Abstract
Children with Attention-Deficit/Hyperactivity Disorder (ADHD) demonstrate increased response variability compared with controls, which is thought to be associated with deficits in attention regulation and response control that subsequently affect performance of more cognitively demanding tasks, such as reading. The present study examined response variability during a computerized simple reaction time (RT) task in 67 children. Ex-Gaussian analyses separated the response time distribution into normal (mu and sigma) and exponential (tau) components; the association of each with reading fluency was examined. Children with ADHD had significantly slower, more variable, and more skewed RTs compared with controls. After controlling for ADHD symptom severity, tau (but not mu or mean RT) was significantly associated with reduced reading fluency, but not with single word reading accuracy. These data support the growing evidence that RT variability, but not simply slower mean response speed, is the characteristic of youth with ADHD and that longer response time latencies (tau) may be implicated in the poorer academic performance associated with ADHD.
Keywords: Attention, Dyslexia, Variability, Processing speed, Executive function, Ex-Gaussian analyses
Attention-Deficit/Hyperactivity Disorder (ADHD) and Reading Disability (RD) have been shown to co-occur much more frequently than would be expected by chance, in both clinical and community samples (Gilger, Pennington, & DeFries, 1992). Both disorders appear highly heritable (e.g., Willcutt, Betjemann, et al., 2010; Ziegler et al., 2005) and an increasing number of genome-wide linkage analyses have identified possible candidate regions or genes that may be shared between the two disorders (see Germanò, Gagliano, & Curatolo, 2010, for a review of these studies). Similarly, imaging studies suggest the possibility of shared neural correlates of both ADHD and RD, including variability in cerebral lateralization (Foster, Hynd, Morgan, & Hugdahl, 2002; Pueyo et al., 2000) and in cerebellar volumes (Castellanos et al., 2002; Eckert et al., 2003; Kronbichler et al., 2008). Not surprisingly, then, there is a growing body of work examining neuropsychological processes that may underlie the shared functional impairments in these two disorders.
Processing speed appears to represent one area of shared neuropsychological deficit between the two disorders (e.g., McGrath et al., 2011; Willcutt, Betjemann, et al., 2010). Children with RD have been shown to have slower naming speed (Compton, Olson, DeFries, & Pennington, 2002) and processing speed (Willcutt, Pennington, Olson, Chhabildas, & Hulslander, 2005). Processing speed has also been described as an area of particular difficulty in youth with ADHD. Specifically, children with ADHD have been shown to demonstrate slower speed of responding, relative to their typically developing peers, across a wide variety of cognitive tasks including: (a) “graphomotor speed,” as measured by the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV) Processing Speed subtests (Chhabildas, Pennington, & Willcutt, 2001; Jacobson et al., 2011; Rucklidge & Tannock, 2002; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005); (b) “naming speed,” as measured by rapid automatized naming tasks (Rucklidge & Tannock, 2002) or tasks such as Stroop color naming or word reading (Shanahan et al., 2006; Willcutt, Betjemann et al., 2010); and (c) “reaction time (RT)” on continuous performance, go–no go, and choice reaction tasks (Andreou et al., 2007; Antonini, Narad, Langberg, & Epstein, 2013; Epstein et al., 2011; Rucklidge & Tannock, 2002; Shanahan et al., 2006; Wodka et al., 2007). Children with ADHD also perform slower than controls on measures of “skeletomotor” (Cole, Mostofsky, Gidley Larson, Denckla, & Mahone, 2008; Klotz, Johnson, Wu, Isaacs, & Gilbert, 2011) and “oculomotor speed” (Mahone, Mostofsky, Lasker, Zee, & Denckla, 2009).
Performance on many of these tasks requires not only a motor response, but also explicit and more effortful cognitive processing of task-related information as well as inhibitory control and/or working memory (e.g., discriminating symbols, identifying colors or letters, determining whether a response is needed, holding response rules in mind). Cognitive processing speed appears separable from motor RT (e.g., Jacobson et al., 2011; Metin et al., 2013), but core deficits in regulation of response control among children with ADHD may contribute to the overall slowing observed on a variety of more cognitively complex timed tasks, since nearly all measures of “response speed” require some type of a motor response/output. Although some recent work suggests that children with ADHD perform more slowly on basic repetitive motor tasks such as finger tapping (Cole et al., 2008; Klotz et al., 2011), few studies have examined whether differences exist in basic response speed on tasks not requiring additional cognitive control for inhibition or response selection. Recent work suggests that youth with ADHD may be not only less efficient in their basic ability to process incoming information, even on tasks with lower demands for executive or inhibitory control, but also tend to take less non-decisional time, or time spent in such tasks as encoding and execution of a motor response, without a significant associated deficit in accuracy (Metin et al., 2013). These findings suggest that the difference in non-decisional time in ADHD may be associated with deficits in motor preparation. Potential differences in motor preparation and control may therefore be an important component of the poorer performance seen in children with ADHD on a variety of timed cognitive tasks, since a motor response of some type (e.g., graphomotor, oral/verbal) is intrinsic to most tasks measuring efficiency of responding.
The complex term “processing speed” contains components of both response preparation and motor execution or reaction speed. Processing speed not only appears to represent a shared neuropsychological deficit in ADHD and RD, but has also been proposed as a promising candidate for a neuropsychological deficit within ADHD that may contribute uniquely to reading difficulties (Denckla & Cutting, 1999; McGrath et al., 2011; Rucklidge & Tannock, 2002; Willcutt, Pennington et al., 2005), through its influence on efficiency of reading fluency among those who can read single words accurately. Slowed response speed may decrease the efficiency of reading fluency, which in turn may hinder development of more complex academic skills such as reading comprehension. In particular, slower processing speed is thought to increase the demand placed upon working memory during reading comprehension tasks, potentially overburdening the child's ability to retain the content long enough to comprehend its meaning. Wolf and Katzir-Cohen (2001) defined reading fluency as the “level of accuracy and rate at which decoding is relatively effortless; at which oral reading is smooth and accurate, with correct prosody; and at which attention can be allocated to comprehension” (p. 219). As reading becomes more automatized, less mental effort and attentional resources are required for ongoing decoding and accurate word reading allowing these resources to be allocated to the task of translating text into meaning. Children with ADHD, even those without co-morbid reading or language disorders, often show weaker reading fluency compared with controls (Ghelani, Sidhu, Jain, & Tannock, 2004; Jacobson et al., 2011; Willcutt, Pennington, Olson, & DeFries, 2007). Further, children with ADHD and children with reading difficulties both demonstrate a common pattern of deficits in both processing speed and working memory, although processing speed deficits may have a more robust association than working memory to both disorders (McGrath et al., 2011). Recently, Willcutt, Doyle, and colleagues (2005) recommended additional research to clarify the taxonomy of processing speed tasks, by examining relationships among measures and by contrasting performance on these measures between clinical groups. At this point, however, much remains unknown about the specific influence of characteristics of ADHD on reading fluency among children without deficits in single word reading or decoding, since most studies of ADHD do not control for the high degree of overlap between ADHD and word reading deficits.
Response Variability in ADHD
In addition to “slower” processing speed, children with ADHD often demonstrate more variable responding, compared with typically developing peers, which is considered to be associated with inefficient response and attentional control (Harris et al., 1995; Vaurio et al., 2009; Wodka et al., 2007), leading to increased moment-to-moment, within-subject variability (Antonini et al., 2013; Di Martino et al., 2008; Epstein et al., 2011; Rommelse et al., 2008; Ryan et al., 2010). While response time distributions are inherently positively skewed, the greater variability observed in children with ADHD leads to an even more skewed distribution of response times, with substantially more “very slow” responses (often interpreted as lapses of attention or lapses “off task”) appearing within the tail of the distribution (for further discussion, see Hervey et al., 2006). This skewness makes traditional statistical analysis techniques (i.e., those based upon mean and SD of a normal distribution; “Gaussian” analyses) problematic. Although some research has addressed this issue by examining the standard deviation of individual RTs (RTSD) or summary measures of intra-subject variability (coefficient of variability [CV]; SDRT/M), ex-Gaussian analysis methods provide a more detailed evaluation of both the normal, or Gaussian, component and the exponential component, or the tail of the distribution (Hervey et al., 2006; Whelan, 2008). Using these analytic methods, mu provides a measure of the mean of the modal portion of the distribution, sigma provides an estimate of the variability within the modal portion, and tau provides a measure of the mean and standard deviation of the exponential component. These measures allow for a more thorough and specific analysis of both the normal and exponential components of the RT distribution in ADHD.
Using these ex-Gaussian analytic methods, children with ADHD have been shown to demonstrate both more variable responding in the normal distribution (e.g., higher values of sigma; Vaurio et al., 2009) and more skewed responding across a variety of RT tasks (greater lapses in performance as represented by higher tau; Epstein et al., 2011; Hervey et al., 2006; Lee et al., 2012; Leth-Steensen, King Elbaz, & Douglas, 2000; Vaurio et al., 2009), with increases in tau the most consistent finding across studies. Although this pattern is evident across a variety of tasks, all the tasks examined previously (i.e., continuous performance tasks, go–no go, choice RT tasks) require efficient cognitive processing and response inhibition prior to the execution of the motor response. The pattern of performance in children with ADHD has not yet been examined on simple motor response tasks without a clear demand for cognitive processing (i.e., simple “go” tasks without demands for inhibition). It remains unclear, therefore, whether the slowed motor responding seen in ADHD is due to slower responding overall or to a similar pattern of more variable/skewed performance across responses.
The present study was designed to better delineate the components of RTs that are actually slowed in children with ADHD, in order to inform more specific interventions. For example, if slowing is observed even at the most basic level of behavioral response among children with ADHD, it should be considered an important endophenotype associated with the condition, and interventions might focus more exclusively on provision of extra time. Conversely, if ADHD-related slowing is only observed under demands for controlled responses (e.g., go–no go tasks, flanker tasks, choice RT tasks), it may be that the deficits are related to the top-down control demands of the tasks. By removing these demands, speed of performance should normalize in children with ADHD. A third alternative is that the ADHD-related “slowing” is simply an artifact of intermittent performance lapses on all types of tasks (resulting in extremely slow individual responses). These intermittent lapses contribute to increased mean RT (MRT) but are not captured in traditional analyses of Gaussian distributions. In such cases, alternatives to these traditional analyses, such as exploration of the ex-Gaussian distribution, may contribute to a clearer understanding of why mean response times are increased (Lee et al., 2012; Leth-Steensen et al., 2000).
Objective
The purpose of the present study was to examine components of response speed and variability among children with ADHD and typically developing controls on a computerized “simple” RT task. Specifically, we hypothesized that children with ADHD would have slower RTs (compared with typically developing controls) on a simple motor RT task, but only for overall MRT values (i.e., not mu). Additionally, we hypothesized that children with ADHD would be more variable in their performance of the RT tasks (i.e., elevated values of sigma and tau) and that variability in performance in ADHD, including intermittent performance lapses, would be associated with the reductions in reading fluency more than actual speed of response.
Method
Participants
Following approval from the Institutional Review Board, participants were recruited from outpatient clinics within a large developmental disabilities center and from local area pediatricians, local chapters of Children and Adults with ADHD (CHADD), schools, social/service organizations (e.g., Boy/Girl Scouts), and community advertisements (e.g., postings at libraries). The sample included 67 children (55.2% men), of whom 39 met criteria for a diagnosis of ADHD. This study is part of a larger project examining brain–behavior relationships in children using magnetic resonance imaging; therefore, participants were screened for co-morbidities commonly observed in ADHD (197 children were screened and 94 enrolled; details below). All participants and their parents signed a consent form that met Institutional Review Board standards. Participants ranged in age from 9 to 14 (M = 11.21, SD = 1.52). The majority (74.6%) was Caucasian, 14.9% African American, 4.5% multiracial, 4.5% Asian, and 1.5% Pacific Islander. One participant (1.5%) reported Hispanic ethnicity.
Children included in the study had intellectual ability scores of 80 or higher on the General Ability Index (GAI) of WISC-IV (Wechsler, 2004). Screening criteria were similar for both groups: children were excluded if they were identified as having a history of speech/language disorder or word reading difficulties, either through telephone screening before the initial visit or based on prior school assessment within the past year. Further exclusion criteria included visual or hearing impairment, history of other neurological disorder, psychotropic medication use other than stimulants, or co-morbid diagnoses other than Oppositional Defiant Disorder (ODD) or Specific Phobia. Demographic information, school, and developmental histories were obtained through telephone screenings with parents of participants. Children with ADHD who were taking stimulant medication were removed from the medication on the day of and day prior to testing.
Screening Measures
Following initial telephone screening, participants were screened for psychiatric diagnoses using a structured parent interview (Diagnostic Interview for Children and Adolescents, Fourth Edition, DICA-IV; Reich, Welner, & Herjanic, 1997). Additionally, parents and teachers of both children with ADHD and controls completed behavior rating scales, including the Conners' Parent/Teacher Rating Scale-Revised (CPRS-R/CTRS-R; Conners, 1997) and ADHD Rating Scale-IV (DuPaul, Power, Anastopoulos, & Reid, 1998). Controls with T-scores greater than 60 on either the DSM-IV Inattentive or Hyperactive/Impulsive scales of the CPRS-R or CTRS-R or item ratings of 2 or greater for four or more symptoms of inattention or hyperactivity/impulsivity from the ADHD Rating Scale-IV were also excluded. The CPRS-R/CTRS-R and ADHD Rating Scale-IV were used to confirm ADHD diagnosis and group assignment using the following criteria: (a) positive DSM-IV ADHD diagnosis on DICA-IV; (b) T-scores greater than 65 on the DSM-IV Hyperactive/Impulsive or Inattentive scales of the CPRS-R or CTRS-R and (c) six of nine DSM-IV symptoms met (item rating of 2 or 3) on the Hyperactive/Impulsive or Inattention scales of the ADHD Rating Scale-IV, home or school version. Positive rating scale responses alone were insufficient for assignment to ADHD group membership; children were required to meet ADHD diagnostic criteria on the DICA, including the pervasiveness criterion. Children with DSM-IV diagnoses other than Oppositional Defiant Disorder and Specific Phobias were excluded from both groups. Additional exclusionary criteria for both groups included history of mental health services for behavior or emotional problems (other than for ADHD-related behaviors in the ADHD group), history of academic problems requiring school-based intervention services, or history of defined primary reading or language-based learning disability. Screening also included subtests of the Clinical Evaluation of Language Fundamentals (Semel, Wiig, & Secord, 2003); any child scoring below −1.5 SD on the receptive or expressive language composites, or below −1.0 SD on both composites, was excluded. Additionally, children scoring below −1.0 SD on the Basic Reading Composite of the Woodcock–Johnson Tests of Achievement (WJ-III; Woodcock, McGrew, & Mather, 2001) were excluded.
A total of 197 children were initially screened for participation in the study. Based upon the aforementioned exclusionary criteria, which were necessarily restrictive as the parent study was designed as an imaging study examining structural differences in ADHD (without common co-morbidities), 94 children were ultimately enrolled in the parent study from which the present sample was drawn. Of those, 67 children completed all of the relevant study measures, including the simple RT test given on the second day of testing; thus, not all of the 94 enrolled children completed all measures and/or returned for day 2 of testing.
All participants were screened to rule out basic word reading difficulties, operationally defined as a score less than 25th percentile on the WJ-III (Woodcock et al., 2001). Participants with Basic Reading scores below the 25th percentile were excluded. Participants were also screened to rule out intellectual impairment (WISC-IV; Wechsler, 2004). Participants with GAI scores below a standard score of 80 were excluded.
Data were collected over 2 days of testing, less than 1 month apart. Assessments included measures of intellectual functioning, reading, and simple motor RT. On the first day, children were administered the WJ-III, Gray Oral Reading Test, Fourth Edition (GORT-IV, Wiederholt & Bryant, 2000), and Test of Word Reading Efficiency (TOWRE; Torgesen, 1999). Measures completed on the second day included the WISC-IV (Wechsler, 2004) and simple RT measures.
Study Measures
Simple RT
Each participant completed a computerized simple RT task designed for the study that assessed attention and simple response speed through repeated presentation of a visual stimulus. The child was required to acknowledge each stimulus by pushing a designated button on the keyboard (e.g., the space bar). The task runs for 6 min and 30 s, presenting 216 total stimuli for 300 ms each, with variable (jittered) interstimulus intervals (ISIs). The ISI was jittered 33% around a median ISI of 1,500 ms (i.e., 1,000, 1,250, 1,500, 1,750, and 2,000 m). Of note, a jittered paradigm was used to increase engagement in the task (e.g., Ryan et al., 2010) as preliminary evidence suggests that a fixed ISI on a simple RT task may not engage children enough for optimal performance, whereas a jittered format has the potential to reduce lapses off-task in children with ADHD (Lee et al., 2012). Furthermore, the use of a fixed ISI on a simple RT task presents the participant with essentially a motor timing task in which demand for response control is greatly reduced. Each stimulus was a “go” presentation in the form of a green spaceship to minimize demands upon working memory. MRT, SD, and CV were calculated for each participant for the entire task. Additionally, performance across the two halves of the task was also examined: MRT, SD, and CV were calculated for each participant across each half of the task in order to examine variability in performance across the course of the task.
The Conners' Parent Rating Scale-Revised
The CPRS-R (Conners, 1997) is a well-recognized and validated parent-report rating scale describing the child's behavior primarily within the symptom domains characteristic of ADHD (e.g., inattentive and hyperactive-impulsive symptoms). Items are rated according to the frequency of occurrence on a four point scale ranging from 0 “never” to 3 “almost always.” The CPRS-R DSM-IV symptom scales were used in screening participants (as described above) and as one component of the procedure used to confirm diagnostic group assignment. The CPRS-R N score, or the total ADHD symptoms score, was used in analyses as a measure of ADHD symptom severity.
Reading Fluency
GORT-IV, Fluency
The GORT-IV (Wiederholt & Bryant, 2000) requires children to read aloud text passages of increasing difficulty, with the instruction to read for comprehension. The Fluency score represents both the child's speed (rate) of reading and accuracy (# of deviations from print) for each passage. Scaled scores were calculated for Rate and Accuracy, based upon age norms, and combined to produce the Fluency score.
WJ-III Reading Fluency
The WJ-III (Woodcock et al., 2001) Reading Fluency subtest is a timed measure of silent contextual reading fluency requiring the child to read simple sentences silently, determine whether the sentence is true, and circle the appropriate corresponding letter (e.g., T or F). The total score represents the number of correct responses within a 3-min time limit, converted to an age-normed standard score.
WJ-III Letter Word Identification
The WJ-III (Woodcock et al., 2001) Letter Word Identification subtest is an untimed measure of non-contextual single word reading ability requiring the child to read a list of increasingly complex English words aloud. The total score represents the number of words read correctly, converted to a standard score based upon age norms.
Test of Word Reading Efficiency
The TOWRE (Torgesen, 1999) is an assessment of the child's single word reading and single pseudo-word decoding isolated (non-contextual) word fluency under timed conditions. The child is asked to read as many individual words (Sight Word Efficiency) or non-words (Phonetic Decoding Efficiency) of increasing length and phonetic difficulty as possible in 45 s. Scaled scores for Sight Word Efficiency and Phonetic Decoding Efficiency represent the number of correctly read words within the time limit, relative to age norms. The TOWRE Total score is a composite of performance on both the Sight Word Efficiency and Phonetic Decoding Efficiency tasks.
Intellectual Ability
WISC-IV General Ability Index
The WISC-IV (Wechsler, 2004) GAI score served as a measure of participants' broad intellectual ability in which requirements for speeded processing of information were reduced. The GAI score is a composite of the Verbal Comprehension and Perceptual Reasoning subtests.
Data Analyses
Group differences in task performance (RT and variability; t-tests) were examined using both Gaussian (e.g., MRT, RTSD, CV SD/M) and ex-Gaussian (e.g., mu, sigma, and tau) approaches. Ex-Gaussian variables were extracted using MATLAB and the EGFit toolkit (Lacouture & Cousineau, 2008). Next, we examined whether inconsistent responding was a consistent characteristic of the sample or a function of time spent on the task (i.e., due to increasing fatigue or “boredom” with the task). For this purpose, the RT task was divided into two halves and additional analyses (repeated-measures ANOVAs) for each dependent variable were conducted to examine the effect of time on task (first versus second half) on ex-Gaussian measures of RT speed (mu) and variability (sigma and tau). Finally, following checks for collinearity (e.g., tolerance range: 0.287–0.619; variance inflation factor range: 1.57–3.49), regression analyses were used to examine effects of RT speed (mu), variability (sigma), and skewness (tau) on oral and silent reading fluency. Effects of RT skewness (tau) on reading fluency were further examined within the ADHD group after controlling for ADHD symptom severity. Supplementary regression analyses examined the effect of across task variability (tau across the two halves of the task) on reading fluency performance. For all RT analyses, only successful trials were used (i.e., omission errors were not included; individual omission error rates ranged from 0% to11.6% in controls and 0.5% to 25.9% in the ADHD group).
Results
Participants
There were no significant differences in racial distribution—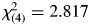 , p = .589—between the ADHD and control groups. Additionally, there were no significant differences between groups in age—t(65) = 0.387, p = .700—or socioeconomic status—t(62) = 0.958, p = .342; however, consistent with a recent meta-analysis examining effects of ADHD-related symptomatology on test-taking behavior (Jepsen, Fagerlund, & Mortensen, 2009), GAI scores were higher for controls than the (un-medicated) ADHD group (Table 1). There was also a larger proportion of boys in the ADHD group than in the control group—
, p = .589—between the ADHD and control groups. Additionally, there were no significant differences between groups in age—t(65) = 0.387, p = .700—or socioeconomic status—t(62) = 0.958, p = .342; however, consistent with a recent meta-analysis examining effects of ADHD-related symptomatology on test-taking behavior (Jepsen, Fagerlund, & Mortensen, 2009), GAI scores were higher for controls than the (un-medicated) ADHD group (Table 1). There was also a larger proportion of boys in the ADHD group than in the control group—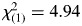 , p = .026.
, p = .026.
Table 1.
Demographic, IQ, and reading information
| ADHD (n = 39) |
Control (n = 28) |
p-value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Sex (% men) | 66.7 | 39.3 | |||
| Age | 11.15 | 1.60 | 11.30 | 1.44 | .70 |
| WISC-IV | |||||
| GAI | 104.23 | 13.37 | 115.86 | 12.43 | .001 |
| Reading Fluency | |||||
| GORT-IV Fluency | 10.86 | 3.42 | 13.39 | 3.36 | .003 |
| WJ-III RF | 99.21 | 14.44 | 108.48 | 18.33 | .025 |
| TOWRE Total | 102.95 | 13.36 | 112.00 | 12.67 | .003 |
| ADHD | |||||
| CPRS-R N Scale | 72.92 | 9.90 | 47.17 | 7.48 | <.001 |
Notes: WISC-IV = Wechsler Intelligence Scale for Children, Fourth Edition; GAI = General Ability Index, standard score; GORT-IV = Gray Oral Reading Test-IV, scaled score; WJ-III RF = Woodcock–Johnson-III Reading Fluency, standard score; TOWRE = Test of Word Reading Efficiency, standard score; CPRS-R N scale: Conners' Parent Rating Scale-Revised, Total ADHD Symptoms scale (N scale), T-score.
RT Performance
Using traditional summative measures (i.e., mean, SD), there were no significant sex differences in mean response time—MRT, t(65) = 0.297, p = .767; mu, t(65) = 0.704, p = .484—nor was age significantly correlated with motor RT or RT variability (MRT, r = −.173, p = .161; mu, r = −.049, p = .691; SDRT, r = .131, p = .508; CV, r = .017, p = .891; tau, r = −.002, p = .993). Thus, age and sex were not included as covariates in further analyses.
Performance on RT variables is listed in Table 2. Although children with ADHD appeared slower than controls based on overall mean response time—MRT, t(1,65) = −2.513, p = .014, when examining response speed within the normal component of the RT distribution, the groups did not differ—mu, t(1,65) = −0.317, p = .752, suggesting that the “slowing” observed in MRT may be due to the presence of outlying (extremely slow) responses. Children with ADHD were significantly more variable than controls, when examining both the overall distribution of responses—SDRT, t(1,65) = −2.62, p = .011—and the normal component of the distribution—sigma, t(1,65) = −2.22, p = .030. Additionally, children with ADHD showed greater RT skewness—tau, t(1,65) = −2.70, p = −.009, suggesting a greater tendency to show infrequent longer response latencies, even on this simple motor RT task.
Table 2.
Group differences in RT variables
| VSRT measure | ADHD |
Control |
p | 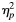 |
||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| MRT | 438.26 | 106.93 | 378.93 | 75.98 | .014 | 0.089* |
| mu | 291.24 | 90.99 | 285.09 | 55.16 | .752 | 0.002 |
| SDRT | 192.48 | 95.92 | 137.28 | 66.86 | .011 | 0.096* |
| CV | 0.432 | 0.17 | 0.359 | 0.16 | .082 | 0.046 |
| Sigma | 77.71 | 34.96 | 60.59 | 24.96 | .030 | 0.070* |
| Tau | 147.03 | 93.41 | 93.89 | 53.61 | .009 | 0.101* |
Notes: RT = reaction time; ADHD = Attention-Deficit/Hyperactivity Disorder; VSRT = Visual simple reaction time task; MRT = mean RT; SDRT = RT standard deviation; CV = coefficient of variability (SD/M).
*Effect size values represent generally medium effect sizes (per Cohen's criteria, as discussed in Fritz, Morris, & Richler, 2012).
In order to determine whether patterns of speed and variability changed over the course of the task, speed (mu), variability (sigma), and skewness (tau) were examined separately in the first and the second half of the task. For mu, repeated-measures ANOVA revealed no significant effects for group, F(1,65) = 0.078, p = .780, 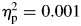 , block, F(1,65) = 0.285, p = .595,
, block, F(1,65) = 0.285, p = .595, 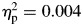 , or a group-by-time interaction, F(1,65) = 0.101, p = .751,
, or a group-by-time interaction, F(1,65) = 0.101, p = .751, 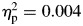 . In contrast, for sigma, there were significant differences for group, ADHD > controls; F(1,65) = 4.70, p = .034,
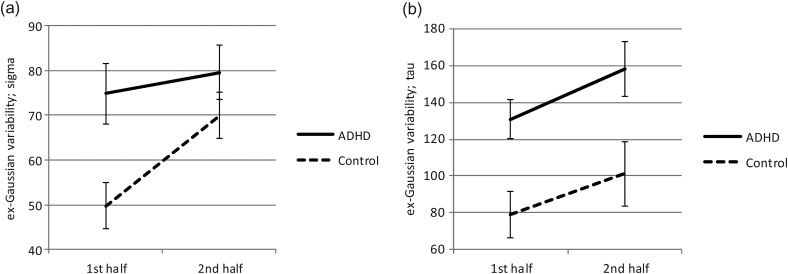
. In contrast, for sigma, there were significant differences for group, ADHD > controls; F(1,65) = 4.70, p = .034, 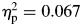 , block, second half > first half; F(1,65) = 11.80, p = .001,
, block, second half > first half; F(1,65) = 11.80, p = .001, 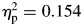 (Fig. 1), as well as a significant group-by-block interaction, F(1,65) = 4.51, p = .037,
(Fig. 1), as well as a significant group-by-block interaction, F(1,65) = 4.51, p = .037, 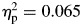 . With regard to the interaction effect on sigma, although children with ADHD were consistently more variable than controls across the task, controls tended to become more variable in their responding during the second half of the task compared with the first half—controls, F(1,27) = 22.71, p < .001,
. With regard to the interaction effect on sigma, although children with ADHD were consistently more variable than controls across the task, controls tended to become more variable in their responding during the second half of the task compared with the first half—controls, F(1,27) = 22.71, p < .001, 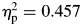 ; ADHD, F(1,38) = .793, p = .379. As a result, performance of controls was more similar to that of the ADHD group in the second half of the task (Fig. 1). For tau, there were also significant effects for group (ADHD > controls), F(1,65) = 8.41, p = .005,
; ADHD, F(1,38) = .793, p = .379. As a result, performance of controls was more similar to that of the ADHD group in the second half of the task (Fig. 1). For tau, there were also significant effects for group (ADHD > controls), F(1,65) = 8.41, p = .005, 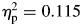 , block (second half > first half), F(1,65) = 12.14, p = .001,
, block (second half > first half), F(1,65) = 12.14, p = .001, 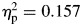 , but no significant group-by-block interaction, F(1,65) = 0.116, p = .735,
, but no significant group-by-block interaction, F(1,65) = 0.116, p = .735, 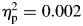 (Fig. 1). Thus, although both groups showed higher tau (greater RT variability) during the second half of the task relative to the first half, children with ADHD showed consistently greater tau than controls.
(Fig. 1). Thus, although both groups showed higher tau (greater RT variability) during the second half of the task relative to the first half, children with ADHD showed consistently greater tau than controls.
Fig. 1.
Ex-Gaussian measures of variability (sigma and tau) by group across the course of the motor RT task.
Reading Fluency
Children with ADHD showed significantly slower reading fluency than controls, across all three measures (Table 1). Additionally, after controlling for GAI, children with ADHD had significantly lower scores on oral non-contextual fluency (TOWRE Total: ΔR2 = .063, p = .031, Model Total R2 = .107), but not on oral or written contextual reading fluency (GORT-IV Fluency: ΔR2 = .038, p = .074, Model Total R2 = .185; WJ-III Reading Fluency: ΔR2 = .007, p = .441, Model Total R2 = .229).
Effects of RT Speed and Variability on Reading Fluency Performance
Examining effects separately by group, within controls, mu contributed to predictions of oral non-contextual reading fluency (TOWRE) but not to oral or silent contextual fluency (Table 3). Within the ADHD group, mu contributed only to predictions of oral contextual fluency (GORT-IV), even after controlling for symptom severity, but not to silent or oral non-contextual fluency. Within groups, variability within the normal component of the response distribution (i.e., sigma) did not significantly predict reading fluency.
Table 3.
Variance in reading fluency accounted for by RT variables, after controlling for ADHD symptom severity (CPRS-R N score)
| Reading fluency Measure | ADHD |
Control |
||
|---|---|---|---|---|
| ΔR2 | p | ΔR2 | p | |
| GORT-IV Fluency | ||||
| Mu | .135** | .030 | .041 | .363 |
| Sigma | .004 | .703 | .000 | .934 |
| Tau | .112* | .040 | .074 | .219 |
| WJ-III RF | ||||
| Mu | .006 | .652 | .113 | .131 |
| Sigma | .017 | .447 | .016 | .575 |
| Tau | .068 | .125 | .124 | .112 |
| TOWRE Total | ||||
| Mu | .089 | .073 | .232** | .027 |
| Sigma | .026 | .338 | .031 | .444 |
| Tau | .164** | .013 | .038 | .397 |
Notes: RT = reaction time; CPRS-R = Conners' Parent Rating Scale, Revised; GORT-IV = Gray Oral Reading Test-IV; WJ-III RF = Woodcock–Johnson-III Reading Fluency; TOWRE = Test of Word Reading Efficiency.
*Significant ΔR2 values represent generally medium effect sizes.
**Significant ΔR2 values represent generally large effect sizes.
Additionally, within the ADHD group, tau was significantly associated with oral reading fluency, with medium to large effect sizes, even after controlling for ADHD symptom severity, as measured by the CPRS-R N score (Table 3). Even after additionally controlling for WISC-IV GAI, tau remained a significant predictor of oral reading fluency in children with ADHD (GORT-IV Fluency: ΔR2 = .102, p = .055, Total Model R2 = .175; TOWRE Total: ΔR2 = .155, p = .016, Total Model R2 = .204; WJ-III Reading Fluency: ΔR2 = .044, p = .170, Total Model R2 = .260).
In contrast to predictions regarding performance on timed reading measures, RT variability was not significantly associated with untimed non-contextual single word reading (Letter Word Identification). Specifically, for single word reading, tau did not add to predictions of basic word reading skills, after controlling for ADHD symptomatology across groups (CPRS-R N score; ΔR2 = .029, p = .178) or within controls (ΔR2 = .034, p = .423). Within the ADHD group alone, the association of RT variability (tau) with non-contextual word reading also was non-significant (ΔR2 = .095, p = .066).
Discussion
The present study examined whether children with ADHD showed similar patterns of deficits on simple RT tasks (i.e., decreased speed and intra-individual variability) that have been observed on response time tasks involving greater demands for cognitive control (i.e., go–no go, choice RT, stop signal). Consistent with our hypothesis, RT performance in children with ADHD was slower than that of typically developing controls, based upon overall MRT values but not within the normal component of the distribution (mu). Additionally, compared with typically developing controls, children with ADHD were more variable within the normal component of the RT distribution (elevated sigma) and showed greater RT skewness (elevated tau). We also found that, even on a relatively brief (6-min) task, children in both groups showed longer periodic response times (elevated tau) during the second half of the simple RT task relative to the first half, with children with ADHD showing consistently greater lapses in performance compared with controls. Interestingly, controls showed greater variability within the normal component of the distribution (i.e., sigma) during the second half of the task relative to their performance during the first half, approaching the variability (sigma) of children with ADHD. Furthermore, tau was significantly associated with slower, more inefficient reading fluency in children with ADHD.
These findings are consistent with much of the recent empirical evidence regarding comprehensive examination of the RT distribution in children with ADHD, but present new findings and suggest important implications of RT variability. Of importance, using traditional summative measures (i.e., MRT), children with ADHD appeared to be slower than controls on the simple RT task; however, by segregating the normal and exponential components of the distribution, we observed that children with ADHD were not actually slower than controls on this RT task (i.e., as measured by mu). Use of the ex-Gaussian analytic approach permits a more specific characterization of performance and suggests that MRT values are affected by the inconsistency of responses and the presence of periodic “very long” response times that may reflect lapses of attention or lapses of on-task behavior (e.g., Antonini et al., 2013). That is, even on a brief, simple motor RT task with minimal cognitive processing demands, children with ADHD were significantly more inconsistent and had more significantly off-task behavior than controls. Although this has been robustly demonstrated on RT tasks with greater demands for cognitive processing (e.g., Epstein et al., 2011), this pattern of inconsistent responding has not previously been demonstrated on a simple motor RT task. It is also striking that this variability was evident even with use of a jittered ISI paradigm, in spite of emerging evidence that jittering may represent a non-pharmacologic method of improving response control (Lee et al., 2012; Ryan et al., 2010), particularly for children with ADHD. Further, presence and severity of these apparent lapses off task (elevated tau) significantly predicted oral reading fluency across groups and oral reading fluency in children with ADHD. In fact, after controlling for both symptom severity and intellectual ability, there was an even greater association between RT skewness (elevated tau) and reading fluency performance among children with ADHD, with this relationship showing a medium to large effect size. Conversely, variability in performance was not significantly associated with untimed single word reading. These findings add to new evidence that RT variability is also associated with attention to and accuracy on math tasks (Antonini et al., 2013).
Furthermore, these findings suggest that periodic longer response times, or lapses off task, are evident across motor response tasks and not only under demands for controlled responses (e.g., go–no go tasks, flanker tasks, choice RT tasks). Removing demands for more complex, top-down, cognitive control did not appear to normalize performance in ADHD. As such, interventions may need to focus on such areas as provision of extra time for academic tasks, particularly those traditionally presenting children with a speed demand (reading fluency, math fluency tasks) as well as methods for facilitating bottom-up regulation of attention.
It is important to note that statistical control of IQ may produce anomalous results when examining group differences in components of executive function or response control (e.g., Dennis et al., 2009). Prior work has suggested that reduced IQ scores in ADHD samples relative to typically developing controls may be specifically related to attentional dysregulation and poor test-taking behavior rather than deficits intelligence, per se (see Jepsen et al., 2009). This issue is even more salient in this sample, since the children with ADHD were tested while off medication, which Jepsen and colleagues have suggested may result in lowered IQ scores. Given these considerations, we ran key statistical analyses both with and without controlling for IQ; findings remained consistent as noted above.
It is not entirely clear why this association was observed in oral reading fluency tasks, but was not evident on the silent reading fluency task. Oral reading involves an overtly motor (oral-motor) response, whereas silent reading is largely a covert motor task. As such, oral reading may place a higher demand upon the white matter circuitry connecting posterior occipital/temporal regions (Wernicke's area) and frontal regions (Broca's area) than silent reading, given the demand for rapid verbal output based upon some level of comprehension of text. Findings of a significant association between RT skewness (tau) and oral reading fluency may implicate inefficiency in the arcuate fasciculus and/or superior longitudinal fasciculus, areas found to show white matter abnormalities in ADHD (Peterson et al., 2011). Furthermore, white matter pathways considered critical for automaticity and response regulation, such as the inferior longitudinal fasciculus and/or cingulate, may also be implicated.
Although a growing body of evidence suggests that the inhibition and working memory difficulties characteristic of many children with ADHD contribute to the slower and more variable pattern of performance seen on tasks requiring both cognitive processing and a motor response (e.g., go–no go, choice reaction tasks), the present findings provide evidence for marked variability in RT performance, including highly inconsistent performance and, possibly, frank attentional lapses, on even simple RT tasks. Notably, there was an interaction effect (group by time block) for sigma but not for tau, suggesting that typically developing children also show increased variability over time spent completing the RT task, but that this variability is confined to the normal component of the distribution (sigma), without the tendency to show periodic longer response latencies characteristic of ADHD. This apparent tendency to show periodic lapses in attention, evident even over relatively short periods of time (e.g., in each half of this 6-min task), likely plays an important role in performance of children with ADHD on more complex tasks over longer periods of time—such as those required when reading and writing in the classroom setting.
This variability in self-regulation of attention may reflect the anomalous development of frontal-striatal and/or cerebellar circuitry in ADHD (see Mahone, 2011, for a review), as these areas have been implicated in timing and automaticity of responding. Furthermore, corpus callosal circumference has been associated with response control and RT variability (McNally et al., 2009). Functional imaging studies also implicate other white matter connections (i.e., left sagittal stratum; Peterson et al., 2011) as well as the oculomotor regions and associated frontal-subcortical connections in ADHD, with oculomotor deficits in ADHD associated with slower processing and automaticity (see Mahone, 2011, for a review). However, this study did not examine neuroimaging correlates of these behavioral findings; thus, further research will be better able to elucidate the nature of these interrelated pathways and their role in the shared functional impairments underlying ADHD and RD.
Additionally, although reading fluency carries demands for both accuracy and speed, and prior work highlights the importance of processing speed for reading fluency (e.g., Jacobson et al., 2011), RT speed per se (e.g., mu) did not contribute to predictions of children's reading fluency scores. Instead, variability/skewness in RT performance (tau) was predictive of oral reading fluency but not of untimed word reading. Thus, this variability in responding (rather than speed) may represent a core phenotype of the ADHD construct, that contributes to the between group differences seen in multiple task domains. Increased RT skewness may represent a basic disruption in behavioral and attentional regulation, likely linked to variability in noradrenergic and/or dopaminergic systems involved in regulating arousal and alertness and in reward-based mediation of response and attentional control.
As such, findings suggest that interventions should focus on strategies to reduce the “attentional” or performance lapses that appear to contribute to the overall slower responding seen in youth with ADHD across a variety of tasks. Although such accommodations as extended time are often recommended to address the pattern of slower task completion, more targeted interventions that address potential variability in bottom-up control of attention (e.g., pharmacological interventions or increasing variability in presentation of information; i.e., “jittering”) may be more effective in reducing these lapses in performance and, therefore, improving overall performance on the task at hand. As noted above, there is emerging evidence that ISI jittering may facilitate engagement and reduce variability (off-task lapses; e.g., Ryan et al., 2010; Wodka, Simmonds, Mahone, & Mostofsky, 2009); it may be that a simple “go” RT task is so intrinsically “uninteresting” to children with ADHD that even jittering does not provide enough extrinsic stimulation to support sustained attentional focus. Further investigation of the level of intrinsic engagement of a task, presence/level of ISI jitter, and children's variability in response preparation and execution will be important to better address these questions. Finally, it is worth noting that the jittered ISI ranged from 1,000 to 2,000 ms, thus truncating the possible length of even longer response times, and the potential variability of tau. Effects of tau may therefore be underestimated in this study and larger effects may be evident when RT is examined on a task with a potentially longer response time “window.”
Further evaluation of RT variability across tasks involving different levels of complexity will be important to better characterize the nature and implications of these findings for children with ADHD. These data represent an important extension of work examining the RT distribution in ADHD; however, given the relatively small size of the current sample, overrepresentation of girls in the control group, and generally above average cognitive ability levels within the control group, results should be replicated in a larger sample. It will be critical to examine these associations in a sample with a more normal distribution of gender and ability levels across groups, as results may be different in such a sample. Furthermore, findings may be limited in their generalization to the larger population of children with ADHD as this study excluded children with identified co-morbidities other than ODD and Specific Phobia. As the majority of children with ADHD do have co-occurring conditions, results may not generalize to all children with ADHD.
Funding
This work was supported by the Intellectual and Developmental Disabilities Research Center (P50 HD 52121, R01 MH085328, and HD-24061) and the University School of Medicine Institute for Clinical and Translational Research, a National Institutes of Health/NIH Clinical Translational Science Awards (CTSA) Program (UL1-RR025005).
Conflict of Interest
None declared.
Acknowledgements
Portions of this manuscript were presented at the 10th annual meeting of the American Academy of Clinical Neuropsychology in Seattle, WA, on June 21, 2012.
References
- Andreou P., Neale B. M., Chen W. A. I., Christiansen H., Gabriels I., Heise A., et al. Reaction time performance in ADHD: Improvement under fast-incentive condition and familial effects. Psychological Medicine. 2007;37:1703–1715. doi: 10.1017/S0033291707000815. doi:10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini T. N., Narad M. E., Langberg J. M., Epstein J. N. Behavioral correlates of reaction time variability in children with and without ADHD. Neuropsychology. 2013;27:201–209. doi: 10.1037/a0032071. doi:10.1037/a0032071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F. X., Lee P. P., Sharp W., Jeffries N. O., Greenstein D. K., Clasen L. S., et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Medical Association. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. doi:10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Chhabildas N., Pennington B. F., Willcutt E. G. A comparison of the neuropsychological profiles of the DSM-IV subtypes of ADHD. Journal of Abnormal Child Psychology. 2001;29:529–540. doi: 10.1023/a:1012281226028. doi:10.1023/A:1012281226028. [DOI] [PubMed] [Google Scholar]
- Cole W. R., Mostofsky S. H., Gidley Larson J. C., Denckla M. B., Mahone E. M. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology. 2008;71:1514–1520. doi: 10.1212/01.wnl.0000334275.57734.5f. doi:10.1212/01.wnl.0000334275.57734.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton D. L., Olson R. K., DeFries J. C., Pennington B. F. Comparing the relationships among two different versions of alphanumeric rapid automatized naming and word level reading skills. Scientific Studies of Reading. 2002;6:343–368. doi:10.1207/S1532799XSSR0604_03. [Google Scholar]
- Conners C. K. Conners' Rating Scales - Revised. North Tonawanda, NY: Multi-Health Systems; 1997. [Google Scholar]
- Denckla M. B., Cutting L. E. History and significance of rapid automatized naming. Annals of Dyslexia. 1999;49:29–42. doi:10.1007/s11881-999-0018-9. [Google Scholar]
- Dennis M., Francis D. J., Cirino P. T., Schachar R., Barnes M. A., Fletcher J. M. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Ghaffari M., Curchack J., Reiss P., Hyde C., Vannucci M., et al. Decomposing intra-subject variability in children with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2008;64:607–614. doi: 10.1016/j.biopsych.2008.03.008. doi:10.1016/j.biopsych.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul G. J., Power T. J., Anastopoulos A. D., Reid R. ADHD Rating Scale-IV: Checklists, norms and clinical interpretation. New York: Guilford; 1998. [Google Scholar]
- Eckert M. A., Leonard C. M., Richards T. L., Aylward E. H., Thomson J., Berninger V. W. Anatomical correlates of dyslexia: Frontal and cerebellar findings. Brain. 2003;126:482–494. doi: 10.1093/brain/awg026. doi:10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- Epstein J. N., Langberg J. M., Rosen P. J., Graham A., Narad M. E., Antonini T. N., et al. Evidence for higher reaction time variability for children with ADHD on a range of cognitive tasks including reward and event rate manipulations. Neuropsychology. 2011;25:427–441. doi: 10.1037/a0022155. doi:10.1037/a0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster L. M., Hynd G., Morgan A. E., Hugdahl K. Planum temporale asymmetry and ear advantage in dichotic listening in developmental dyslexia and ADHD. Journal of the International Neuropsychological Society. 2002;8:22–36. doi:10.1017/S1355617702811031. [PubMed] [Google Scholar]
- Fritz C. O., Morris P. E., Richler J. J. Effect size estimates: Current use, calculations, and interpretation. Journal of Experimental Psychology: General. 2012;141:2–18. doi: 10.1037/a0024338. [DOI] [PubMed] [Google Scholar]
- Germanò E., Gagliano A., Curatolo P. Comorbidity of ADHD and dyslexia. Developmental Neuropsychology. 2010;35:475–493. doi: 10.1080/87565641.2010.494748. doi:10.1080/87565641.2010.494748. [DOI] [PubMed] [Google Scholar]
- Ghelani K., Sidhu R., Jain U., Tannock R. Reading comprehension and reading related abilities in adolescents with reading disabilities and attention-deficit/hyperactivity disorder. Dyslexia. 2004;10:364–384. doi: 10.1002/dys.285. doi:10.1002/dys.285. [DOI] [PubMed] [Google Scholar]
- Gilger J. W., Pennington B. F., DeFries J. C. A twin study of the etiology of comorbidity: ADHD and dyslexia. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:343–348. doi: 10.1097/00004583-199203000-00024. doi:10.1097/00004583-199203000-00024. [DOI] [PubMed] [Google Scholar]
- Harris E. L., Schuerholz L. J., Singer H. S., Reader M. J., Brown J. E., Cox C., et al. Executive function in children with Tourette syndrome and/or attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society. 1995;1:511–516. doi: 10.1017/s1355617700000631. doi:10.1017/S1355617700000631. [DOI] [PubMed] [Google Scholar]
- Hervey A. S., Epstein J. N., Curry J. F., Tonev S., Arnold L. E., Conners C. K., et al. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychology. 2006;12:125–140. doi: 10.1080/09297040500499081. doi:10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- Jacobson L. A., Ryan M., Martin R. B., Ewen J., Mostofsky S. H., Denckla M. B., et al. Working memory influences processing speed and reading fluency in ADHD. Child Neuropsychology. 2011;17:209–224. doi: 10.1080/09297049.2010.532204. doi:10.1080/09297049.2010.532204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jepsen J. R. M., Fagerlund B., Mortensen E. L. Do attention deficits influence IQ in children and adolescents with ADHD? Journal of Attention Disorders. 2009;12:551–562. doi: 10.1177/1087054708322996. doi:10.1177/1087054708322996. [DOI] [PubMed] [Google Scholar]
- Klotz J. M., Johnson M. D., Wu S. W., Isaacs K. M., Gilbert D. L. Relationship between reaction time variability and motor skill development in ADHD. Child Neuropsychology. 2012;18:576–585. doi: 10.1080/09297049.2011.625356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M., Wimmer H., Staffen W., Hutzler F., Mair A., Ladurner G. Developmental dyslexia: Gray matter abnormalities in the occipitotemporal cortex. Human Brain Mapping. 2008;29:613–625. doi: 10.1002/hbm.20425. doi:10.1002/hbm.20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacouture Y., Cousineau D. How to use MATLAB to fit the ex-Gaussian and other probability functions to a distribution of response times. Tutorials in Quantitative Methods for Psychology. 2008;4:35–45. [Google Scholar]
- Lee R. W. Y., Jacobson L. A., Pritchard A. E., Ryan M., Denckla M. B., Mostofsky S. H., et al. Jitter reduces response time variability in ADHD: An ex-Gaussian analysis. Journal of Attention Disorders. 2012 doi: 10.1177/1087054712464269. doi:10.1177/1087054712464269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leth-Steensen C., King Elbaz Z., Douglas V. I. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychologica. 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. doi:10.1016/S0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Mahone E. M. ADHD: Volumetry, motor, and oculomotor functions. Current Topics in Behavioral Neurosciences. 2011;9:17–47. doi: 10.1007/7854_2011_146. [DOI] [PubMed] [Google Scholar]
- Mahone E. M., Mostofsky S. H., Lasker A. G., Zee D., Denckla M. B. Oculomotor anomalies in Attention-Deficit/Hyperactivity Disorder: Evidence for deficits in response preparation and inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:749–756. doi: 10.1097/CHI.0b013e3181a565f1. doi:10.1097/CHI.0b013e3181a565f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath L. M., Pennington B. F., Shanahan M. A., Santerre-Lemmon L. E., Barnard H. D., Willcutt E. G., et al. A multiple deficit model of reading disability and attention-deficit/hyperactivity disorder: Searching for shared cognitive deficits. Journal of Child Psychology and Psychiatry. 2011;52:547–557. doi: 10.1111/j.1469-7610.2010.02346.x. doi:10.1111/j.1469-7610.2010.02346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally M. A., Crocetti D., Mahone E. M., Denckla M. B., Suskauer S. J., Mostofsky S. H. Corpus callosum segment circumference is associated with response control in children with Attention Deficit Hyperactivity Disorder (ADHD) Journal of Child Neurology. 2010;25:453–462. doi: 10.1177/0883073809350221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metin B., Roeyers H., Wiersema J. R., van der Meere J. J., Thompson M., Sonuga-Barke E. ADHD performance reflects inefficient but not impulsive information processing: A diffusion model analysis. Neuropsychology. 2013;27:193–200. doi: 10.1037/a0031533. doi:10.1037/a0031533. [DOI] [PubMed] [Google Scholar]
- Peterson D. J., Ryan M., Rimrodt S. L., Cutting L. E., Denckla M. B., Kaufmann W. E., et al. Increased regional fractional anisotropy in highly screened attention-deficit hyperactivity disorder (ADHD) Journal of Child Neurology. 2011;26:1296–1302. doi: 10.1177/0883073811405662. doi:10.1177/0883073811405662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueyo R., Maneru C., Vendrell P., Mataro M., Estevez Gonzales A., Garcia-Sanchez C., et al. Attention deficit hyperactivity disorder. Cerebral asymmetry observed on magnetic resonance. Revista de Neurología. 2000;30:920–925. [PubMed] [Google Scholar]
- Reich W., Welner Z., Herjanic B. The Diagnostic Interview for Children and Adolescents-IV. North Tonawanda: Multi-Health Systems; 1997. [Google Scholar]
- Rommelse N. N., Altink M. E., Oosterlaan J., Beem L., Buschgens C. J., Buitelaar J., et al. Speed, variability, and timing of motor output in ADHD: Which measures are useful for endophenotypic research? Behavioral Genetics. 2008;38:121–132. doi: 10.1007/s10519-007-9186-8. doi:10.1007/s10519-007-9186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucklidge J. J., Tannock R. Neuropsychological profiles of adolescents with ADHD: Effects of reading difficulties and gender. Journal of Child Psychology and Psychiatry. 2002;43:988–1003. doi: 10.1111/1469-7610.00227. doi:10.1111/1469-7610.00227. [DOI] [PubMed] [Google Scholar]
- Ryan M., Martin R., Denckla M. B., Mostofsky S. H., Mahone E. M. Interstimulus jitter facilitates response control in children with ADHD. Journal of the International Neuropsychological Society. 2010;16:388–393. doi: 10.1017/S1355617709991305. doi:10.1017/S1355617709991305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E., Wiig E. H., Secord W. A. Clinical Evaluation of Language Fundamentals, Fourth Edition. San Antonio, TX: Harcourt Assessment; 2003. [Google Scholar]
- Shanahan M. A., Pennington B. F., Yerys B. E., Scott A., Boada R., Willcutt E. G., et al. Processing speed deficits in Attention Deficit/Hyperactivity Disorder and reading disability. Journal of Abnormal Child Psychology. 2006;34:585–602. doi: 10.1007/s10802-006-9037-8. doi:10.1007/s10802-006-9037-8. [DOI] [PubMed] [Google Scholar]
- Torgesen J. K., Wagner R. K., Rashotte C. A. Test of word reading efficiency. Austin, TX: Pro-Ed; 1999. [Google Scholar]
- Vaurio R. G., Simmonds D. J., Mostofsky S. H. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2009;47:2389–2396. doi: 10.1016/j.neuropsychologia.2009.01.022. doi:10.1016/j.neuropsychologia.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. L. Wechsler Intelligence Scale for Children - Fourth Edition Technical and interpretive manual. San Antonio, TX: Harcourt Assessment; 2004. [Google Scholar]
- Whelan R. Effective analysis of reaction time data. The Psychological Record. 2008;58:475–482. [Google Scholar]
- Wiederholt L., Bryant B. Examiner's Manual: Gray Oral Reading Test-Fourth Edition. Austin, TX: Pro-Ed; 2000. [Google Scholar]
- Willcutt E. G. Attention-Deficit/Hyperactivity Disorder. In: Yeates K. O., Ris M. D., Taylor M. G., Pennington B. F., editors. Pediatric neuropsychology. 2nd ed. New York: Guilford; 2010. pp. 393–417. [Google Scholar]
- Willcutt E. G., Betjemann R. S., McGrath L. M., Chhabildas N. A., Olson R. K., DeFries J. C., et al. Etiology and neuropsychology of comorbidity between RD and ADHD: The case for multiple-deficit models. Cortex. 2010;46:1345–1361. doi: 10.1016/j.cortex.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt E. G., Doyle A. E., Nigg J. T., Faraone S. V., Pennington B. F. Validity of the executive function theory of Attention-Deficit/Hyperactivity Disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. doi:10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willcutt E. G., Pennington B. F., Olson R. K., Chhabildas N., Hulslander J. Neuropsychological analyses of comorbidity between reading disability and Attention Deficit Hyperactivity Disorder: In search of the common deficit. Developmental Neuropsychology. 2005;27:35–78. doi: 10.1207/s15326942dn2701_3. doi:10.1207/s15326942dn2701_3. [DOI] [PubMed] [Google Scholar]
- Willcutt E. G., Pennington B. F., Olson R. K., DeFries J. C. Understanding comorbidity: A twin study of reading disability and Attention-Deficit/Hyperactivity Disorder. American Journal of Medical Genetics Part B. 2007;144:709–714. doi: 10.1002/ajmg.b.30310. doi:10.1002/ajmg.b.30310. [DOI] [PubMed] [Google Scholar]
- Wodka E. L., Mahone E. M., Blankner J. G., Gidley Larson J. C., Fotedar S., Denckla M. B., et al. Evidence that response inhibition is a primary deficit in ADHD. Journal of Clinical and Experimental Neuropsychology. 2007;29:345–356. doi: 10.1080/13803390600678046. doi:10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]
- Wodka E. L., Simmonds D. J., Mahone E. M., Mostofsky S. H. Moderate variability in stimulus presentation improves motor response control. Journal of Clinical and Experimental Neuropsychology. 2009;31:483–488. doi: 10.1080/13803390802272036. doi:10.1080/13803390802272036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M., Katzir-Cohen T. Reading fluency and its intervention. Scientific Studies of Reading. 2001;5:211–238. doi:10.1207/S1532799XSSR0503_2. [Google Scholar]
- Woodcock R. W., McGrew K. S., Mather N. Woodcock-Johnson – III. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- Ziegler A., Konig I. R., Deimel W., Plume E., Nothen M. M., Propping P., et al. Developmental dyslexia—Recurrence risk estimates from a German bi-center study using the single proband sib pair design. Human Heredity. 2005;59:136–143. doi: 10.1159/000085572. doi:10.1159/000085572. [DOI] [PubMed] [Google Scholar]