Abstract
Although amyloid deposition remains a marker of the development of Alzheimer's disease, results linking amyloid and cognition have been equivocal. Twenty-five community-dwelling non-demented older adults were examined with 18F-flutemetamol, an amyloid imaging agent, and a cognitive battery, including an estimate of premorbid intellect and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). In the first model, 18F-flutemetamol uptake significantly correlated with the Delayed Memory Index of the RBANS (r = −.51, p = .02) and premorbid intellect (r = .43, p = .03). In the second model, the relationship between 18F-flutemetamol and cognition was notably stronger when controlling for premorbid intellect (e.g., three of the five RBANS Indexes and its Total score significantly correlated with 18F-flutemetamol, r's = −.41 to −.58). Associations were found between amyloid-binding 18F-flutemetamol and cognitive functioning in non-demented older adults. These associations were greatest with delayed memory and stronger when premorbid intellect was considered, suggesting that cognitive reserve partly compensates for the symptomatic expression of amyloid pathology in community-dwelling elderly.
Keywords: Amyloid, Neuroimaging, Neuropsychology, Alzheimer's disease, Premorbid intellect
Introduction
The deposition of amyloid within the cerebral cortex is hypothesized to be a primary pathologic substrate in the development of Alzheimer's disease (AD; Citron, 2010; Hardy & Selkoe, 2002). Several molecular neuroimaging radioligands have been developed to determine levels of amyloid deposition in vivo. 11C-PIB was the first amyloid imaging agent used in studies of normal aging, Mild Cognitive Impairment (MCI), and AD (Mormino et al., 2009; Rowe et al., 2010). However, the short half-life of 11C-PIB made it challenging to use in many research and clinical settings. The development of 18F amyloid-binding radiopharmaceuticals with longer half-lives has largely overcome these barriers. For example, 18F-AV-45 (florbetapir, Amyvid) has also been implemented in trials of normal aging, MCI, and AD (Fleisher et al., 2011; Rodrigue et al., 2012), and it was recently approved by the Food and Drug Administration to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for AD and other causes of cognitive decline.
Another 18F amyloid imaging agent with a growing body of evidence is 18F-flutemetamol. This compound has a similar molecular structure as 11C-PIB, but its longer half-life makes it more practical as a research tool for identifying amyloid pathology. Despite its promise, empirical support for 18F-flutemetamol is limited. In a Phase I study, 18F-flutemetamol uptake discriminated patients with AD from healthy controls across a variety of brain regions (Nelissen et al., 2009). In a larger Phase II study, 18F-flutemetamol uptake showed strong sensitivity and specificity in separating probable AD from healthy controls compared with a clinical diagnosis (Vandenberghe et al., 2010). A cortical composite measure of 18F-flutemetamol strongly correlated with 11C-PIB in a subset of these same patients. In a small study of elderly patients, histopathological evidence of amyloid plaques was found in all patients with a “positive” 18F-flutemetamol scan (Wolk et al., 2011). Finally, in the follow-up of 19 MCI subjects of the Phase II study, those that converted to AD were more likely to show increased 18F-flutemetamol uptake (Thurfjell et al., 2012).
Despite the associations between 18F-flutemetamol uptake and clinical diagnosis and histopathological results, no studies have reported the association between this amyloid-binding agent and cognition. Additionally, the majority of the studied patients to date have been demented, and there is less information about 18F-flutemetamol in non-demented seniors. The primary purpose of this study was to examine the relationship between 18F-flutemetamol and cognitive performances in non-demented older adults. Although cognitive studies with 18F-flutemetamol are lacking, cognitive studies with other amyloid imaging agents have found mixed results. Some have found associations with memory (Mormino et al., 2009; Perrotin, Mormino, Madison, Hayenga, & Jagust, 2012; Pike et al., 2007), whereas others have not (Mormino et al., 2009; Rodrigue et al., 2012). Non-memory cognitive domains (e.g., processing speed, attention, premorbid intellect) have also been inconsistently linked to the uptake of amyloid imaging agents (Mormino et al., 2009; Pike et al., 2007; Rodrigue et al., 2012). Overall, it was hypothesized that 18F-flutemetamol would be associated with delayed recall memory, but probably not other cognitive domains in this non-demented cohort. A secondary purpose of the current study was to see if premorbid intellect moderates the relationship between amyloid deposition and cognition, as prior studies have observed that measures of cognitive reserve have been linked to brain amyloid levels (Rentz et al., 2010; Roe et al., 2008; Vemuri et al., 2011).
Methods
Participants
Twenty-five older adults (18 women/7 men; mean age = 74.6 [6.8] years, range = 65–90; mean education = 16.1 [3.2] years, range = 12–22) were enrolled. These individuals were all recruited from senior centers and independent living facilities to participate in studies on memory and aging. Inclusion into the study required each participant complain of memory problems yet declare functional independence in activities of daily living, which was corroborated by a knowledgeable informant. Based on objective cognitive testing, the majority of these individuals were classified as cognitively intact (n = 15), with the remainder characterized as MCI (Winblad et al., 2004). More specifically, individuals who performed below expectations on cognitive testing were classified as MCI, whereas individuals who met or exceeded expectations were classified as intact. This classification scheme yielded a primarily amnestic subtype of MCI, with those classified as MCI performing significantly below those classified as intact on memory measures, but not other cognitive tests. Exclusion criteria for this study included: history of neurological disease know to affect cognition (e.g., stroke, head injury with loss of consciousness of >30 min, seizure disorder, demyelinating disorder, etc.); dementia based on Diagnostic and Statistical Manual of Mental Disorders-IV criteria; current or past major psychiatric illness (e.g., schizophrenia, bipolar affective disorder); 30-item Geriatric Depression Score >15 (mean GDS = 4.0 [3.9], range = 0–14); history of substance abuse; current use of cholinesterase inhibitors, other cognitive enhancers, antipsychotics, or anticonvulsant medications; history of radiation therapy to the brain; history of significant major medical illnesses, such as cancer or AIDS; and currently pregnant.
Procedures
The local institutional review board approved all procedures and all participants provided informed consent before data collection commenced. As part of a larger study, all participants completed a neuropsychological battery designed to characterize their cognitive status across several relevant domains. The battery contained a measure of premorbid intellect (Wide Range Achievement Test-4 Reading [WRAT-4 Reading] subtest) and measures tapping immediate and delayed memory, visuospatial perception and construction, attention, and language (Repeatable Battery for the Assessment of Neuropsychological Status [RBANS]; Randolph, 1998). Depressive symptoms were also assessed with the Geriatric Depression Scale. All tests were administered, scored, and normed per their respective manuals.
Following the completion of the cognitive battery, participants underwent 18F-flutemetamol imaging. 18F-flutemetamol was produced under positron emission tomography (PET) current good manufacturing practices standards and the studies were conducted under an approved investigational new drug. Imaging was performed 90 min after the injection of 185 mBq (5 mCi) of 18F-flutemetamol. Emission imaging time was approximately 30 min. Two scanners were used for imaging, a general electric (GE) Advance PET scanner and a GE ST PET/computed tomography scanner. The full width at half maximum spatial resolution of these two scanners is almost identical, 4.8 and 5.0 mm respectively. The field of view for reconstruction was set to 25.6 cm on each scanner to generate a pixel size 2.0 × 2.0 mm (image matrix size 128 × 128), independent of which scanner was used. Therefore, spatial resolution is identical for these two scanners. The same matrix size was used for both scanners. Brain imaging protocols were created using a set of parameters that would produce images that were virtually identical. The image reconstruction was done using settings that were as close as possible to each other to produce as identical as possible image noise and contrast. The native slice thickness between the two scanners is 4.25 mm for the GE Advance and 3.27 mm for the GE ST system. Although these values are a bit different, the analysis of the images was not done on individual slices, but rather on the brain volume. Volumes of interest (VOIs) were automatically generated by the analysis software and the z-axis dimensions of these VOIs are substantially larger than the slice thickness. 18F-flutemetamol scans were quantified using a fully automated method that has been previously described in detail (Lundqvist et al., in press). Using this method, PET scans were spatially normalized into Montreal Neurologic Institute space and a VOI template was applied to the spatially normalized data. Counts were extracted for regions corresponding to the frontal, lateral temporal and parietal cortices, as well as anterior and posterior cingulate. In addition, reference regions corresponding to the cerebellar cortex and pons were defined. Standard uptake value ratios (SUVRs) were computed by dividing counts in the target regions with counts in the reference regions. In addition, we computed SUVR values for a composite neocortical value as an average of the above-mentioned cortical VOIs.
Statistical Plan
Two models were examined in these analyses. The first model compared the global composite of SUVRs with age-corrected cognitive performances on RBANS via the Pearson correlations. The second model added premorbid intellect (WRAT-4 Reading) as a covariate, since premorbid intellect can affect cognitive performance and is often used as a measure of cognitive reserve (Duff, 2010).
Results
No adverse events were reported during the injection, uptake time, or imaging studies with the experimental 18F-flutemetamol. The mean global composite of SUVRs normalized to the cerebellar cortex was 1.65 (SD = 1.30, range = 1.05–7.66). One participant was an outlier with a very high level of 18F-flutemetamol uptake (i.e., 7.66), possibly due to significant cerebellar atrophy. When these participant's data were removed, the mean global composite decreased to 1.40 (SD = 0.37, range = 1.05–2.37). Including this outlier, 8 of the 25 scans were categorized as “positive” for tracer uptake, using a cutoff of 1.56 for the composite score (Vandenberghe et al., 2010). Of these eight scans with notable uptake, four were from participants categorized as cognitively intact and four were from participants categorized as MCI.
For the entire group, the mean cognitive performances on the RBANS were in the average range (e.g., Total score: M = 104.4, SD = 15.7), although there was some variability. The mean estimate of premorbid intellect was high average (WRAT-4 Reading: M = 111.2, SD = 9.9). Relevant cognitive scores are presented in Table 1.
Table 1.
Cognitive performances and correlations with 18F-flutemetamol global composite
| Cognitive measure | Mean (SD) (range) |
r
|
|
|---|---|---|---|
| Model 1 | Model 2 | ||
| Premorbid intellect | 111.2 (9.9) (82–126) | .43* | n/a |
| RBANS | |||
| Immediate Memory | 109.2 (18.1) (73–142) | −.08 | −.22 |
| Visuospatial Construction | 97.4 (15.2) (66–116) | −.05 | −.51* |
| Language | 101.4 (11.4) (75–120) | −.30 | −.38 |
| Attention | 101.6 (15.1) (72–128) | −.37 | −.41* |
| Delayed Memory | 103.5 (11.7) (78–122) | −.51* | −.58* |
| Total Scale | 104.4 (15.7) (75–137) | −.29 | −.49* |
Notes: All cognitive scores have M = 100; SD = 15; Model 1 corrects for age. Model 2 corrects for age and premorbid intellect. RBANS = Repeatable Battery for the Assessment of Neuropsychological Status. r = Pearson's correlation.
*p < .05.
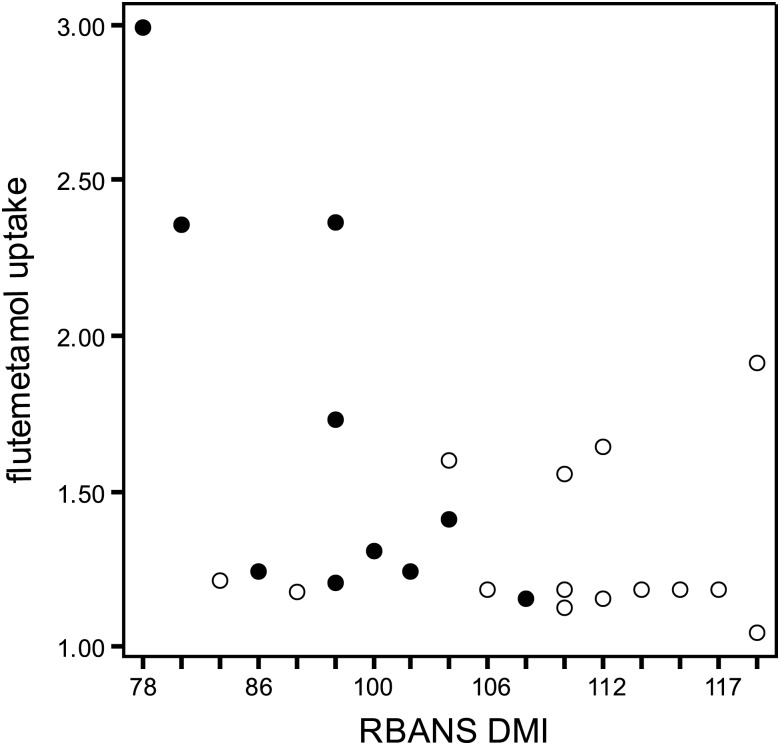
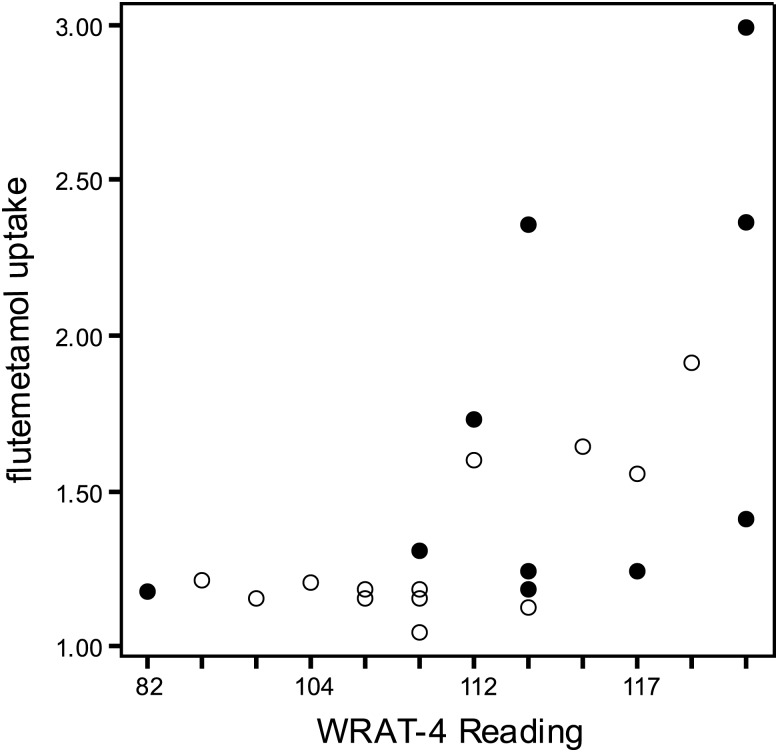
In the first model, 18F-flutemetamol uptake significantly correlated with the Delayed Memory Index from the RBANS (r = −.51, p = .01, see Fig. 1), with greater uptake being associated with lower memory scores. Other RBANS Indexes did not correlate with the global composite score of 18F-flutemetamol. WRAT-4 Reading was significantly, but positively, correlated with 18F-flutemetamol uptake (r = .43, p = .03, see Fig. 2), so that greater uptake was associated with higher scores of premorbid intellect. Neither age nor education (another proxy measure of cognitive reserve) significantly correlated with 18F-flutemetamol uptake in this sample (p's > .25).
Fig. 1.
Relationship between flutemetamol uptake and RBANS Delayed Memory Index. RBANS DMI = Repeatable Battery for the Assessment of Neuropsychological Status Delayed Memory Index. Open circles are cognitively intact, and closed circles are MCI. The one outlier's flutemetamol uptake composite was recoded as 3.00 for this figure.
Fig. 2.
Relationship between flutemetamol uptake and WRAT-4 Reading. WRAT-4 = Wide Range Achievement Test-4. Open circles are cognitively intact, and closed circles are MCI. The one outlier's flutemetamol uptake composite was recoded as 3.00 for this figure.
In the second model, the relationship between 18F-flutemetamol and cognition was notably stronger when controlling for premorbid intellect. In these analyses, 18F-flutemetamol uptake significantly correlated with three of the five RBANS Indexes and the Total score (see Table 1). For all significant correlations, greater 18F-flutemetamol uptake (i.e., greater amyloid deposition) was associated with poorer cognition. The overall pattern of these results was similar when the one outlier with very high 18F-flutemetamol uptake was removed from the analyses. Scatterplots of the relationships from Model 2 can be obtained from the first author.
In secondary analyses, the relationships between cognition and amyloid uptake appeared to be more strongly driven by those classified as MCI. Although both subgroups showed relationships with premorbid intellect (MCI r = .41, intact r = .57), the Delayed Memory and amyloid associations seemed most influenced by the MCI subgroup (MCI r = −.65, intact r = −.17). This same pattern was observed in the second model correlations (i.e., MCI subgroup yielded stronger correlations with amyloid than intact group when WRAT was controlled).
Conclusions
In this sample of non-demented community-dwelling older adults, 18F-flutemetamol was negatively correlated with performance on a composite score of delayed memory, with greater tracer uptake being associated with worse memory. Our findings differ from those examining the association between another 18F agent (florbetapir) and cognitive performances (Rodrigue et al., 2012). In that study, tracer uptake did not correlate with a composite memory score. Furthermore, Rodrigue and colleagues did find associations between their 18F agent and processing speed, fluid reasoning, and working memory. We do not believe these differences are due to differences in the tracers used, since these agents have quite similar properties. Instead, there are some notable differences in the two studies that likely explain these disparate findings. Rodrigue and colleagues examined associations across the lifespan (e.g., 20–89-year olds), while we examined only older adults (e.g., 65–82 year olds). Since amyloid deposition seems to occur later in life, age cohort effects on cognitive performance are likely to have played a much greater role in the Rodrigue and colleagues study than in ours. Additionally, the participants in Rodrigue and colleagues were carefully screened for cognitive intactness, whereas our subjects had more variable cognitive status. Finally, many of the tasks employed by Rodrigue and colleagues were experimental measures, while we used the standard clinical tasks that have more practical relevance.
Most of our results are consistent with studies examining the relationship between 11C-PIB and cognition in older individuals (Mormino et al., 2009; Perrotin et al., 2012; Pike et al., 2007). That is, the greater the uptake of the amyloid imaging agent, the poorer the performance on a variety of memory tests. The methodological similarities between the current study and these 11C-PIB studies likely contribute to their unitary message and also strengthen the ability to draw conclusions across amyloid imaging agents.
With the exception of our one outlier who showed extensive 18F-flutemetamol uptake, composite SUVRs were comparable with those obtained in prior studies with this agent (Thurfjell et al., 2012; Vandenberghe et al., 2010; Wolk et al., 2011). Consistent with studies using other amyloid imaging agents, 27% of our cognitively intact participants demonstrated “positive” amyloid scans (Pike et al., 2007; Rowe et al., 2010). Tracking these individuals across time may tell if this pathology has some diagnostic implications (e.g., eventually they do develop AD dementia). Additionally, 40% of our participants categorized with MCI displayed “positive” amyloid scans, which is also consistent with existing literature (Mormino et al., 2009; Pike et al., 2007) and may reflect the heterogeneity of this diagnostic classification.
Results of our second model indicated that premorbid intellect may be an important moderator of the relationship between amyloid and cognition. When we controlled for premorbid intellect with a test of current reading abilities, three of the five cognitive domains (i.e., delayed memory, visuospatial construction, language) and the overall cognitive score were significantly correlated with 18F-flutemetamol uptake, with all correlations going in the expected direction. Few prior studies have corrected for premorbid intellect in the examination of amyloid imaging agents. Roe and colleagues (2008) found that their proxy of cognitive reserve (i.e., years of education) moderated the relationship between their amyloid imaging agent (11C-PIB) and cognition. Similarly, Rentz and colleagues (2010) also found that the relationship between 11C-PIB and cognition was affected by their measure of cognitive reserve (i.e., an mini mental status examination-adjusted North American Adult Reading Test). More recently, when Rodrigue and colleagues (2012) examined the relationship between their 18F tracer and “crystallized intelligence” (using two vocabulary tasks), they found no relationship (r = −.06). Conversely, in our sample, the correlation between premorbid intellect and 18F-flutemetamol uptake was moderate (r = .43) and statistically significant. As with the other discrepant findings mentioned earlier, methodological differences (e.g., different measures, different age ranges, different levels of cognitive impairment, different amyloid tracers) may be partially responsible.
In addition to the potentially moderating effects of premorbid intellect, it is worth noting that this association was positive (i.e., greater 18F-flutemetamol uptake was associated with higher premorbid intellect). This is the opposite relationship that was observed between 18F-flutemetamol uptake and cognition. One possible explanation for this positive association between 18F-flutemetamol uptake and premorbid intellect is that premorbid intellect may be a proxy measure of cognitive reserve. Several investigations have used reading tests, similar to the one used in our study, to estimate cognitive reserve (Jefferson et al., 2011). Higher cognitive reserve has also been posited as a protective factor in the development of dementia (Stern, 2006). In our study, it is possible that higher cognitive reserve is temporarily mitigating the effects of high amyloid burden in these non-demented older adults. Although the replication of this hypothesis is needed, future amyloid imaging studies should consider accounting for premorbid intellect/cognitive reserve.
Despite the interesting findings, some limitations of this study should be acknowledged. First, these results should be viewed cautiously as the sample size was relatively small. Larger studies with a wider range of cognitive functioning (both current and premorbid) would better test this hypothesis. Second, the sample was relatively homogeneous (e.g., all Caucasian, highly educated, mostly men, healthy enough to complete a PET scan), and the ability to generalize these findings to a more diverse group is unknown. Third, structural imaging was not part of this research protocol. Thurfjell and colleagues (2012) has shown that combining 18F-flutemetamol and structural MRI can better characterize disease states and predict progression. We are starting to collect these structural scans on this cohort and encourage future investigations to do the same. Fourth, we characterized subjects as intact or MCI largely based on a psychometric approach, which has limitations. Future studies might examine clinically diagnosed cases of MCI. Finally, the RBANS has some limitations. It is typically used as a screening measure or for other briefer evaluations. It may not comprehensively assess each individual cognitive domain. Additionally, it does not specifically assess executive functioning. Since executive dysfunction can occur in a number of late life dementing illnesses, it is worth examining its relationship with 18F-flutemetamol uptake. Regardless of these limitations, the current study found notable relationships between amyloid-binding 18F-flutemetamol and cognitive functioning in non-demented community-dwelling older adults, which were highlighted when we controlled for premorbid intellect.
Funding
Funding for this project was provided by an anonymous foundation, GE Healthcare, NIH NIA K23 AG028417, the Molecular Imaging Program at the Huntsman Cancer Institute, and the University of Utah Center for Alzheimer's Care, Imaging and Research.
Conflict of Interest
Research support for this project was provided by GE Heathcare to some of the authors (KD, NLF, JMH).
References
- Citron M. Alzheimer's disease: Strategies for disease modification. Nature Reviews: Drug Discovery. 2010;9(5):387–398. doi: 10.1038/nrd2896. doi:10.1038/nrd2896. [DOI] [PubMed] [Google Scholar]
- Duff K. Predicting premorbid memory functioning in older adults. Applied Neuropsychology. 2010;17(4):278–282. doi: 10.1080/09084282.2010.525113. doi:10.1080/09084282.2010.525113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher A. S., Chen K., Liu X., Roontiva A., Thiyyagura P., Ayutyanont N., et al. Using positron emission tomography and florbetapir F18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Archives of Neurology. 2011;68(11):1404–1411. doi: 10.1001/archneurol.2011.150. doi:10.1001/archneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D. J. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. doi:10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Jefferson A. L., Gibbons L. E., Rentz D. M., Carvalho J. O., Manly J., Bennett D. A., et al. A life course model of cognitive activities, socioeconomic status, education, reading ability, and cognition. Journal of the American Geriatrics Society. 2011;59(8):1403–1411. doi: 10.1111/j.1532-5415.2011.03499.x. doi:10.1111/j.1532-5415.2011.03499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist R., Lilja J., Thomas B., Lötjönen J., Villemagne V., Rowe C., et al. Implementation and validation of an adaptive template registration method for [18F]flutemetamol imaging data. Journal of Nuclear Medicine. doi: 10.2967/jnumed.112.115006. in press. [DOI] [PubMed] [Google Scholar]
- Mormino E. C., Kluth J. T., Madison C. M., Rabinovici G. D., Baker S. L., Miller B. L., et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132(Pt 5):1310–1323. doi: 10.1093/brain/awn320. doi:10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen N., Van Laere K., Thurfjell L., Owenius R., Vandenbulcke M., Koole M., et al. Phase 1 study of the Pittsburgh compound B derivative 18F-flutemetamol in healthy volunteers and patients with probable Alzheimer disease. Journal of Nuclear Medicine. 2009;50(8):1251–1259. doi: 10.2967/jnumed.109.063305. doi:10.2967/jnumed.109.063305. [DOI] [PubMed] [Google Scholar]
- Perrotin A., Mormino E. C., Madison C. M., Hayenga A. O., Jagust W. J. Subjective cognition and amyloid deposition imaging: A Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Archives of Neurology. 2012;69(2):223–229. doi: 10.1001/archneurol.2011.666. doi:10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike K. E., Savage G., Villemagne V. L., Ng S., Moss S. A., Maruff P., et al. Beta-amyloid imaging and memory in non-demented individuals: Evidence for preclinical Alzheimer's disease. Brain. 2007;130(Pt 11):2837–2844. doi: 10.1093/brain/awm238. doi:10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- Rentz D. M., Locascio J. J., Becker J. A., Moran E. K., Eng E., Buckner R. L., et al. Cognition, reserve, and amyloid deposition in normal aging. Annals of Neurology. 2010;67(3):353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue K. M., Kennedy K. M., Devous M. D., Sr, Rieck J. R., Hebrank A. C., Diaz-Arrastia R., et al. Beta-Amyloid burden in healthy aging: Regional distribution and cognitive consequences. Neurology. 2012;78(6):387–395. doi: 10.1212/WNL.0b013e318245d295. doi:10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe C. M., Mintun M. A., D'Angelo G., Xiong C., Grant E. A., Morris J. C. Alzheimer disease and cognitive reserve: Variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Archives of Neurology. 2008;65(11):1467–1471. doi: 10.1001/archneur.65.11.1467. doi:10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe C. C., Ellis K. A., Rimajova M., Bourgeat P., Pike K. E., Jones G., et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiological Aging. 2010;31(8):1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. doi:10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Disease and Associated Disorder. 2006;20(2):112–117. doi: 10.1097/01.wad.0000213815.20177.19. doi:10.1097/01.wad.0000213815.20177.19. [DOI] [PubMed] [Google Scholar]
- Thurfjell L., Lotjonen J., Lundqvist R., Koikkalainen J., Soininen H., Waldemar G., et al. Combination of biomarkers: PET [F]Flutemetamol Imaging and structural MRI in Dementia and Mild Cognitive Impairment. Neuro-degenerative Diseases. 2012;10(1–4):246–249. doi: 10.1159/000335381. doi:10.1159/000335381. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R., Van Laere K., Ivanoiu A., Salmon E., Bastin C., Triau E., et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: A phase 2 trial. Annals of Neurology. 2010;68(3):319–329. doi: 10.1002/ana.22068. doi:10.1002/ana.22068. [DOI] [PubMed] [Google Scholar]
- Vemuri P., Weigand S. D., Przybelski S. A., Knopman D. S., Smith G. E., Trojanowski J. Q., et al. Cognitive reserve and Alzheimer's disease biomarkers are independent determinants of cognition. Brain. 2011;134(Pt 5):1479–1492. doi: 10.1093/brain/awr049. doi:10.1093/brain/awr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B., Palmer K., Kivipelto M., Jelic V., Fratiglioni L., Wahlund L. O., et al. Mild cognitive impairment–beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. Journal of International Medicine. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. doi:10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Wolk D. A., Grachev I. D., Buckley C., Kazi H., Grady M. S., Trojanowski J. Q., et al. Association between in vivo fluorine 18-labeled flutemetamol amyloid positron emission tomography imaging and in vivo cerebral cortical histopathology. Archives of Neurology. 2011;68(11):1398–1403. doi: 10.1001/archneurol.2011.153. doi:10.1001/archneurol.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]