a major public health issue that is prevalent in more than 5 million people over the age of 65 years with associated healthcare costs estimated at $183 billion annually (Alzheimer's Association, 2011). Current treatment options are in large part ineffective at arresting or slowing disease progression (Williams, 2009). Thus, the identification of risk factors, particularly modifiable vascular risk factors, is critical for long-term risk reduction and prevention of dementia.
One purported vascular risk factor for dementia is elevated blood pressure and hypertension (Bermejo-Pareja et al., 2010; Kivipelto, Laakso, Tuomilehto, Nissinen, & Soininen, 2002; Skoog et al., 1996; Whitmer, Sidney, Selby, Johnston, & Yaffe, 2005). Hypertension is itself a major public health issue for older adults, affecting an estimated 65% of adults over 65 years of age (Kearney et al., 2005) and is a risk factor for many poor health outcomes, including heart failure (Levy, Larson, Vasan, Kannel, & Ho, 1996), stroke (Kannel, Wolf, Verter, & McNamara, 1970; Strandgaard, 1996), and dementia (Skoog, 1997).
A majority of studies suggest that older adults with elevated blood pressure are at greater risk for developing Alzheimer's disease (AD) when compared with adults with normal blood pressure (Bermejo-Pareja et al., 2010; Kivipelto et al., 2002). The association between hypertension and incident AD appears to begin as early as mid-life with uncontrolled blood pressure in mid-life being associated with an increased risk of developing AD in later life (Launer et al., 2010). Among individuals already diagnosed with AD, hypertension is associated with a faster rate of cognitive decline (Bellew et al., 2004). Clinicopathological evidence suggests elevated blood pressure in mid-life is related to increased post-mortem neuropathological signs of AD in later life (i.e., neuritic plaques and neurofibrillary tangles). Post-mortem animal studies link hypertension with increased deposits of β-amyloid (Gentile et al., 2009), suggesting that blood pressure may be associated with the pathogenesis of AD, particularly within the medial temporal lobe (Petrovitch et al., 2000).
Elevated blood pressure has also been linked to an increased risk of stroke (Kannel et al., 1970; Strandgaard, 1996) and cerebrovascular changes, including small vessel ischemic disease (Hachinski, Potter, & Merskey, 1987), cortical infarcts (Leys et al., 1999; Suter et al., 2002), and subcortical white matter hyperintensities as seen on T2-weighted brain magnetic reasoning imaging (MRI) scans (de Leeuw et al., 2001; Raz, Rodrigue, & Haacke, 2007; Thomas, O'Brien, Barber, McMeekin, & Perry, 2003). Mechanistically, elevated blood pressure affects vascular structural integrity in multiple ways, including alteration in dilatory functioning (Yang, Mayhan, Faraci, & Heistad, 1991), dysregulation of cerebral perfusion (Jennings, 2003), and microvascular infarcts (White et al., 2002). These cerebrovascular changes are related to the development of vascular cognitive impairment (Bowler & Hachinski, 1995), a term encompassing a spectrum of cognitive changes due to cerebrovascular disease, including vascular cognitive impairment no dementia (Nyenhuis et al., 2004), subcortical ischemic vascular disease (for review, see Skoog, 1998), and vascular dementia (VaD; Hayden et al., 2006; Posner et al., 2000; Skoog, 1994).
Overall, the mechanism by which blood pressure contributes to cognitive decline, incident dementia, and symptom progression is likely multifactorial. Blood pressure purportedly affects the central nervous system through two pathways, including altering the cerebral vasculature (Moss & Jonak, 2007) and contributing to the pathogenesis of AD (Hall, Oostveen, Dunn, & Carter, 1995; Kitaguchi et al., 2009). It is well known that AD and vascular pathology frequently co-exist with estimates suggesting that 60%–90% of individuals with AD also have concomitant cerebrovascular disease post-mortem (Kalaria, 2000). As such, the clinical manifestation of these pathologies (i.e., neuropsychological profiles or symptomatology) can overlap (Erkinjuntti, 2001; Libon et al., 1998).
Elevated blood pressure is a modifiable vascular risk factor (Chobanian et al., 2003). Both epidemiological (Launer et al., 2010; Peila, White, Masaki, Petrovitch, & Launer, 2006) and clinical trial data (Poon, 2008) suggest that the management of blood pressure may reduce the risk of dementia over the life course. Thus, understanding how blood pressure relates to cognition prior to clinical dementia and stroke is important for early identification and prevention efforts.
In the absence of clinical dementia and stroke, elevated blood pressure has been linked to cognitive impairment (Knopman et al., 2001). Specifically, global cognition, as defined by either a composite of individual test scores (Elias, Wolf, D'Agostino, Cobb, & White, 1993; Knecht et al., 2008) or a single test assessing global cognition (Gupta, Solanki, & Pathak, 2008; Tsivgoulis et al., 2009), is lower among hypertensive when compared with normotensive elders. With respect to domain-specific impairments, lower performances in episodic memory (Elias et al., 1993; Swan, Carmelli, & Larue, 1998), attention (Elias et al., 1993; Madden & Blumenthal, 1998), and executive functioning (Bucur & Madden, 2010; Vicario, Martinez, Baretto, Diaz Casale, & Nicolosi, 2005; Waldstein et al., 1996) have been consistently observed in hypertensive when compared with normotensive samples.
In contrast, blood pressure has been inconsistently linked to language. Some studies suggest a minimal association (Waldstein, 2003; Waldstein et al., 2008), whereas others have reported an increase in blood pressure is correlated with poorer language abilities (Brady, Spiro, & Gaziano, 2005; Nation et al., 2010). Less consistently, blood pressure has been linked to decrements in information processing speed (Bucur & Madden, 2010), and blood pressure appears relatively unrelated to visuoperceptual abilities (Brady et al., 2005; Sands & Meredith, 1992; Swan, DeCarli, et al., 1998).
Mechanistically, elevated blood pressure may contribute to decrements in cognitive performance by altering the cerebral vasculature (Moss & Jonak, 2007) and white matter integrity (Longstreth et al., 1996) as well as by contributing to the pathogenesis of AD (Hall et al., 1995; Kitaguchi et al., 2009). Specifically, decreased episodic memory performance has been linked to the presence of vascular changes (Bucur et al., 2008; Gunning-Dixon & Raz, 2000) and AD pathology (O'Brien, Desmond, Ames, Schweitzer, & Tress, 1997; Scheltens et al., 1992). Executive functioning deficits have also been seen in individuals with white matter disease (O'Sullivan et al., 2001) and preclinical AD (Twamley, Ropacki, & Bondi, 2006). Language performances have been linked to white matter disease (Carew, Lamar, Cloud, Grossman, & Libon, 1997; Delano-Wood et al., 2009), though not all studies support this observation (Au et al., 2006; Mungas et al., 2001). Similarly, evidence suggests language function declines in early AD (Backman, Jones, Berger, Laukka, & Small, 2005; Jacobs et al., 1995; Mickes et al., 2007; Saxton et al., 2004); however, other studies have not supported this finding (Albert, Moss, Tanzi, & Jones, 2001; Fox, Warrington, Seiffer, Agnew, & Rossor, 1998). Decreased attention (Sierra et al., 2004) and information processing speed performances (Gunning-Dixon & Raz, 2000) have been linked to poor white matter integrity and are present in the preclinical presentation of AD (Twamley et al., 2006).
Despite a growing literature, it remains unclear which cognitive system may be most strongly affected by elevations in blood pressure (for review, see Waldstein, 2003). Studies examining the effect of blood pressure on specific cognitive domains lack agreement and consistency regarding which specific cognitive systems are in fact affected. For example, compared with normotensives, hypertensives show a longitudinal decline in processing speed and phonemic fluency (Alves de Moraes, Szklo, Knopman, & Sato, 2002) and a decline in non-verbal reasoning (Elias, Elias, Robbins, & Budge, 2004) but both studies evidence no decline in episodic memory (Alves de Moraes et al., 2002; Elias et al., 2004). Conversely, another study suggests that high blood pressure is associated with poorer episodic memory performance (Elias et al., 1993). As noted above, similar inconsistencies are seen within the literature relating blood pressure to language (Nation et al., 2010; Waldstein et al., 2008).
Given the overlap in clinical symptomatology and the common co-existence of vascular and AD pathology in older adults with cognitive decline, this meta-analysis aims to better understand and clarify how blood pressure affects specific cognitive systems independent of etiology (e.g., AD, microvascular pathology) in older individuals without a history of clinical stroke or dementia. Because of the multiple pathways in which high blood pressure affects the central nervous system, we hypothesized that blood pressure would be related to poorer cognitive performance in neuropsychological domains sensitive to these pathological mechanisms, including global cognition (Gupta et al., 2008; Knecht, Wersching, Lohmann, Berger, & Ringelstein, 2009), episodic memory (Gunning-Dixon & Raz, 2000; Scheltens et al., 1992), executive functioning (Bucur & Madden, 2010; Twamley et al., 2006), attention (Elias et al., 1993; Madden & Blumenthal, 1998), information processing speed (Gunning-Dixon & Raz, 2000; Twamley et al., 2006), and language (Backman et al., 2005; Brady et al., 2005).
Methods
Selection of Studies
Our methods for selecting relevant studies included three steps based on criteria and strategies outlined in the literature (Demakis, 2006; Rosenthal & DiMatteo, 2001; Small, Rosnick, Fratiglioni, & Backman, 2004; Stroup et al., 2000). First, using PubMed (with articles indexed beginning in 1966) and PsychINFO (with articles indexed beginning in 1887) as the primary databases, a search was performed on 1 October 2013 to identify relevant articles. Results from the search terms, which focused on identifying all relevant studies assessing the association between blood pressure and hypertension and cognition, are summarized in Table 1. After excluding duplicate records generated by the two databases, the search returned 9,395 unique articles (i.e., n = 7,113 studies indexed in PubMed, n = 2,282 studies indexed in PsycINFO). We excluded unrelated publications (e.g., “The old lady who liked liquorice: hypertension due to chronic intoxication in a memory-impaired patient”), resulting in 230 articles. In the second step, the following inclusion and exclusion criteria were applied in which selected studies must:
be published, peer-reviewed data (e.g., no reviews, no dissertations) from an observational study (i.e., cross-sectional or longitudinal prospective study but not a randomized controlled trial or intervention study),
include one or more objective cognitive measure(s),
include participants with hypertension as defined by elevated systolic blood pressure (>140 mmHg) and/or elevated diastolic blood pressure (>90 mmHg; Chobanian et al., 2003),
provide average systolic and diastolic blood pressure values for sample,
be available in the English language,
include study participants with a mean age of at least 55 years,
explicitly exclude participants with clinical dementia/AD (determined in each study by cognitive data, medical history),
explicitly exclude participants with clinical stroke (determined in each study by self-report, clinical evidence, or review of medical history), and
report sufficient statistical data to allow for the calculation of a correlation coefficient (e.g., at least two groups with means, standard deviations, and beta values presented).
Table 1.
Search scripts and results
| Database | Script | # of results |
|---|---|---|
| PubMed | (cognit* or memor* or neuropsycholog*) and (hypertens* or “blood pressure”) | 6,356 |
| PubMed | Hypertension[Mesh] AND (“Cognition” [Mesh] OR “Neuropsychological Tests” [Mesh]) | 757 |
| PsycINFO | (cognit* or memor* or neuropsycholog*) and (hypertens* or “blood pressure”) | 2,259 |
| PsycINFO | (DE “Hypertension”) AND ((DE “Cognition”) OR (DE “Neuropsychological Assessment”)) | 23 |
Two independent raters (MB and ALJ) reviewed the articles independently, and following a consensus discussion, a total of 12 unique studies were identified meeting all inclusion/exclusion criteria listed above, which are summarized in Table 2. In two cases, more than one study included in the meta-analysis came from the same or overlapping cohort:
community-dwelling participants from Baltimore, MD, USA (Waldstein, Brown, Maier, & Katzel, 2005; Waldstein & Katzel, 2004) and
the Systematic Evaluation and Alteration of Risk Factors for Cognitive Health (SEARCH-Health) study (Knecht et al., 2008, 2009).
Table 2.
Characteristics for selected studies (n = 12)
| Study | Sample size | Age (M ± SD)a | Age rangea | Cognitive domains |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GC | MEM | LG | AT | EF | PS | VS | |||||
| 1 | Brady and colleagues (2005) | 357 | 67 ± 7 | 52–85 | ✓ | ✓ | ✓ | ✓ | |||
| 2 | Elias and colleagues (2010) | 1,025 | 61 ± 13 | — | ✓ | ✓ | ✓ | ✓ | |||
| 3 | Goldstein and colleagues (2013) | 1,385 | — | — | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 4 | Gunstad and colleagues (2009) | 99 | 69 ± 8 | — | ✓ | ✓ | ✓ | ||||
| 5 | Hannesdottir and colleagues (2009) | 70 | 65 ± 9 | — | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 6 | Izquierdo-Porrera and Waldstein (2002) | 43 | 59 ± 11 | 43–82 | ✓ | ✓ | ✓ | ||||
| 7 | Knecht and colleagues (2008) | 377 | 64 ± 7 | 44–82 | ✓ | ||||||
| 8 | Knecht and colleagues (2009) | 377 | 64 ± 7 | 44–82 | ✓ | ||||||
| 9 | Kuo and colleagues (2004) | 70 | 72 ± 4 | 65–85 | ✓ | ✓ | |||||
| 10 | Waldstein and Katzel (2004) | 98 | — | 55–83 | ✓ | ✓ | ✓ | ✓ | |||
| 11 | Waldstein and colleagues (2005) | 101 | 67 ± 7 | 53–84 | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| 12 | Yeung and Thornton (2011) | 74 | 66 ± 8 | 51–91 | ✓ | ✓ | ✓ | ||||
Notes: M = mean; SD = standard deviation; ✓ = domain assessed by specific study; GC = global cognition; MEM = episodic memory; LG = language; AT = attention; EF = executive functioning; PS = information processing speed; VS = visuoperceptual skills.
aMean age or age range not reported in all studies.
Blood Pressure Measurements
All studies included in this meta-analysis assessed blood pressure using a brachial artery measurement.
Cognitive Domains
Based on the available descriptions from the papers' authors and standard categorizations of neuropsychological tests (Greenaway, Smith, Tangalos, Geda, & Ivnik, 2009; Lezak, Howieson, & Loring, 2004; McCabe, Roediger, McDaniel, Balota, & Hambrick, 2010), the cognitive test data were coded into one of seven a priori domains for each selected paper, including global cognition, episodic memory, language, attention, executive functioning, information processing speed, and visuoperceptual skills. Global cognition was defined based upon the authors' delineation and may have included a single test (i.e., the Mini-Mental State Examination [Goldstein, Levey, & Steenland, 2013; Hannesdottir et al., 2009]) or a composite score based on multiple cognitive tests (i.e., Elias, Dore, Davey, Robbins, & Elias, 2010; Knecht et al., 2008, 2009; see Table 3 for a description of the cognitive tests that comprise each domain by a specific study).
Table 3.
Neuropsychological tests by the cognitive domain assessed in each study
| Cognitive domain | Neuropsychological test | Brady and colleagues (2005) | Elias and colleagues (2010) | Goldstein and colleagues (2013) | Gunstad and colleagues (2009) | Hannesdottir and colleagues (2009) | Izquierdo-Porrera and Waldstein (2002) | Knecht and colleagues (2008) | Knecht and colleagues (2009) | Kuo and colleagues (2004) | Waldstein and Katzel (2004) | Waldstein and colleagues (2005) | Yeung and Thorton (2011) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global cognition | Composite Score | ✓a | ✓b | ✓b | |||||||||
| MMSE | ✓ | ✓ | |||||||||||
| Episodic memory | California Verbal Learning Test-II | ✓ | |||||||||||
| CERAD Word List Immediate and Delay Recall | ✓ | ||||||||||||
| Composite Score | ✓c | ✓d | |||||||||||
| WMS-R Logical Memory I and II | ✓ | ✓ | ✓ | ||||||||||
| WMS-III Logical Memory I and II | ✓ | ✓ | |||||||||||
| Morris Word List Immediate and Delay Recall | ✓ | ||||||||||||
| WMS-R Visual Reproduction I and II | ✓ | ✓ | |||||||||||
| WMS-III Visual Reproduction I and II | ✓ | ||||||||||||
| Language | Animal Fluency | ✓ | |||||||||||
| Boston Naming Test | ✓ | ||||||||||||
| Category Fluency | ✓ | ||||||||||||
| Composite Scoree | ✓ | ||||||||||||
| Attention | WAIS or WMS-R digit span forward | ✓ | ✓ | ✓ | ✓ | ||||||||
| WAIS or WMS-III digit span forward | ✓ | ||||||||||||
| WMS-R Visual Span-Tapping Forward | ✓ | ||||||||||||
| Executive functioning | Clock Drawing | ✓ | |||||||||||
| Composite Score | ✓f | ✓g | |||||||||||
| WAIS or WMS-R digit span backward | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| WAIS or WMS-III digit span backward | ✓ | ||||||||||||
| Everyday Problem Solving | ✓ | ||||||||||||
| EXIT 25 | ✓ | ||||||||||||
| Letter Fluency | |||||||||||||
| Stroop Color-Word Test | ✓ | ✓ | |||||||||||
| Trails B | ✓ | ✓ | ✓ | ✓ | |||||||||
| Verbal Fluency | ✓ | ||||||||||||
| WMS-R Visual Span-Tapping Backward | ✓ | ||||||||||||
| Word Fluency | ✓ | ||||||||||||
| Working Memory Compositeh | ✓ | ||||||||||||
| Information processing speed | Composite Scorei | ✓ | |||||||||||
| Trails (Motor speed and scanning subtests) | ✓ | ||||||||||||
| Trails A | ✓ | ✓ | |||||||||||
| WAIS-R Digit Symbol Coding | ✓ | ||||||||||||
| WAIS-III Digit Symbol Coding | ✓ | ||||||||||||
| Visuoperceptual skills | Composite Scorej | ✓ | |||||||||||
| WAIS-R Block Design | ✓ | ✓ | |||||||||||
| Figure-copying Test | ✓ | ||||||||||||
| Judgment of Line Orientation | ✓ | ✓ | |||||||||||
| Pattern Comparison Test | ✓ |
Notes: MMSE = Mini Mental State Examination; CERAD = Consortium to Establish a Registry for Alzheimer's Disease; WMS = Wechsler Memory Scale; WAIS = Wechsler Adult Intelligence Scale; Logical Memory I = Immediate Recall; Logical Memory II = Delayed Recall; Visual Reproductions I = Immediate; Visual Reproduction II = Delayed Recall; EXIT = The Executive Interview;
aComposite comprised of: tests included in the episodic memory, executive functioning, working memory, and information processing speed composites.
bComposite comprised of: Auditory Verbal Learning Test, WMS-R Digit Span, Rey Complex Figure, Color-Word Interference, Digit-Symbol Substitution Test, Trail Making Test A&B, Category and Letter fluency, and Boston Naming Test-Short Version.
cComposite comprised of: Hopkins Verbal Learning Test and WMS-R Logical Memory I and II.
dComposite comprised of: California Verbal Learning Test and Brief Visual Memory Test-Revised.
eComposite comprised of: Animal Naming and Boston Naming Test.
fComposite comprised of: Trails B and Controlled Oral Word Association Test.
gComposite comprised of: Trail Making Test B, Stroop Color-Word, and WAIS-III Digit Symbol Coding.
hComposite comprised of: WAIS digit span forward and backward, WAIS letter number sequencing, and COWAT.
iComposite comprised of: WAIS Digit Symbol Substitution, WAIS-III Symbol Search, Trails A, and Trails B.
jComposite comprised of: WAIS-III Block Design, Hooper Visual Organization Test, and Rey Complex Figure.
Data Extraction
Data extracted from each article included descriptive statistics, means, and standard deviations for the neuropsychological outcome measures, average, and standard deviation of the systolic and diastolic blood pressures for each group, and statistical results, including beta values, standard error measurements, odds ratio, and p-values. Table 2 summarizes each study and the cognitive domains assessed.
Clinical Covariates
Table 4 summarizes the vascular comorbidity exclusions and statistical covariates considered in each of the 12 selected studies. One study did not adjust for age (Goldstein et al., 2013) and 2 of the 12 studies did not adjust for education (Goldstein et al., 2013; Hannesdottir et al., 2009). Three studies did not statistically adjust for vascular risk factors other than hypertension, such as diabetes, cholesterol, or prevalent cardiovascular disease (Goldstein et al., 2013; Knecht et al., 2008; Yeung & Thornton, 2011). The clinical sample within one study included only participants with prevalent cardiovascular disease (Gunstad et al., 2009). The remaining eight studies methodologically considered vascular risk factors that might confound associations between blood pressure and cognition (e.g., diabetes, heart disease, cholesterol) either statistically or by excluding participants with medical/vascular comorbidities. Because a majority of studies included in the meta-analysis controlled for relevant demographic and/or vascular factors that might confound blood pressure and cognition associations, the current study did not apply further adjustments.
Table 4.
Demographic and clinical covariate considerations for selected studies (n = 12)
| Study |
Statistical covariates |
Sample exclusion | ||||
|---|---|---|---|---|---|---|
| Age | Education | Sex | Vascular factors | Vascular/medical comorbidities | ||
| 1 | Brady and colleagues (2005) | ✓ | ✓ | ✓e | ||
| 2 | Elias and colleagues (2010) | ✓ | ✓ | ✓ | ✓a | |
| 3 | Goldstein and colleagues (2013) | |||||
| 4 | Gunstad and colleagues (2009) | ✓ | ✓ | |||
| 5 | Hannesdottir and colleagues (2009) | ✓ | ✓f | |||
| 6 | Izquierdo-Porrera and Waldstein (2002) | ✓ | ✓ | ✓b | ||
| 7 | Knecht and colleagues (2008) | ✓ | ✓ | ✓ | ||
| 8 | Knecht and colleagues (2009) | ✓ | ✓ | ✓ | ✓c | |
| 9 | Kuo and colleagues (2004) | ✓ | ✓ | ✓ | ✓d | ✓g |
| 10 | Waldstein and Katzel (2004) | ✓ | ✓ | ✓h | ||
| 11 | Waldstein and colleagues (2005) | ✓ | ✓ | ✓h | ||
| 12 | Yeung and Thornton (2011) | ✓ | ✓ | |||
Note: ✓ = covariate or exclusion in specific study .
aSpecific covariate: body mass index (BMI), cigarette use, alcohol use, arthritis, and cardiovascular disease (myocardial infarction, coronary artery disease, heart failure, angina, TIA).
bSpecific covariate: antihypertensive use, hemoglobin A1C.
cSpecific covariate: smoking pack years, cholesterol, BMI, hemoglobin A1C, C-reactive protein.
dSpecific covariate: BMI, alcohol use, total cholesterol, antihypertensive use.
eSpecific covariate: “cardiovascular disease” and diabetes.
fSpecific covariate: cardiovascular disease (angina, myocardial infarction).
gSpecific covariate: congestive heart failure, valvular heart disease, carotid stenosis, coronary artery disease, chronic lung disease, smoking history, diabetes, severe hypertension, peripheral vascular disease.
hSpecific covariate: “cardiovascular disease,” diabetes, major medical disease (renal, hepatic, pulmonary), alcohol use.
Statistical Analyses
To test the association between blood pressure and cognition, we calculated a correlation coefficient (i.e., r-value; Diener, Hilsenroth, & Weinberger, 2009) where r represents the direction and strength of association between blood pressure (defined for each selected study as systolic or diastolic blood pressure) and cognition, weighted by the sample size of each individual study (Borenstein, Hedges, Higgings, & Rothstein, 2009). A correlation coefficient was used to compare results across studies because a majority of the 12 studies statistically adjusted for or excluded relevant and potentially confounding demographic (n = 10) or vascular factors (n = 8).
Four types of association measures between blood pressure and cognition were reported directly or could be obtained directly from the selected studies: (a) Pearson's correlation, (b) beta estimates from linear regressions, (c) odds ratios from logistic regression in which the outcome was hypertension status, and (d) subcategory mean blood pressure and mean cognitive performance, categorized by diagnostic or treatment status of hypertension. All association measures were converted into a Pearson correlation to compare association measures across domains (Borenstein et al., 2009).
The beta estimate was converted to the standardized beta estimate, which is equivalent to the Pearson correlation coefficient, by multiplying the standard deviation of blood pressure and dividing by the standard deviation of cognitive performance. Odds ratios were converted to Pearson's correlation (Borenstein et al., 2009). For the fourth type of the association measure, a linear random effect model was applied with the mean cognitive performance of each group as the outcome and the mean blood pressure of each group as the random effect to obtain the beta estimates. The corresponding Pearson correlation was then obtained by applying the conversion method to the beta estimate described above. Many published studies did not provide cognitive performance standard deviation information; in these cases, a quarter of the raw score ranges of those neuropsychological measures was used as an approximate of standard deviation.
The resulting correlation coefficients between blood pressure and cognition were pooled across studies by generating an overall correlation coefficient for each cognitive system using a random-effects model to adjust for variation between the 12 studies and to account for the repeated measures of the same study in a cognitive domain. Heterogeneity of effect sizes were accounted for by the random effect model used for the meta-analysis (Berkey, Hoaglin, Mosteller, & Colditz, 1995). Next, to better understand how blood pressure related to cognition statistically independent of demographic and other medical or vascular factors, the correlation coefficient for each cognitive domain was obtained from the meta-analysis limited to the eight studies that systematically adjusted for demographic variables (i.e., age, education) and adjusted or excluded for relevant medical factors (i.e., diabetes, cholesterol, cardiac disease). For all analyses (i.e., unadjusted and adjusted), correlation coefficients were considered small at 0.10, moderate at 0.30, and large at ≥0.50 (Cohen, 1988).
For both unadjusted and adjusted analyses, preferential association between blood pressure and cognition across the seven cognitive domains was examined by comparing the 99% confidence intervals (CIs) for the calculated correlation coefficients. If the corresponding CIs overlapped for any two cognitive domains, no statistical difference existed between the correlation coefficients; thus, there was no evidence for any difference in association between the two domains. Similarly, differential associations between systolic versus diastolic blood pressure and cognition were also assessed using the same strategy.
Analyses were conducted using R 2.12.1 (www.r-project.org) with functions “escalc” and “rma” from metafor package for random effect models in meta-analysis.
Results
Study Characteristics
Twelve studies were included in the meta-analysis, with a total of 4,076 participants. Table 2 summarizes each study, including sample size, clinical characteristics, and cognitive domains assessed.
Unadjusted Results Between Blood Pressure and Cognition
Global cognition
A small correlation between blood pressure and global cognition was noted (n = 2,866, r = −.07, p < .001, 99% CI = −0.13 to −0.02). This finding was based on the following studies: Elias and colleagues (2010), Goldstein and colleagues (2013), Hannesdottir and colleagues (2009), Knecht and colleagues (2009), and Knecht and colleagues (2008). Results were essentially the same when excluding Mini Mental State Examination (MMSE) results from the analysis (i.e., limiting the analysis to composite measures of global cognition; n = 1,402, r = −.10, p < .001, 99% CI = −0.17 to −0.03; Elias et al., 2010; Knecht et al., 2008, 2009).
Episodic memory
A modest correlation was observed between blood pressure and episodic memory performance (n = 3,331, r = −.18, p < .001, 99% CI = −0.25 to −0.12). This finding was based on the following studies: Brady and colleagues (2005), Elias and colleagues (2010), Goldstein and colleagues (2013), Gunstad and colleagues (2009), Hannesdottir and colleagues (2009), Izquierdo-Porrera and Waldstein (2002), Kuo and colleagues (2004), Waldstein and colleagues (2005), Waldstein and Katzel (2004), and Yeung and Thornton (2011).
Language
No correlation was found between blood pressure and language (n = 1,841, r = −.03, p = .62, 99% CI = −0.18 to 0.12). This finding was based on the following studies: Brady and colleagues (2005), Goldstein and colleagues (2013), and Gunstad and colleagues (2009).
Attention
A trend for a small correlation was found between blood pressure and attention (n = 1,706, r = .09, p = .02, 99% CI = −0.01 to 0.19). This finding was based on the following studies: Goldstein and colleagues (2013), Hannesdottir and colleagues (2009), Izquierdo-Porrera and Waldstein (2002), Waldstein and colleagues (2005), and Waldstein and Katzel (2004).
Executive functioning
Blood pressure and executive functioning were not statistically correlated (n = 3,232, r = −.08, p = .21, 99% CI = −0.25 to 0.09). This finding was based on the following studies: Brady and colleagues (2005), Elias and colleagues (2010), Goldstein and colleagues (2013), Hannesdottir and colleagues (2009), Izquierdo-Porrera and Waldstein (2002), Kuo and colleagues (2004), Waldstein and colleagues (2005), Waldstein and Katzel (2004), and Yeung and Thornton (2011).
Information processing speed
No correlation was observed between blood pressure and information processing speed (n = 2,664, r = −.03, p = .02, 99% CI = −0.06 to 0.00). This finding was based on the following studies: Elias and colleagues (2010), Goldstein and colleagues (2013), Hannesdottir and colleagues (2009), Waldstein and colleagues (2005), and Yeung and Thornton (2011).
Visuoperceptual abilities
Blood pressure was not related to visuoperceptual abilities (n = 655, r = .00, p = .98, 99% CI = −0.12 to 0.13). This finding was based on the following studies: Brady and colleagues (2005), Gunstad and colleagues (2009), Waldstein and colleagues (2005), and Waldstein and Katzel (2004).
Comparison across cognitive domains
Blood pressure had a stronger correlation with episodic memory when compared with attention, information processing speed, and visuospatial skills.
Systolic versus diastolic blood pressure
When associations between systolic and diastolic blood pressure and cognition were compared, results suggested that diastolic blood pressure was more strongly correlated with episodic memory (r = −.29, p < .001, 99% CI = −0.38 to −0.18) compared with systolic blood pressure (r = −.09, p < .001, 99% CI = −0.12 to −0.05).
Adjusted Results Between Blood Pressure and Cognition
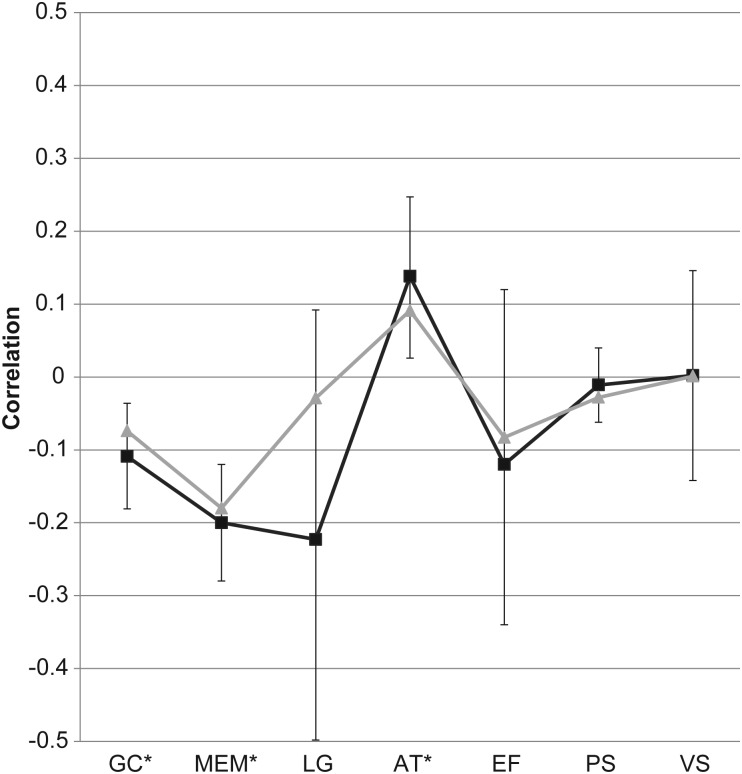
When correlations were recalculated limiting analyses to the eight studies (see Table 3 for details) that statistically adjusted for demographic variables or excluded or statistically adjusted for vascular factors, findings were as follows (see Figure 1 for the depiction of these results).
Fig. 1.
Adjusted meta-analysis correlation results by the cognitive domain with 99% confidence intervals. GC = global cognition; MEM = episodic memory; LG = language; AT = attention; EF = executive functioning; PS = information processing speed; VS = visuoperceptual skills; *p < .01. Gray line = unadjusted results. Black line = adjusted results.
Global cognition
A modest correlation was observed between blood pressure and global cognition (n = 1,481, r = −.11, p < .001, 99% CI = −0.18 to −0.04). Studies included were: Elias and colleagues (2010), Hannesdottir and colleagues (2009), and Knecht and colleagues (2009). Compared with unadjusted analyses, the correlation coefficient became somewhat stronger, suggesting that when prevalent cardiovascular disease is adjusted for, blood pressure is modestly correlated with global cognition.
Episodic memory
A modest correlation between blood pressure and episodic memory was revealed (n = 1,773, r = −.20, p < .001, 99% CI = −0.28 to −0.12). Studies included were: Brady and colleagues (2005), Elias and colleagues (2010), Hannesdottir and colleagues (2009), Izquierdo-Porrera and Waldstein (2002), Kuo and colleagues (2004), Waldstein and colleagues (2005), and Waldstein and Katzel (2004). Compared with unadjusted analyses, the negative correlation was even stronger when including studies that adjusted for or excluded vascular factors, suggesting that blood pressure is negatively associated with episodic memory.
Language
A trend for a moderate association was found between blood pressure and language (n = 357, r = −.22, p = .07, 99% CI = −0.50 to 0.09). This finding was based on only one study: Brady and colleagues (2005).
Attention
A significant small positive correlation was observed between blood pressure and attention (n = 321, r = .14, p = .002, 99% CI = 0.03 to 0.25). This finding was based on the following studies: Hannesdottir and colleagues (2009), Izquierdo-Porrera and Waldstein (2002), Waldstein and colleagues (2005), and Waldstein and Katzel (2004). Compared with the unadjusted analyses, when adjusting for excluding vascular factors, the association between blood pressure and attention became stronger, suggesting that blood pressure may be positively associated with attention.
Executive functioning
Results revealed a modest but not statistically significant correlation between blood pressure and executive functioning (n = 1,773, r = −.12, p = .20, 99% CI = −0.34 to 0.12). Studied included were: Brady and colleagues (2005), Elias and colleagues (2010), Hannesdottir and colleagues (2009), Izquierdo-Porrera and Waldstein (2002), Kuo and colleagues (2004), Waldstein and colleagues (2005), and Waldstein and Katzel (2004). Compared with the unadjusted analyses, the correlation coefficient became stronger although not statistically significant, suggesting that when vascular factors are adjusted for, blood pressure may have a small correlation with executive functioning.
Information processing speed
No correlation was observed between blood pressure and information processing speed (n = 1,205, r = −.01, p = .47, 99% CI = −0.07 to 0.04). This finding was based on the following studies: Elias and colleagues (2010), Hannesdottir and colleagues (2009), and Waldstein and colleagues (2005).
Visuoperceptual abilities
Blood pressure was not related to visuoperceptual abilities (n = 556, r = .00, p = .97, 99% CI = −0.14 to 0.15). This finding was based on the following studies: Brady and colleagues (2005), Waldstein and colleagues (2005), and Waldstein and Katzel (2004).
Comparison across cognitive domains
When associations between blood pressure and cognitive domains were compared, results revealed that blood pressure was more negatively correlated with episodic memory when compared with attention or processing speed. Blood pressure was also more negatively correlated with global cognition when compared with attention.
Systolic versus diastolic blood pressure
When associations between systolic versus diastolic blood pressure and cognition were compared, results suggested that diastolic blood pressure was more negatively correlated with episodic memory (r = −.32, p < .001, 99% CI = −0.43 to −0.21) when compared with systolic blood pressure (r = −.10, p < .001, 99% CI = −0.15 to −0.05).
Discussion
Using meta-analysis, we tested the association between blood pressure and cognition among older individuals free of clinical stroke or dementia at risk for cognitive decline as a function of their increasing age. Unadjusted results yielded a modest correlation between increasing blood pressure and poorer episodic memory performance and a small correlation of increasing blood pressure and decreased global cognitive performances. In contrast, unadjusted analyses revealed a small correlation between increasing blood pressure and stronger attention performances. In adjusted analyses, small to modest correlations persisted between increasing blood pressure and poorer episodic memory and global cognitive performances as well as the modest correlation between increasing blood pressure and enhanced attention performances. Of note, the correlation coefficients generated in this study are small to modest, a finding that is consistent with previous literature (Waldstein, 2003); additionally, given that participants included in the meta-analysis were purposely free of clinical dementia and stroke, we would not expect to see large effects between blood pressure and cognition.
The present meta-analysis offers some insight into the cognitive aging literature by suggesting that elevated blood pressure is associated with poorer aspects of cognitive aging prior to the onset of clinical dementia or stroke, independent of important demographic variables (e.g., age, education) and confounding medical or vascular comorbidities (e.g., diabetes, cholesterol, other prevalent cardiovascular disease). Interestingly, the cognitive systems correlated with blood pressure are ostensibly linked to neuroanatomical areas affected by dementia and cerebrovascular disease. Thus, the mechanism that underlies relations between blood pressure and reduced cognition may reflect a combination of etiologies, including vascular and AD neuropathological mechanisms. For example, blood pressure is associated with AD neuropathology. Post-mortem studies suggest that hypertensive mice evidence greater amyloid deposition than normotensive mice (Carnevale et al., 2012), and neurofibrillary tangles and neuritic plaques are increased among hypertensive versus normotensive elders post-mortem (Petrovitch et al., 2000). These pathological changes have been linked to decrements in episodic memory (Nagy et al., 1996). Furthermore, in an epidemiological context, elevated blood pressure is a risk factor for incident AD (Bermejo-Pareja et al., 2010).
Elevated blood pressure is also associated with cerebrovascular disease. That is, neuropathological data from animal studies suggest that hypertensive rats show more vascular changes (e.g., atherosclerosis, permeability alterations) and infarctions within the cerebral cortex when compared with normotensive rats (Nag, 1984), and hypertensive primates show a greater preponderance of gray and white matter infarctions than normotensive primates (Kemper, Blatt, Killiany, & Moss, 2001). These neuropathological changes have clinical implications, as infarcts in both the gray and white matter are associated with episodic memory impairments among hypertensive primates (Moss & Jonak, 2007). Elevated blood pressure is associated with white matter hyperintensities, and though their pathological nature is not fully understood (Black, Gao, & Bilbao, 2009), white matter changes are associated with compromised vascular integrity (Young, Halliday, & Kril, 2008). These pathological mechanisms may have clinical implications. Hypertensive elders have more white matter hyperintensities on structural MRI than their normotensive counterparts (de Leeuw et al., 2002), and such hyperintensities are associated with poorer global cognition (Longstreth et al., 2005), episodic memory (Gunning-Dixon & Raz, 2000), and executive functioning (Paul et al., 2005). High blood pressure has also been linked to incident VaD (Posner et al., 2002).
Our observation that, when vascular health covariates (e.g., diabetes, cholesterol, or prevalent cardiovascular disease) are statistically adjusted for, blood pressure is related to impairments in global cognition and individual cognitive systems (e.g., episodic memory, language) suggests that multiple pathological processes may underlie the association between blood pressure and cognitive aging prior to overt signs of cognitive impairment. The likelihood that multiple pathological processes are at play is further supported by evidence that AD and VaD share cognitive features (Groves et al., 2000; Mungas et al., 2001) and VaD and AD pathology commonly co-occur in clinically demented samples (Englund, 1998).
It is important to note that while there was an association between elevated blood pressure and poorer language and executive functioning performances in the adjusted analyses, these associations were not statistically significant. The absence of statistical significance may be explained by the multi-faceted nature of both domains, which is supported by the variability across study results. Specifically, language assessment can include various lexical retrieval measures, such as confrontation naming or semantic fluency (i.e., category naming), whereas executive functioning assessment might include inhibition or set-shifting measures. Also, cognitive tasks designed to measure one domain may tap other cognitive processes, increasing variability in findings. Finally, test sensitivity, sample selection, and the specific task implemented in each study may have contributed to variability in our results. Nevertheless, this study suggests that correlations between elevated blood pressure and poorer language and executive functioning performances may in part be driven by comorbid vascular health issues. Furthermore, results from these analyses highlight the importance of individual study methodology (i.e., sample selection, type of cognitive tests used) when evaluating findings across the literature.
Contrary to expectation, results suggested a positive association between blood pressure and attention. This unexpected finding may be related to a few factors. The majority of studies that assessed attention (i.e., four of the five studies) used the same test (i.e., digit span forward). It is plausible that in our dementia and stroke-free cohort, attention processes affected by vascular changes are not sufficiently detectable by this single (albeit common) measure. Future studies may wish to examine attention more comprehensively (e.g., using measures of selected, divided, and sustained attention) to better capture the early pathological changes associated with attention, such as white matter hyperintensities (Burton et al., 2004; Schmidt et al., 1993) or strategically located infarcts (Van der Werf et al., 2003). Although this finding was unexpected, cross-sectional analyses have found a positive correlation between blood pressure and attention (Gunstad et al., 2009) while longitudinal analyses suggest a negative association (Goldstein et al., 2013; Sands & Meredith, 1992). Our observation of a positive cross-sectional association may be due to alterations in autonomic nervous system functioning (e.g., heart rate variability, heart rate) that enhance cognitive functioning as suggested by previous literature (Gunstad et al., 2009; Hansen, Johnsen, Sollers, Stenvik, & Thayer, 2004; Thayer, Hansen, Saus-Rose, & Johnsen, 2009). Another possibility is that increased blood pressure affects cerebral perfusion that has a positive impact on attention, which is supported by reperfusion studies where increasing blood pressure results in cognitive improvements (Hillis et al., 2001, 2003).
The lack of association between blood pressure and information processing speed was inconsistent with our hypotheses. However, the lack of association between blood pressure and information processing speed provides some resolution to inconsistencies in the literature with some prior studies reporting an association (Gunning-Dixon & Raz, 2000), whereas others do not (Madden, Langley, Thurston, Whiting, & Blumenthal, 2003). Our findings suggest that in individuals free of dementia and clinical stroke, information processing speed may not represent one of the initial cognitive manifestations of high blood pressure.
There are a number of strengths associated with the current study. First, based on 12 empirical studies, the sample size was large (n = 4,076). Second, we considered blood pressure as a continuous variable rather than categorizing individuals as normotensive or hypertensive. Third, we maximized the information provided from each study, so when the respective study did not report a correlation or beta coefficient or odds ratio (i.e., Brady et al., 2005; Goldstein et al., 2013; Hannesdottir et al., 2009; Waldstein et al., 2005; Waldstein and Katzel, 2004), we used the established range for each cognitive test to obtain a Pearson correlation. Otherwise, these three studies (representing n = 2,011 participants) would have been excluded. Plus, we minimized variance within the effect sizes for each domain, which allowed for the provision of a more valid estimate of the association between blood pressure and cognition. Fourth, correlations were calculated on a subset of studies that statistically adjusted for key demographic (e.g., age, education) and vascular factors (e.g., diabetes, cholesterol, cardiac disease), suggesting that some associations between blood pressure and cognition are statistically independent of confounding vascular factors. Finally, we comprehensively considered a number of different cognitive domains in order to specifically understand which cognitive system is most affected by elevations in blood pressure.
Despite numerous strengths, several caveats must be considered when interpreting the results. First and foremost, our selection criteria for studies included only peer-review journal articles published in English. Although there are limitations to including unpublished work, such as methodological bias in locating unpublished work and methodological and analytical limitations of results not subject to peer review (Thornton & Lee, 2000), the omission of unpublished results increases the probability of committing a Type I error due to a positive publication bias. Given our exclusion of unpublished work, the results of this meta-analysis represent the strongest possible scenario in the association between blood pressure and cognition. Additionally, we applied a strict exclusion criterion in which each study's methodology must explicitly state the exclusion of stroke and dementia in the study cohort, which limited the amount of studies eligible to be included in this meta-analysis. Second, we considered information processing speed and attention as two separate domains. Within the attention domain, four of the five studies included in the meta-analysis relied on a single test of attention (i.e., digit span forward). These methodological issues may have minimized the association between blood pressure and attention and information processing speed. Third, all studies included in our meta-analysis assessed blood pressure using brachial artery measurement. Although the use of a single measure could limit our understanding of how different types of blood pressure measurements relate to cognition, the brachial artery measurement is the most common assessment of blood pressure, particularly within clinical practice, and may in fact increase the generalizability of our results. Fourth, the majority of studies included in this meta-analysis are cross-sectional in design, which limits making conclusions regarding causality between blood pressure and cognition. Next, factors such as dose effect, chronicity of blood pressure abnormalities, or the potential mediating effect of incipient dementia on blood pressure levels (Qiu, Winblad, & Fratiglioni, 2005) were not investigated within this meta-analysis. Similarly, we focused on elevated blood pressure and its association with cognition, though a curvilinear effect of age on blood pressure has been shown in the literature (Qiu et al., 2005) though inconsistently (Hebert et al., 2004). Longitudinal analyses may be helpful in clarifying the role of these potential mediating factors on the relation of blood pressure and cognition. Lastly, this meta-analysis included people free of dementia and stroke, but did not explicitly exclude individuals that may have had some degree of early cognitive decline or mild cognitive impairment.
With these limitations in mind, results from the current meta-analysis offer some insight into the specific cognitive domains affected by blood pressure and suggest that in a group of at-risk older adults free of clinical dementia and stroke brachial artery, blood pressure is modestly correlated with lower cognitive performances, specifically within the areas of global cognition, episodic memory, language, and executive functioning. Such observations offer to reconcile the specific cognitive domains affected by elevated blood pressure and may suggest a causal pathway between blood pressure and cognition versus an epiphenomenon (e.g., increasing age) or a mediating pathway (e.g., prevalent cardiovascular disease); replication of findings using longitudinal studies may be important to validate our observations. The mechanism accounting for our observations is likely multifactorial and includes a combination of cerebrovascular damage (e.g., infarcts and white matter disease) and AD pathological mechanisms (Schneider & Bennett, 2010). However, our discussion of potential mechanisms is theoretical, and given that AD and vascular pathology are frequently concomitant and the clinical manifestations of these pathologies can overlap, more research is needed to fully understand the underlying mechanism(s) accounting for associations between blood pressure and abnormal cognitive aging. Additionally, further study is necessary to better understand how disease modifying treatments or lifestyle interventions for elevations in blood pressure may have a beneficial impact on brain health prior to the onset of clinical dementia.
Funding
This work was supported by the National Institute on Aging (K23-AG030962 [Paul B. Beeson Career Development Award in Aging], R03-AG027480, and R01-AG034962) to ALJ, National Heart Lung & Blood Institute (R01-HL11516) to ALJ, the Alzheimer's Association (IIRG-08-88733) to ALJ, and the American Federation for Aging Research Medical Student Training in Aging Research (MSTAR) Program to MB.
Conflict of Interest
None declared.
References
*Studies included in this meta-analysis.
- Albert M. S., Moss M., Tanzi R., Jones K. Preclinical prediction of AD using neuropsychological tests [Research Support, U.S. Gov't, P.H.S.] Journal of the International Neuropsychological Society. 2001;7(5):631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- Alves de Moraes S., Szklo M., Knopman D. S., Sato R. The relationship between temporal changes in blood pressure and changes in cognitive function: Atherosclerosis risk in communities (ARIC) study. Preventive Medicine. 2002;35(3):258–263. doi: 10.1006/pmed.2002.1077. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association. 2011 Alzheimer's disease facts and figures. Alzheimer's and Dementia. 2011;7(2):208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Au R., Massaro J. M., Wolf P. A., Young M. E., Beiser A., Seshadri S., et al. Association of white matter hyperintensity volume with decreased cognitive functioning: The Framingham Heart Study. Archives of Neurology. 2006;63(2):246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- Backman L., Jones S., Berger A. K., Laukka E. J., Small B. J. Cognitive impairment in preclinical Alzheimer's disease: A meta-analysis [Comparative Study Meta-Analysis Research Support, Non-U.S. Gov't] Neuropsychology. 2005;19(4):520–531. doi: 10.1037/0894-4105.19.4.520. doi:10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- Bellew K. M., Pigeon J. G., Stang P. E., Fleischman W., Gardner R. M., Baker W. W. Hypertension and the rate of cognitive decline in patients with dementia of the Alzheimer type. Alzheimer Disease and Associated Disorders. 2004;18(4):208–213. doi:00002093-200410000-00009. [PubMed] [Google Scholar]
- Berkey C. S., Hoaglin D. C., Mosteller F., Colditz G. A. A random-effects regression model for meta-analysis [Comparative Study Research Support, U.S. Gov't, P.H.S.] Statistics in Medicine. 1995;14(4):395–411. doi: 10.1002/sim.4780140406. [DOI] [PubMed] [Google Scholar]
- Bermejo-Pareja F., Benito-Leon J., Louis E., Trincado R., Carro E., Villarejo A., et al. Risk of incident dementia in drug-untreated arterial hypertension: A population based study. Journal of Alzheimer's Disease. 2010;22(3):949–958. doi: 10.3233/JAD-2010-101110. [DOI] [PubMed] [Google Scholar]
- Black S., Gao F., Bilbao J. Understanding white matter disease: Imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40(3 Suppl.):S48–S52. doi: 10.1161/STROKEAHA.108.537704. doi:STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- Borenstein M., Hedges L., Higgings J., Rothstein H. Introduction to meta-analysis. Chichester, UK: Wiley; 2009. [Google Scholar]
- Bowler J. V., Hachinski V. Vascular cognitive impairment: A new approach to vascular dementia. Bailliere's Clinical Neurology. 1995;4(2):357–376. [PubMed] [Google Scholar]
- *Brady C. B., Spiro A., 3rd, Gaziano J. M. Effects of age and hypertension status on cognition: The Veterans Affairs Normative Aging Study. Neuropsychology. 2005;19(6):770–777. doi: 10.1037/0894-4105.19.6.770. [DOI] [PubMed] [Google Scholar]
- Bucur B., Madden D. J. Effects of adult age and blood pressure on executive function and speed of processing. Experimental Aging Research. 2010;36(2):153–168. doi: 10.1080/03610731003613482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucur B., Madden D. J., Spaniol J., Provenzale J. M., Cabeza R., White L. E., et al. Age-related slowing of memory retrieval: Contributions of perceptual speed and cerebral white matter integrity [Research Support, N.I.H., Extramural] Neurobiology of Aging. 2008;29(7):1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. doi:0.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton E. J., Kenny R. A., O'Brien J., Stephens S., Bradbury M., Rowan E., et al. White matter hyperintensities are associated with impairment of memory, attention, and global cognitive performance in older stroke patients. Stroke. 2004;35(6):1270–1275. doi: 10.1161/01.STR.0000126041.99024.86. doi:10.1161/01.STR.0000126041.99024.86. [DOI] [PubMed] [Google Scholar]
- Carew T. G., Lamar M., Cloud B. S., Grossman M., Libon D. J. Impairment in category fluency in ischemic vascular dementia [Research Support, U.S. Gov't, P.H.S.] Neuropsychology. 1997;11(3):400–412. doi: 10.1037//0894-4105.11.3.400. [DOI] [PubMed] [Google Scholar]
- Carnevale D., Mascio G., Ajmone-Cat M. A., D'Andrea I., Cifelli G., Madonna M., et al. Role of neuroinflammation in hypertension-induced brain amyloid pathology [Research Support, Non-U.S. Gov't] Neurobiology of Aging. 2012;33(1):205 e219–e229. doi: 10.1016/j.neurobiolaging.2010.08.013. doi:10.1016/j.neurobiolaging.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Chobanian A. V., Bakris G. L., Black H. R., Cushman W. C., Green L. A., Izzo J. L., Jr., et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. Journal of the American Medical Association. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. doi:10.1001/jama.289.19.2560289.19.2560. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Delano-Wood L., Bondi M. W., Sacco J., Abeles N., Jak A. J., Libon D. J., et al. Heterogeneity in mild cognitive impairment: Differences in neuropsychological profile and associated white matter lesion pathology. Journal of the International Neuropsychological Society. 2009;15(6):906–914. doi: 10.1017/S1355617709990257. doi:10.1017/S1355617709990257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw F. E., de Groot J. C., Achten E., Oudkerk M., Ramos L. M., Heijboer R., et al. Prevalence of cerebral white matter lesions in elderly people: A population based magnetic resonance imaging study. The Rotterdam Scan Study. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;70(1):9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw F. E., de Groot J. C., Oudkerk M., Witteman J. C., Hofman A., van Gijn J., et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125(Pt 4):765–772. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- Demakis G. J. Meta-analysis in neuropsychology: Basic approaches, findings, and applications. Clinical Neuropsychology. 2006;20(1):10–26. doi: 10.1080/13854040500203282. [DOI] [PubMed] [Google Scholar]
- Diener M. J., Hilsenroth M. J., Weinberger J. A primer on meta-analysis of correlation coefficients: The relationship between patient-reported therapeutic alliance and adult attachment style as an illustration. Psychotherapy Research: Journal of the Society for Psychotherapy Research. 2009;19(4–5):519–526. doi: 10.1080/10503300802491410. [DOI] [PubMed] [Google Scholar]
- *Elias M. F., Dore G. A., Davey A., Robbins M. A., Elias P. K. From blood pressure to physical disability: The role of cognition. Hypertension. 2010;55(6):1360–1365. doi: 10.1161/HYPERTENSIONAHA.110.149823. doi:HYPERTENSIONAHA.110.149823. [DOI] [PubMed] [Google Scholar]
- Elias M. F., Wolf P. A., D'Agostino R. B., Cobb J., White L. R. Untreated blood pressure level is inversely related to cognitive functioning: The Framingham Study. American Journal of Epidemiology. 1993;138(6):353–364. doi: 10.1093/oxfordjournals.aje.a116868. [DOI] [PubMed] [Google Scholar]
- Elias P. K., Elias M. F., Robbins M. A., Budge M. M. Blood pressure-related cognitive decline: Does age make a difference? Hypertension. 2004;44(5):631–636. doi: 10.1161/01.HYP.0000145858.07252.99. doi:01.HYP.0000145858.07252.99. [DOI] [PubMed] [Google Scholar]
- Englund E. Neuropathology of white matter changes in Alzheimer's disease and vascular dementia. Dementia and Geriatric Cognitive Disorders. 1998;9(Suppl. 1):6–12. doi: 10.1159/000051183. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T. Clinical deficits of Alzheimer's disease with cerebrovascular disease and probable VaD [Review] International Journal of Clinical Practice: Supplement. 2001;120:14–23. [PubMed] [Google Scholar]
- Fox N. C., Warrington E. K., Seiffer A. L., Agnew S. K., Rossor M. N. Presymptomatic cognitive deficits in individuals at risk of familial Alzheimer's disease. A longitudinal prospective study [Research Support, Non-U.S. Gov't] Brain. 1998;121(Pt 9):1631–1639. doi: 10.1093/brain/121.9.1631. [DOI] [PubMed] [Google Scholar]
- Gentile M. T., Poulet R., Di Pardo A., Cifelli G., Maffei A., Vecchione C., et al. Beta-amyloid deposition in brain is enhanced in mouse models of arterial hypertension [Research Support, Non-U.S. Gov't] Neurobiology in Aging. 2009;30(2):222–228. doi: 10.1016/j.neurobiolaging.2007.06.005. doi:10.1016/j.neurobiolaging.2007.06.005. [DOI] [PubMed] [Google Scholar]
- *Goldstein F. C., Levey A. I., Steenland N. K. High blood pressure and cognitive decline in mild cognitive impairment. Journal of the American Geriatrics Society. 2013;61(1):67–73. doi: 10.1111/jgs.12067. doi:10.1111/jgs.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenaway M. C., Smith G. E., Tangalos E. G., Geda Y. E., Ivnik R. J. Mayo older Americans normative studies: Factor analysis of an expanded neuropsychological battery. Clinical Neuropsychology. 2009;23(1):7–20. doi: 10.1080/13854040801891686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves W. C., Brandt J., Steinberg M., Warren A., Rosenblatt A., Baker A., et al. Vascular dementia and Alzheimer's disease: Is there a difference? A comparison of symptoms by disease duration. Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12(3):305–315. doi: 10.1176/jnp.12.3.305. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon F. M., Raz N. The cognitive correlates of white matter abnormalities in normal aging: A quantitative review. Neuropsychology. 2000;14(2):224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- *Gunstad J., Keary T. A., Spitznagel M. B., Poppas A., Paul R. H., Sweet L. H., et al. Blood pressure and cognitive function in older adults with cardiovascular disease. International Journal of Neuroscience. 2009;119(12):2228–2242. doi: 10.3109/00207450903139713. doi:10.3109/00207450903139713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Solanki R. K., Pathak V. Blood pressure is associated with cognitive impairment in young hypertensives. World Journal of Biological Psychiatry. 2008;9(1):43–50. doi: 10.1080/15622970601187784. [DOI] [PubMed] [Google Scholar]
- Hachinski V. C., Potter P., Merskey H. Leuko-araiosis [Research Support, Non-U.S. Gov't] Archives of Neurology. 1987;44(1):21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- Hall E. D., Oostveen J. A., Dunn E., Carter D. B. Increased amyloid protein precursor and apolipoprotein E immunoreactivity in the selectively vulnerable hippocampus following transient forebrain ischemia in gerbils. Experimental Neurology. 1995;135(1):17–27. doi: 10.1006/exnr.1995.1062. doi:S0014-4886(85)71062-X. [DOI] [PubMed] [Google Scholar]
- *Hannesdottir K., Nitkunan A., Charlton R. A., Barrick T. R., MacGregor G. A., Markus H. S. Cognitive impairment and white matter damage in hypertension: A pilot study. Acta Neurologica Scandinavica. 2009;119(4):261–268. doi: 10.1111/j.1600-0404.2008.01098.x. [DOI] [PubMed] [Google Scholar]
- Hansen A. L., Johnsen B. H., Sollers J. J., 3rd, Stenvik K., Thayer J. F. Heart rate variability and its relation to prefrontal cognitive function: The effects of training and detraining [Research Support, Non-U.S. Gov't] European Journal of Applied Physiology. 2004;93(3):263–272. doi: 10.1007/s00421-004-1208-0. [DOI] [PubMed] [Google Scholar]
- Hayden K. M., Zandi P. P., Lyketsos C. G., Khachaturian A. S., Bastian L. A., Charoonruk G., et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: The Cache County study. Alzheimer Disease and Associated Disorders. 2006;20(2):93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- Hebert L. E., Scherr P. A., Bennett D. A., Bienias J. L., Wilson R. S., Morris M. C., et al. Blood pressure and late-life cognitive function change: A biracial longitudinal population study. Neurology. 2004;62(11):2021–2024. doi: 10.1212/01.wnl.0000129258.93137.4b. [DOI] [PubMed] [Google Scholar]
- Hillis A. E., Kane A., Tuffiash E., Ulatowski J. A., Barker P. B., Beauchamp N. J., et al. Reperfusion of specific brain regions by raising blood pressure restores selective language functions in subacute stroke. Brain and Language. 2001;79(3):495–510. doi: 10.1006/brln.2001.2563. [DOI] [PubMed] [Google Scholar]
- Hillis A. E., Ulatowski J. A., Barker P. B., Torbey M., Ziai W., Beauchamp N. J., et al. A pilot randomized trial of induced blood pressure elevation: Effects on function and focal perfusion in acute and subacute stroke. Cerebrovascular Diseases. 2003;16(3):236–246. doi: 10.1159/000071122. [DOI] [PubMed] [Google Scholar]
- *Izquierdo-Porrera A. M., Waldstein S. R. Cardiovascular risk factors and cognitive function in African Americans. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2002;57(4):P377–P380. doi: 10.1093/geronb/57.4.p377. [DOI] [PubMed] [Google Scholar]
- Jacobs D. M., Sano M., Dooneief G., Marder K., Bell K. L., Stern Y. Neuropsychological detection and characterization of preclinical Alzheimer's disease. Neurology. 1995;45(5):957–962. doi: 10.1212/wnl.45.5.957. [DOI] [PubMed] [Google Scholar]
- Jennings J. R. Autoregulation of blood pressure and thought: Preliminary results of an application of brain imaging to psychosomatic medicine. Psychosomatic Medicine. 2003;65(3):384–395. doi: 10.1097/01.psy.0000062531.75102.25. [DOI] [PubMed] [Google Scholar]
- Kalaria R. N. The role of cerebral ischemia in Alzheimer's disease. Neurobiology of Aging. 2000;21(2):321–330. doi: 10.1016/s0197-4580(00)00125-1. doi:S0197-4580(00)00125-1. [DOI] [PubMed] [Google Scholar]
- Kannel W. B., Wolf P. A., Verter J., McNamara P. M. Epidemiologic assessment of the role of blood pressure in stroke. The Framingham study. Journal of the American Medical Association. 1970;214(2):301–310. [PubMed] [Google Scholar]
- Kearney P. M., Whelton M., Reynolds K., Muntner P., Whelton P. K., He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. doi:S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- Kemper T. L., Blatt G. J., Killiany R. J., Moss M. B. Neuropathology of progressive cognitive decline in chronically hypertensive rhesus monkeys. Acta Neuropathologica. 2001;101(2):145–153. doi: 10.1007/s004010000278. [DOI] [PubMed] [Google Scholar]
- Kitaguchi H., Tomimoto H., Ihara M., Shibata M., Uemura K., Kalaria R. N., et al. Chronic cerebral hypoperfusion accelerates amyloid beta deposition in APPSwInd transgenic mice. Brain Research. 2009;1294:202–210. doi: 10.1016/j.brainres.2009.07.078. doi:S0006-8993(09)01572-8. [DOI] [PubMed] [Google Scholar]
- Kivipelto M., Laakso M. P., Tuomilehto J., Nissinen A., Soininen H. Hypertension and hypercholesterolaemia as risk factors for Alzheimer's disease: potential for pharmacological intervention. CNS Drugs. 2002;16(7):435–444. doi: 10.2165/00023210-200216070-00001. [DOI] [PubMed] [Google Scholar]
- *Knecht S., Wersching H., Lohmann H., Berger K., Ringelstein E. B. How much does hypertension affect cognition?: Explained variance in cross-sectional analysis of non-demented community-dwelling individuals in the SEARCH study. Journal of the Neurological Sciences. 2009;283(1–2):149–152. doi: 10.1016/j.jns.2009.02.362. doi:S0022-510X(09)00428-6. [DOI] [PubMed] [Google Scholar]
- *Knecht S., Wersching H., Lohmann H., Bruchmann M., Duning T., Dziewas R., et al. High-normal blood pressure is associated with poor cognitive performance. Hypertension. 2008;51(3):663–668. doi: 10.1161/HYPERTENSIONAHA.107.105577. doi:HYPERTENSIONAHA.107.105577. [DOI] [PubMed] [Google Scholar]
- Knopman D., Boland L. L., Mosley T., Howard G., Liao D., Szklo M., et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56(1):42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- *Kuo H. K., Sorond F., Iloputaife I., Gagnon M., Milberg W., Lipsitz L. A. Effect of blood pressure on cognitive functions in elderly persons. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2004;59(11):1191–1194. doi: 10.1093/gerona/59.11.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer L. J., Hughes T., Yu B., Masaki K., Petrovitch H., Ross G. W., et al. Lowering midlife levels of systolic blood pressure as a public health strategy to reduce late-life dementia: Perspective from the Honolulu Heart Program/Honolulu Asia Aging Study. Hypertension. 2010;55(6):1352–1359. doi: 10.1161/HYPERTENSIONAHA.109.147389. doi:HYPERTENSIONAHA.109.147389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D., Larson M. G., Vasan R. S., Kannel W. B., Ho K. K. The progression from hypertension to congestive heart failure. Journal of the American Medical Association. 1996;275(20):1557–1562. [PubMed] [Google Scholar]
- Leys D., Erkinjuntti T., Desmond D. W., Schmidt R., Englund E., Pasquier F., et al. Vascular dementia: The role of cerebral infarcts. Alzheimer Disease and Associated Disorders. 1999;13(Suppl. 3):S38–S48. doi: 10.1097/00002093-199912003-00007. [DOI] [PubMed] [Google Scholar]
- Lezak M. D., Howieson D. B., Loring D. W. Neuropsychological assessment (4th ed.) New York: Oxford University Press; 2004. [Google Scholar]
- Libon D. J., Bogdanoff B., Cloud B. S., Skalina S., Giovannetti T., Gitlin H. L., et al. Declarative and procedural learning, quantitative measures of the hippocampus, and subcortical white alterations in Alzheimer's disease and ischaemic vascular dementia. Journal of Clinical and Experimental Neuropsychology. 1998;20(1):30–41. doi: 10.1076/jcen.20.1.30.1490. [DOI] [PubMed] [Google Scholar]
- Longstreth W. T., Jr., Arnold A. M., Beauchamp N. J., Jr., Manolio T. A., Lefkowitz D., Jungreis C., et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: The Cardiovascular Health Study [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, P.H.S.] Stroke. 2005;36(1):56–61. doi: 10.1161/01.STR.0000149625.99732.69. doi:10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- Longstreth W. T., Jr., Manolio T. A., Arnold A., Burke G. L., Bryan N., Jungreis C. A., et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27(8):1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- Madden D. J., Blumenthal J. A. Interaction of hypertension and age in visual selective attention performance. Health Psychology. 1998;17(1):76–83. doi: 10.1037//0278-6133.17.1.76. [DOI] [PubMed] [Google Scholar]
- Madden D. J., Langley L. K., Thurston R. C., Whiting W. L., Blumenthal J. A. Interaction of blood pressure and adult age in memory search and visual search performance. Aging Neuropsychology and Cognition. 2003;10(4):241–254. [Google Scholar]
- McCabe D. P., Roediger H. L., McDaniel M. A., Balota D. A., Hambrick D. Z. The relationship between working memory capacity and executive functioning: Evidence for a common executive attention construct [Research Support, N.I.H., Extramural] Neuropsychology. 2010;24(2):222–243. doi: 10.1037/a0017619. doi:10.1037/a0017619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickes L., Wixted J. T., Fennema-Notestine C., Galasko D., Bondi M. W., Thal L. J., et al. Progressive impairment on neuropsychological tasks in a longitudinal study of preclinical Alzheimer's disease [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, Non-P.H.S.] Neuropsychology. 2007;21(6):696–705. doi: 10.1037/0894-4105.21.6.696. doi:10.1037/0894-4105.21.6.696. [DOI] [PubMed] [Google Scholar]
- Moss M. B., Jonak E. Cerebrovascular disease and dementia: A primate model of hypertension and cognition. Alzheimer’s and Dementia. 2007;3(2 Suppl.):S6–S15. doi: 10.1016/j.jalz.2007.01.002. doi:S1552–5260(07)00005-2. [DOI] [PubMed] [Google Scholar]
- Mungas D., Jagust W. J., Reed B. R., Kramer J. H., Weiner M. W., Schuff N., et al. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer's disease. Neurology. 2001;57(12):2229–2235. doi: 10.1212/wnl.57.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S. Cerebral changes in chronic hypertension: Combined permeability and immunohistochemical studies. Acta Neuropathologica. 1984;62(3):178–184. doi: 10.1007/BF00691850. [DOI] [PubMed] [Google Scholar]
- Nagy Z., Jobst K. A., Esiri M. M., Morris J. H., King E. M., MacDonald B., et al. Hippocampal pathology reflects memory deficit and brain imaging measurements in Alzheimer's disease: Clinicopathologic correlations using three sets of pathologic diagnostic criteria. Dementia. 1996;7(2):76–81. doi: 10.1159/000106857. [DOI] [PubMed] [Google Scholar]
- Nation D. A., Wierenga C. E., Delano-Wood L., Jak A. J., Delis D. C., Salmon D. P., et al. Elevated pulse pressure is associated with age-related decline in language ability [Research Support, N. I. H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.] Journal of the International Neuropsychological Society. 2010;16(5):933–938. doi: 10.1017/S1355617710000548. doi:10.1017/S1355617710000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyenhuis D. L., Gorelick P. B., Geenen E. J., Smith C. A., Gencheva E., Freels S., et al. The pattern of neuropsychological deficits in Vascular Cognitive Impairment-No Dementia (Vascular CIND) [Comparative Study Research Support, U.S. Gov't, P.H.S.] Clinical Neuropsychology. 2004;18(1):41–49. doi: 10.1080/13854040490507145. doi:10.1080/13854040490507145. [DOI] [PubMed] [Google Scholar]
- O'Brien J. T., Desmond P., Ames D., Schweitzer I., Tress B. Magnetic resonance imaging correlates of memory impairment in the healthy elderly: Association with medial temporal lobe atrophy but not white matter lesions [Research Support, Non-U.S. Gov't] International Journal of Geriatric Psychiatry. 1997;12(3):369–374. [PubMed] [Google Scholar]
- O'Sullivan M., Jones D. K., Summers P. E., Morris R. G., Williams S. C., et al. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57(4):632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Paul R. H., Haque O., Gunstad J., Tate D. F., Grieve S. M., Hoth K., et al. Subcortical hyperintensities impact cognitive function among a select subset of healthy elderly. Archives of Clinical Neuropsychology. 2005;20(6):697–704. doi: 10.1016/j.acn.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peila R., White L. R., Masaki K., Petrovitch H., Launer L. J. Reducing the risk of dementia: Efficacy of long-term treatment of hypertension. Stroke. 2006;37(5):1165–1170. doi: 10.1161/01.STR.0000217653.01615.93. doi:01.STR.0000217653.01615.93. [DOI] [PubMed] [Google Scholar]
- Petrovitch H., White L. R., Izmirilian G., Ross G. W., Havlik R. J., Markesbery W., et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: The HAAS. Honolulu-Asia aging Study . Neurobiology of Aging. 2000;21(1):57–62. doi: 10.1016/s0197-4580(00)00106-8. doi:S0197458000001068. [DOI] [PubMed] [Google Scholar]
- Poon I. O. Effects of antihypertensive drug treatment on the risk of dementia and cognitive impairment. Pharmacotherapy. 2008;28(3):366–375. doi: 10.1592/phco.28.3.366. doi:10.1592/phco.28.3.366. [DOI] [PubMed] [Google Scholar]
- Posner H. B., Tang M. X., Luchsinger J., Lantigua R., Stern Y., Mayeux R. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology. 2000;58(8):1175–1181. doi: 10.1212/wnl.58.8.1175. [DOI] [PubMed] [Google Scholar]
- Posner H. B., Tang M. X., Luchsinger J., Lantigua R., Stern Y., Mayeux R. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology. 2002;58(8):1175–1181. doi: 10.1212/wnl.58.8.1175. [DOI] [PubMed] [Google Scholar]
- Qiu C., Winblad B., Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurology. 2005;4(8):487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- Raz N., Rodrigue K. M., Haacke E. M. Brain aging and its modifiers: insights from in vivo neuromorphometry and susceptibility weighted imaging. Annals of the New York Academy of Sciences. 2007;1097:84–93. doi: 10.1196/annals.1379.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R., DiMatteo M. R. Meta-analysis: Recent developments in quantitative methods for literature reviews. Annual Review of Psychology. 2001;52:59–82. doi: 10.1146/annurev.psych.52.1.59. doi:10.1146/annurev.psych.52.1.5952/1/59. [DOI] [PubMed] [Google Scholar]
- Sands L. P., Meredith W. Blood pressure and intellectual functioning in late midlife [Research Support, U.S. Gov't, P.H.S.] Journals of Gerontology. 1992;47(2):P81–P84. doi: 10.1093/geronj/47.2.p81. [DOI] [PubMed] [Google Scholar]
- Saxton J., Lopez O. L., Ratcliff G., Dulberg C., Fried L. P., Carlson M. C., et al. Preclinical Alzheimer disease: Neuropsychological test performance 1.5–8 years prior to onset [Comparative Study Multicenter Study Research Support, N.I.H., Extramural Research Support, U.S. Gov't, P.H.S.] Neurology. 2004;63(12):2341–2347. doi: 10.1212/01.wnl.0000147470.58328.50. [DOI] [PubMed] [Google Scholar]
- Scheltens P., Leys D., Barkhof F., Huglo D., Weinstein H. C., Vermersch P., et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: Diagnostic value and neuropsychological correlates [Research Support, Non-U.S. Gov't] Journal of Neurology, Neurosurgery, and Psychiatry. 1992;55(10):967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Fazekas F., Offenbacher H., Dusek T., Zach E., Reinhart B., et al. Neuropsychologic correlates of MRI white matter hyperintensities: A study of 150 normal volunteers. Neurology. 1993;43(12):2490–2494. doi: 10.1212/wnl.43.12.2490. [DOI] [PubMed] [Google Scholar]
- Schneider J. A., Bennett D. Where vascular meets neurodegenerative disease. Stroke. 2010;41:S144–S146. doi: 10.1161/STROKEAHA.110.598326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra C., De La Sierra A., Salamero M., Sobrino J., Gomez-Angelats E., Coca A. Silent cerebral white matter lesions and cognitive function in middle-aged essential hypertensive patients. American Journal of Hypertension. 2004;17(6):529–534. doi: 10.1016/j.amjhyper.2004.02.014. doi:10.1016/j.amjhyper.2004.02.014S0895706104000652. [DOI] [PubMed] [Google Scholar]
- Skoog I. Risk factors for vascular dementia: A review. Dementia. 1994;5(3–4):137–144. doi: 10.1159/000106711. [DOI] [PubMed] [Google Scholar]
- Skoog I. The relationship between blood pressure and dementia: A review [Review] Biomedicine and Pharmacotherapy. 1997;51(9):367–375. doi: 10.1016/s0753-3322(97)89428-0. [DOI] [PubMed] [Google Scholar]
- Skoog I. A review on blood pressure and ischaemic white matter lesions [Research Support, Non-U.S. Gov't Review] Dementia and Geriatric Cognitive Disorders. 1998;9(Suppl. 1):13–19. doi: 10.1159/000051184. [DOI] [PubMed] [Google Scholar]
- Skoog I., Lernfelt B., Landahl S., Palmertz B., Andreasson L. A., Nilsson L., et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347(9009):1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- Small B. J., Rosnick C. B., Fratiglioni L., Backman L. Apolipoprotein E and cognitive performance: a meta-analysis. Psychology and Aging. 2004;19(4):592–600. doi: 10.1037/0882-7974.19.4.592. [DOI] [PubMed] [Google Scholar]
- Strandgaard S. Hypertension and stroke [Review] Journal of Hypertension. Supplement: Official Journal of the International Society of Hypertension. 1996;14(3):S23–S27. doi: 10.1097/00004872-199610003-00005. [DOI] [PubMed] [Google Scholar]
- Stroup D. F., Berlin J. A., Morton S. C., Olkin I., Williamson G. D., Rennie D., et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Journal of the American Medical Association. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Suter O. C., Sunthorn T., Kraftsik R., Straubel J., Darekar P., Khalili K., et al. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke. 2002;33(8):1986–1992. doi: 10.1161/01.str.0000024523.82311.77. [DOI] [PubMed] [Google Scholar]
- Swan G. E., Carmelli D., Larue A. Systolic blood pressure tracking over 25 to 30 years and cognitive performance in older adults [Research Support, U.S. Gov't, P.H.S.] Stroke. 1998;29(11):2334–2340. doi: 10.1161/01.str.29.11.2334. [DOI] [PubMed] [Google Scholar]
- Swan G. E., DeCarli C., Miller B. L., Reed T., Wolf P. A., Jack L. M., et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology [Multicenter Study Research Support, U.S. Gov't, P.H.S. Twin Study] Neurology. 1998;51(4):986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- Thayer J. F., Hansen A. L., Saus-Rose E., Johnsen B. H. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine. 2009;37(2):141–153. doi: 10.1007/s12160-009-9101-z. doi:10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Thomas A. J., O'Brien J. T., Barber R., McMeekin W., Perry R. A neuropathological study of periventricular white matter hyperintensities in major depression. Journal of Affective Disorders. 2003;76(1–3):49–54. doi: 10.1016/s0165-0327(02)00064-2. [DOI] [PubMed] [Google Scholar]
- Thornton A., Lee P. Publication bias in meta-analysis: Its causes and consequences [Research Support, Non-U.S. Gov't Review] Journal of Clinical Epidemiology. 2000;53(2):207–216. doi: 10.1016/s0895-4356(99)00161-4. [DOI] [PubMed] [Google Scholar]
- Tsivgoulis G., Alexandrov A. V., Wadley V. G., Unverzagt F. W., Go R. C., Moy C. S., et al. Association of higher diastolic blood pressure levels with cognitive impairment. Neurology. 2009;73(8):589–595. doi: 10.1212/WNL.0b013e3181b38969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley E. W., Ropacki S. A., Bondi M. W. Neuropsychological and neuroimaging changes in preclinical Alzheimer's disease [Research Support, N.I.H., Extramural Review] Journal of the International Neuropsychological Society. 2006;12(5):707–735. doi: 10.1017/S1355617706060863. doi:10.1017/S1355617706060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf Y. D., Scheltens P., Lindeboom J., Witter M. P., Uylings H. B., Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions [Case Reports Research Support, Non-U.S. Gov't] Neuropsychologia. 2003;41(10):1330–1344. doi: 10.1016/s0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- Vicario A., Martinez C. D., Baretto D., Diaz Casale A., Nicolosi L. Hypertension and cognitive decline: Impact on executive function. Journal of Clinical Hypertension (Greenwich, Conn.) 2005;7(10):598–604. doi: 10.1111/j.1524-6175.2005.04498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstein S. R. The relation of hypertension to cognitive function. Current Directions in Psychological Science. 2003;12(1):9–12. [Google Scholar]
- *Waldstein S. R., Brown J. R., Maier K. J., Katzel L. I. Diagnosis of hypertension and high blood pressure levels negatively affect cognitive function in older adults. Annals of Behavioral Medicine. 2005;29(3):174–180. doi: 10.1207/s15324796abm2903_3. doi:10.1207/s15324796abm2903_3. [DOI] [PubMed] [Google Scholar]
- Waldstein S. R., Jennings J. R., Ryan C. M., Muldoon M. F., Shapiro A. P., Polefrone J. M., et al. Hypertension and neuropsychological performance in men: Interactive effects of age. Health Psychology. 1996;15(2):102–109. doi: 10.1037//0278-6133.15.2.102. [DOI] [PubMed] [Google Scholar]
- *Waldstein S. R., Katzel L. I. Gender differences in the relation of hypertension to cognitive function in older adults. Neurological Research. 2004;26(5):502–506. doi: 10.1179/016164104225016173. doi:10.1179/016164104225016173. [DOI] [PubMed] [Google Scholar]
- Waldstein S. R., Rice S. C., Thayer J. F., Najjar S. S., Scuteri A., Zonderman A. B. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51(1):99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- White L., Petrovitch H., Hardman J., Nelson J., Davis D. G., Ross G. W., et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Annals of the New York Academy of Sciences. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- Whitmer R. A., Sidney S., Selby J., Johnston S. C., Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- Williams M. Progress in Alzheimer's disease drug discovery: An update. Current Opinion in Investigational Drugs. 2009;10(1):23–34. [PubMed] [Google Scholar]
- Yang S. T., Mayhan W. G., Faraci F. M., Heistad D. D. Mechanisms of impaired endothelium-dependent cerebral vasodilatation in response to bradykinin in hypertensive rats. Stroke. 1991;22(9):1177–1182. doi: 10.1161/01.str.22.9.1177. [DOI] [PubMed] [Google Scholar]
- *Yeung S., Thornton W. Age-related effects of blood pressure on everyday cognitive function in community-dwelling women. Aging, Neuropsychology, and Cognition. 2011;18(6):733–755. doi: 10.1080/13825585.2011.609882. [DOI] [PubMed] [Google Scholar]
- Young V. G., Halliday G. M., Kril J. J. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008;71(11):804–811. doi: 10.1212/01.wnl.0000319691.50117.54. doi:01.wnl.0000319691.50117.54. [DOI] [PubMed] [Google Scholar]