Abstract
Sleep responses to chronic sleep restriction may be very different from those observed after acute total sleep deprivation. Specifically, when sleep restriction is repeated for several consecutive days, animals express attenuated compensatory increases in sleep time and intensity during daily sleep opportunities. The neurobiological mechanisms underlying these adaptive, or more specifically, allostatic, changes in sleep homeostasis are unknown. Several lines of evidence indicate that norepinephrine may play a key role in modulating arousal states and NREM EEG delta power, which is widely recognized as a marker for sleep intensity. Therefore, we investigated time course changes in brain adrenergic receptor mRNA levels in response to chronic sleep restriction using a rat model. Here, we observed that significantly altered mRNA levels of the α1- adrenergic receptor in the basal forebrain as well as α2- and β1-adrenergic receptor in the anterior cingulate cortex only on the first sleep restriction day. On the other hand, the frontal cortex α1-, α2-, and β1- adrenergic receptor mRNA levels were reduced throughout the period of sleep restriction. Combined with our earlier findings on EEG that sleep time and intensity significantly increased only on the first sleep restriction days, these results suggest that alterations in the brain norepinephrine system in the basal forebrain and cingulate cortex may mediate allostatic changes in sleep time and intensity observed during chronic sleep restriction.
Keywords: chronic sleep restriction, allostasis, norepinephrine, rat
1. Introduction
Sleep responses to chronic sleep restriction (CSR) may be very different from those observed after acute total sleep deprivation (SD). For example, short-term SD (i.e., 24 h or less) in animals as well as humans normally produces robust compensatory increases in sleep time and/or sleep intensity in the sleep episodes following the SD. However, when sleep time is reduced for several consecutive days, several studies have reported that rats adapt to the new sleep restriction (SR) condition by exhibiting attenuated (or non-significant) increases in sleep time or intensity during the daily sleep opportunities (Deurveilher et al., 2012; Kim et al., 2007; Kim et al., 2012; Lancel and Kerkhof, 1989; Rechtschaffen et al., 1999). Human CSR studies have also found adaptive sleep responses in that subjective sleepiness stabilizes at a mildly elevated level within the first 3 days of SR. However, objective sleepiness and neurobehavioral performance continue to worsen across SR days in humans and animals (Belenky et al., 2003; Carskadon and Dement, 1981; Kim et al., 2012; McCoy et al., 2013; Van Dongen et al., 2003). We recently reported that the brain adenosine system may mediate the continuous elevation in objective sleepiness observed during CSR in rats (Kim et al., 2012). However, it is still unknown what neurochemical mechanisms mediate the allostatic sleep responses, specifically the rapid adaptation of sleep time/intensity, observed during CSR.
Accumulating evidence suggests that locus coeruleus (LC) norepinephrine (NE) neurons may play a key role in regulating sleep duration and sleep intensity. For example, LC NE neurons in rodents stop firing before the transition from waking to sleep (Aston-Jones and Bloom, 1981) and before sleep-active neurons in the basal forebrain or preoptic hypothalamic neurons exhibit elevated discharge activities (Takahashi et al., 2010). These results suggest that sleep may be initiated by silencing wake-promoting neurons in the LC (Berridge, 2008; Takahashi et al., 2010). Even though electrical and pharmacological stimulation or inhibition of LC neurons alters arousal state, chemical or genetic ablation of LC NE neurons produces only small effects in sleep-wake amount, (Berridge, 2008; Blanco-Centurion et al., 2004; Gompf et al., 2010). This is likely due to compensation by other arousal promoting neuronal populations or within the NE system itself (Abercrombie and Zigmond, 1989; Harik et al., 1981), since maintaining wakefulness is critical for an animals’ survival. However, selective lesion of LC NE neurons using the neurotoxin DSP-4 reduces NREM delta power in low frequency ranges (< 1.5Hz) during subsequent recovery sleep after 6-h SD (Cirelli et al., 2005), implying that NE tone during wakefulness affects NREM delta power during subsequent sleep. A recent optogenetic study also confirmed that LC NE neurons are actively involved in modulating sleep/wake time and EEG power of different arousal states (Carter et al., 2010). Therefore, the combined evidence indicates that the NE system is a good candidate for a neuronal mechanism underlying the adaptive changes in sleep time and intensity observed in the CSR condition.
We investigated the time course of changes in adrenergic receptor (AR) mRNA levels before, during, and after 5 days of SR. The findings reveal that the pattern of brain AR mRNA changes in specific brain areas parallels the adaptive changes of sleep time/intensity observed in CSR, suggesting the norepinephrine system as a possible mediator of sleep allostasis.
2. Results
Following a 24-h baseline sleep (BL) day, each day rats underwent 18-h SD each day followed by 6-h sleep opportunity (SO). The SO was given during the first 6 h of the light period (10 AM to 4 PM, or zeitgeber time (ZT) 0-6). This SR protocol was repeated for 5 consecutive days, followed by 3 unrestricted recovery sleep days (R1-R3). Brain samples for mRNA analysis were collected at the light onset (i.e. immediately following 18-h SD on SR days) on 6 selected experimental days (BL, SR1, SR3, SR5, R1, R3).
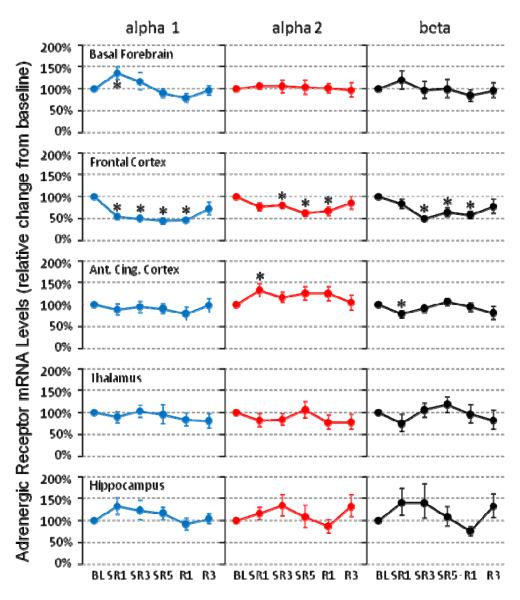
As shown in the Figure 1, α1-AR mRNA levels in the basal forebrain were significantly increased only on SR1 (+36%, N = 8, P = 0.025) and returned to the baseline level for remaining SR days as well as during recovery days. Similar single day changes were also observed for α2-AR (+33%, N = 12, P = 0.034) and β1-AR (-22%, N = 10, P = 0.047) in the anterior cingulate cortex. In contrast, the frontal cortex showed continuously reduced levels of all 3 subtypes of AR mRNA examined throughout SR days and R1 day [α1-AR (N = 8): −46% on SR1, −50% on SR3, −55% on SR5 and −53% on R1 (all P = 0.012); α2-AR (N = 9): −20% on SR3 (P = 0.028), −37% on SR5 (P = 0.008) and −33% on R1 (P = 0.038); β1-AR (N = 7): −50% on SR3 (P = 0.018), −37% on SR5 (P = 0.043) and −42% on R1 (P = 0.028)]. The other brain areas (i.e., hippocampus and thalamus) did not show any significant changes for any of the 3 AR mRNA levels examined.
Figure 1. Adrenergic alpha 1, alpha 2 and beta receptor mRNA levels during chronic sleep restriction.
The brain tissue was collected at the light onset, which is immediately after 18-h sleep deprivation on sleep restriction (SR) days. The time course of changes (mean ± s.e.m.) show 2 major patterns: allostatic (basal forebrain alpha 1 and anterior cingulate cortex alpha 2 and beta) and homeostatic (all 3 receptor types in the frontal cortex). The asterisk (*) indicates statistical significance (P < 0.05, N=7~12) compared to the BL.
The exercise control group (Fig. 2) showed significant increases in α2-AR mRNA levels only on SR1 in the basal forebrain (+22%, N = 7, P = 0.028). In the anterior cingulate cortex, significant increases were observed in α1-AR mRNA levels on SR3 (+25%, N = 8, P = 0.050), α2-AR (N = 8) on SR3 (+38%, P = 0.025), R1 (+67%, P = 0.018) and R3 (+61%, P = 0.050), and β1-AR on SR3 (+47%, N = 8, P = 0.017). However, there were no overlapping significant changes in AR mRNA levels between the CSR group and the exercise control group.
Figure 2. Adrenergic alpha 1, alpha 2 and beta receptor mRNA levels in the exercise control group.
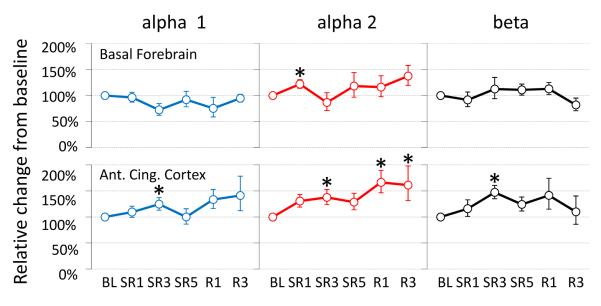
The brain tissue was collected at the light onset following the 30 min-on and 90 min-off cycle of sleep deprivation wheel rotation. The time course of changes (mean ± s.e.m.) show no specific patterns. The asterisk (*) indicates statistical significance (P < 0.05, N=7~8) compared to the baseline (BL).
3. Discussion
Selective changes of AR mRNA levels following 18 h of SD per day were observed only on the first SR day for α1-AR in the basal forebrain and for α2- and β1-AR in the anterior cingulate cortex. In contrast, the frontal cortex α1-, α2-, and β1-AR mRNA levels were reduced throughout the 5 days of SR. The thalamus and hippocampus did not show any significant changes at all. We have previously reported that during daily 6-h SO, significant compensatory increases in total sleep time and NREM delta power were observed only on the first SR day and were absent on SR days 2 to 5 (Kim et al., 2012). Taken together, these results suggest that alterations in the brain NE system in the basal forebrain and cingulate cortex may mediate allostatic changes in sleep time and intensity observed during CSR.
3.1. Homeostasis vs. allostasis
In everyday life, organisms exert a coordinated physiological process to maintain most stable states. This process is called “homeostasis” (Cannon, 1929), which is observed in various physiological systems such as pH, body temperature, glucose levels and daily sleep-wake amount. To survive, however, all organisms also need to actively adapt to changes in the environment, especially when the environmental changes are persistent. Animals change their morphology, physiology and behavior in response to seasonal change or as they go about their life cycle (McEwen and Wingfield, 2003). Achieving stability through change is called “allostasis” (Sterling and Eyer, 1988). Allostasis is a broader concept than simple ‘adaptation’ in that it includes both adaptive changes and the cost of adaptation to the body system. When environmental changes are unpredictable but long lasting, such as storms or natural disasters, animals experience chronic stress, which may induce a continuous high levels of the mediators of allostasis (including glucocorticosteroids, catecholamines and inflammatory cytokines), which can result in negative impacts on the body (McEwen and Wingfield, 2003). The cumulative cost to the body system in the process of adaptation is called “allostatic load” (McEwen, 1998). Continuous high level of allostatic load often leads to developing serious pathophysiology such as obesity, hypertension, neuronal death (McEwen and Wingfield, 2003). Recently, the existence of sleep allostasis during CSR has been reported (Deurveilher et al., 2012; Kim et al., 2007; Kim et al., 2012; McEwen, 2006).
3.2. Changes in receptor expression may reflect altered ligand levels
In this study, we observed that the AR mRNA levels were changed only on the SR day 1 and returned to the baseline level by SR day 3 for α1-AR in the basal forebrain and for both α2- and β1-AR in the anterior cingulate cortex (Fig. 1). It remains unknown precisely how CSR induces these allostatic changes in AR levels, especially in different brain areas. To postulate from existing lines of evidence, during wake or short-term SD, LC neurons increase their activity (Kalen et al., 1989; Shouse et al., 2000), which may in turn induce changes in its receptor number or affinity. Receptor desensitization or downregulation is very common in G-protein coupled receptors, which includes adrenergic and adenosine receptors (Grady et al., 1997; Sibley and Lefkowitz, 1985). Therefore, it is likely that NE release increases immediately when animals confronts a new SD condition, but the increased NE output rapidly returns to the baseline level as animals adapt to the repeated SR schedule. Returning of NE ligand level brings its receptor levels back to the baseline level too, as seen in the anterior cingulate cortex and the basal forebrain in this study (Fig. 1). In support of this model, 3 d of REM SD decreased NE tissue levels in the rat frontal cortex during the first 24 h and then returned to the baseline level (Porkka-Heiskanen et al., 1995). A similar adaptive pattern in the activation of LC neurons during 5 d of REM SD was also reported as assessed by c-Fos activity: significant increases on the SD day 1 and no difference on day 5 (Basheer et al., 1998). This highly adaptive characteristics of NE neurons may explain why many previous studies using 2 days or longer of selective REM or total SD protocols have failed to find alterations in NE ligand or receptor levels when these measures are assessed only after the long-term sleep loss (Abel et al., 1983; Bergmann et al., 1994; Brock et al., 1994; Mogilnicka, 1981; Radulovacki and Micovic, 1982; Tsai et al., 1993).
Norepinephrine acts through 3 major types of receptors in the brain: α1, α2 and β. In general, α1- (depolarizing, excitatory) and β-AR are present primarily postsynaptically whereas α2-AR (hyperpolarizing, inhibitory) exist both pre- and postsynaptically (Berridge et al., 2012; Jones, 2005). α1- and β-AR are suggested as wake-promoting while α2-ARs are sleep-promoting, especially in the preoptic-basal forebrain area (Berridge et al., 2012; Manns et al., 2003). We have observed that direction of change of β-AR and α2-AR are opposite in the anterior cingulate cortex: downregulation and upregulation, respectively. This is also consistent with the findings in adenosine receptors; downregulation of postsynaptic (excitatory) A2a receptors and upregulation of presynaptic (inhibitory) A1 receptors during CSR (Kim et al., 2012). However, the upregulation of α1-AR mRNA levels observed in the basal forebrain (Fig. 1) are in contrast to this pattern. Evidence suggests that α1-AR is wake-promoting in the medial preoptic area and medial septum area, but not in the substantia innominata (Berridge et al., 2012). Interestingly, α1-AR may also have potential sleep-promoting actions in the medial preoptic area since infusion of α1-AR antagonist increased wake amount (Kumar et al., 2006). Unfortunately, since the basal forebrain tissue collected in this study includes all 4 subdivisions of the basal forebrain (i.e., the medial septum, diagonal band of Broca, magnocellular preoptic nucleus, and substantia innominata), further studies are needed to find out basal forebrain subarea-specific changes in the AR density during CSR.
We found that the exercise control group did not show any specific pattern of changes in the basal forebrain and anterior cingulate cortex (Fig. 2), where we observed the distinct adaptive pattern of mRNA changes in the CSR group (Fig. 1). The reason why the exercise control group exhibited such an unspecific pattern of change is unknown. However, we rule out the possibility that it is due to the effect of locomotor activity because the exercise control group had experienced the exactly same distance of wheel rotation movement during 18-h SD (4-s on & 12-s off in CSR group vs. 30-min on & 90-min off in exercise control group).
3.3. Possible neurochemical mechanism of sleep allostasis
Our working hypotheses are that 1) NE release is homeostatically regulated in that elevated release of NE during the active period is followed by prolonged depletion during the inactive period, and 2) the NE tone during wakefulness affects the magnitude of sleep time and intensity during following sleep. When sleep time is reduced to only a few hours on the first SR day, we speculate that LC neuronal activity and NE release, during forced wakefulness, increase, which is counteracted by downregulation of NE receptors, as observed in β1-AR mRNA levels of the anterior cingulate cortex (Fig. 1). However, net NE tone (i.e., combination of ligand levels and receptor density) is still elevated as receptor downregulation can only partially offset the effects of elevated ligand levels. The elevated NE tone during wakefulness induces longer quiescence in LC neuronal activity during the following sleep opportunity, generating strong homeostatic sleep drive (HSD), expressed as a longer sleep time and higher sleep intensity. However, when SR is repeated the next day, we predict that less NE is released and less receptor downregulation occurs, leading to reduced HSD. From the third day, no significant changes in NE and AR receptor mRNA levels and HSD are observed. In other words, rats’ HSD has adapted to the new SR condition (i.e., sleep allostasis has occurred) by SR day 3. Interestingly, in humans, self-rated feeling of sleepiness also does not significantly increase after 3 days of SR (Belenky et al., 2003; Van Dongen et al., 2003), which indicates a similar time course of rapid adaptive changes.
The inhibitory neuromodulator adenosine is suggested as an endogenous sleep factor. During periods of spontaneous or forced wakefulness, extracellular adenosine levels rise in the basal forebrain, which inhibits wake-active neurons (Basheer et al., 2004). The repeated wake extensions and shorter sleep amount may induce cumulative increases in brain adenosine levels, especially in the basal forebrain and the frontal cortex, which is accompanied by continuous changes in adenosine receptor mRNA levels throughout 5 days of SR (Kim et al., 2012). CSR also induces a continuously high corticosterone level in the system, which may last more than a few weeks. For example, increases in the corticosterone level in chronically sleep restricted rats were highest on day 1 and persisted to day 6 although with a lesser degree (Roman et al., 2006), and were still elevated at weeks 4 and 11 (Zielinski et al., 2012).
Therefore, sleep allostasis may be very different from existing allostasis models reported in many other biological systems (McEwen, 1998) in that it has 2 distinct phases: rapid adaptation and slow adaptation. Animals rapidly adapt (i.e. within 3 days) their daily amount of sleep to a new sleep condition, stabilizing their sleep time and intensity to a new homeostatic set point. Meanwhile, reduced sleep amount-related stress may persist for several weeks or longer as indicated by prolonged elevated levels of corticosterone (Roman et al., 2006; Zielinski et al., 2012). The rapid adaptation of HSD appears to paradoxically cause animals not to utilize their limited sleep opportunity maximally to compensate for the sleep loss they experienced during the daily SD periods of CSR. This behavior is also observed in humans that people sacrifice their sleep amount to do pleasure-seeking activities such as drinking, smoking, eating, watching TV or playing games, which all can be interpreted as counter-stress behaviors (Dallman et al., 2003; Lazarus and Folkman, 1984; Sinha, 2008).
The persistent high level of mediators of allostasis, including corticosterone and possibly adenosine tone, results in cumulative increases in allostatic load to the animals, which includes increased objective sleepiness (Carskadon and Dement, 1981; Kim et al., 2012) and neuronal dendritic atrophy especially in the frontal cortex and hippocampus (McEwen, 2006; Meerlo et al., 2009). Throughout the 5 SR days, we have observed consistently low mRNA levels in most receptors that we measured in the frontal cortex: adenosine A2a (Kim et al., 2012) and NE α1, α2, and β1 receptors (Fig. 1). This might be explained as loss of synapses or diminished arborization of neurons in the frontal cortex as observed in chronic stress studies (McEwen, 1998; McEwen, 2006). However, we found no significant decreases in any receptor expression we measured in the hippocampus (Fig. 1 and Kim et al., 2012). Both increased objective sleepiness and neuronal atrophy in the frontal cortex may underlie the cumulative impairment in daily neurocognitive performance (Belenky et al., 2003; Van Dongen et al., 2003). Continuing SR without fully recovering sleep loss may ultimately lead to severe detrimental effects on health including hypertension, obesity, neuronal atrophy or loss, and even mortality (Dinges et al., 2005).
3.4. Sleep allostasis in Wistar-Kyoto rats
Adaptive changes in sleep time and intensity during CSR have been observed in most rat strains studied including Sprague-Dowley (Kim et al., 2012; Rechtschaffen et al., 1999), Wistar (Deurveilher et al., 2012; Lancel and Kerkhof, 1989), and F344 (Kim et al., 2007). Interestingly, such adaptive sleep responses to CSR were not observed in Wistar-Kyoto (WKY) rats (Leemburg et al., 2010). Our working model on the change of norepinephrine tone and HSD during CSR may explain why WKY rats (Leemburg et al., 2010) did not show such allostatic changes. Animals’ responses to a stressor consist of activation of 2 major body systems: the hypothalamic-pituitary-adrenocortical and sympatho-adrenomedullary axes. It is not clear yet whether circulating corticosteroids and adrenaline are directly associated with NREM delta power because adrenalectomy reduces baseline SWA (Bradbury et al., 1998) while SWA rebound following SD are intact in adrenalectomized rats (Bradbury et al., 1998) and mice (Mongrain et al.). However, stress-induced alterations in sleep duration and fragmentation are well known in humans and rodents (Mezick et al., 2009; Pawlyk et al., 2008). WKY rats are widely known to exhibit high susceptibility to stressor and inability to adapt to stress (Morilak et al., 2005; Pare, 1989; Pare and Redei, 1993; Redei et al., 1994). Thus, it is possible that that WKY rats do not adapt to repeated sleep restriction due to elevated norepinephrine tone (in addition to corticosterone) throughout the period of CSR especially when the sleep deprivation method is fairly stressful. For example, the disk-over-water technique used by Leemburg et al. (2010) is potentially more stressful than other sleep deprivation methods since it involved continuous arousal stimulation by human experimenters in addition to rats’ frequent falling into the water during the entire 20-h SD periods. Our model suggests that continuous high level of NE tone produced by stressful condition during SD periods results in elevated SWA during following sleep. Nonetheless, it remains important to determine at the mechanistic level why the sleep responses of WKY rats to CSR do not resemble those of other rat strains. For example, it would be very interesting to compare brain NE tone during CSR produced by different SD methods in WKY rats to find out the cause of the discrepancy in sleep responses.
3.5. Conclusions
Based on our previous CSR study (Kim et al., 2012) and the current study, we propose that allostatic sleep responses may be mediated by the NE system in a brain area and receptor subtype specific manner. The consequent allostatic load may be mediated by the adenosine system, in addition to other well known mediators of allostasis such as corticosterone and cytokines. Future studies are needed to confirm the functional importance of the mRNA changes observed in this study. Although changes in mRNA levels are not always reflected in actual changes in the receptor protein, acute sleep deprivation-induced changes in adenosine receptor mRNA and membrane receptor density have been shown to occur in parallel (Basheer et al., 2001; Basheer et al., 2005; Basheer et al., 2007). Also needed are follow-up studies to test if the NE system directly mediates or causally regulates the rapid adaptive changes in homeostatic sleep drive. Although further studies are needed, the present study is a key first step in revealing a possible neurochemical mechanism underlying sleep allostasis observed during CSR.
4. Experimental procedure
4.1. Subjects
Three-month-old male Sprague-Dawley rats (Charles River, Wilmington, MA, USA) were housed individually and maintained on a 12:12h light-dark cycle (light on at 10 AM) with free access to food and water. 120 rats in total were used in this study. All animal procedures were approved by the Institutional Animal Care and Use Committee of the VA Boston Healthcare System, and were in accordance with National Institutes of Health guidelines for the treatment of animals.
4.2. Sleep Deprivation
Animals (N=12) were sleep deprived by placing each animal in a periodically rotating wheel (product #80860, Lafayette Instrument Lafayette, IN, USA) programmed on a repeated cycle of 4-s on (3 m/min) and 12-s off during the daily 18-h periods of SD (Animal Wheel Monitor software, Lafayette Instrument, Lafayette, IN, USA). The exercise control group (N=8) was on 30-min on and 90-min off schedule to give an extended sleep opportunity with the same distance of wheel movement. Animals had free access to food and water throughout the SD and SO periods. After the daily 18-h SD period, animals were quickly returned to their sleep recording home cage for the 6-h SO.
4.3. qRT-PCR
Brain tissue samples were punched (2mm in diameter) from brain slices (2mm in thickness) for the 5 different brain areas. The brain coordinates (AP, ML, and DV in mm) were 4.5, 1.5, 2.0 for the frontal cortex; 0.5, 0.0, 2.0 for the anterior cingulate cortex; 0.5, 2.5, 8.5 for the basal forebrain; −2.5, 0.0, 5.5 for the thalamus; and the whole hippocampus (from −2.5 to −4.5 in AP). The RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcribed using Oligo(dT)20 and SuperScript III (Invitrogen). Real time PCR was performed using Taqman Gene Expression Assays (Applied Biosystems, Foster City, CA, USA) on rat α1-AR (Cat# Rn00567876_m1), α2-AR (Cat# Rn00562488_s1) and β1-AR (Cat# Rn00824536_s1), as well as beta-actin (Cat# 4352340E) to serve as an internal control to normalize RNA concentration variation among samples. Relative quantification was done using the comparative Ct method (ΔΔCt method) (Livak and Schmittgen, 2001). The fold-difference in the levels of mRNA expression was calculated as described previously (Chen et al., 2006). Individual samples were excluded from analysis based on the following exclusion criteria: 1) if the cDNA in the sample was too low to produce linear amplification within 35 PCR cycles, or, 2) if the mRNA levels assessed in the sample were regarded as an outlier (3 standard deviations away from a group mean). To avoid the potential confounding factor that PCR amplification efficiency varies between runs, samples from 5 different brain regions were grouped into matching sets and the same set was always run on the same plate. PCR is based on exponential amplification and the fold differences are not normally distributed. Hence, the Wilcoxon signed-rank test, a non-parametric paired test, was used to compare ΔΔCt values from experimental groups to the baseline values. Comparisons were considered significant if p < 0.05.
Highlights.
Changes in norepinephrine tone show an adaptive pattern during sleep restriction.
Changes in sleep time and intensity also showed an adaptive pattern.
Therefore, norepinephrine system may mediate sleep allostasis.
Acknowledgements
This research was supported by: Department of Veterans Affairs Medical Research Service Award (RES), HL060292 (RES & RWM), MH039683 & HL095491 (RWM), NHLBI - T32 HL07901 (YK). We thank Yunren Bolortuya for technical assistance and Drs. Radhika Basheer and Ritchie E. Brown for helpful discussions.
Abbreviations
- AR
adrenergic receptor
- BL
baseline
- CSR
chronic sleep restriction
- HSD
homeostatic sleep derive
- LC
locus coeruleus
- NE
norepinephrine
- NREM
non-rapid eye movement
- qRT-PCR
quantitative reverse transcription-polymerase chain reaction
- R
recovery sleep
- REM
rapid eye movement
- SD
sleep deprivation
- SO
sleep opportunity
- SR
sleep restriction
- WKY
Wistar-Kyoto
- ZT
zeitgeber time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: There are no conflicts of interest to disclose for any of the authors related to this work.
References
- Abel MS, Villegas F, Abreu J, Gimino F, Steiner S, Beer B, Meyerson LR. The effect of rapid eye movement sleep deprivation on cortical beta-adrenergic receptors. Brain Res Bull. 1983;11:729–34. doi: 10.1016/0361-9230(83)90015-1. [DOI] [PubMed] [Google Scholar]
- Abercrombie ED, Zigmond MJ. Partial injury to central noradrenergic neurons: reduction of tissue norepinephrine content is greater than reduction of extracellular norepinephrine measured by microdialysis. J Neurosci. 1989;9:4062–7. doi: 10.1523/JNEUROSCI.09-11-04062.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–86. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basheer R, Magner M, McCarley RW, Shiromani PJ. REM sleep deprivation increases the levels of tyrosine hydroxylase and norepinephrine transporter mRNA in the locus coeruleus. Brain Res Mol Brain Res. 1998;57:235–40. doi: 10.1016/s0169-328x(98)00088-6. [DOI] [PubMed] [Google Scholar]
- Basheer R, Halldner L, Alanko L, McCarley RW, Fredholm BB, Porkka-Heiskanen T. Opposite changes in adenosine A1 and A2A receptor mRNA in the rat following sleep deprivation. Neuroreport. 2001;12:1577–80. doi: 10.1097/00001756-200106130-00013. [DOI] [PubMed] [Google Scholar]
- Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–96. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Basheer R, Brown R, Ramesh V, Begum S, McCarley RW. Sleep deprivation-induced protein changes in basal forebrain: implications for synaptic plasticity. J Neurosci Res. 2005;82:650–8. doi: 10.1002/jnr.20675. [DOI] [PubMed] [Google Scholar]
- Basheer R, Bauer A, Elmenhorst D, Ramesh V, McCarley RW. Sleep deprivation upregulates A1 adenosine receptors in the rat basal forebrain. Neuroreport. 2007;18:1895–9. doi: 10.1097/WNR.0b013e3282f262f6. [DOI] [PubMed] [Google Scholar]
- Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, Russo MB, Balkin TJ. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- Bergmann BM, Seiden LS, Landis CA, Gilliland MA, Rechtschaffen A. Sleep deprivation in the rat: XVIII. Regional brain levels of monoamines and their metabolites. Sleep. 1994;17:583–9. doi: 10.1093/sleep/17.7.583. [DOI] [PubMed] [Google Scholar]
- Berridge CW. Noradrenergic modulation of arousal. Brain Res Rev. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Schmeichel BE, Espana RA. Noradrenergic modulation of wakefulness/arousal. Sleep Med Rev. 2012;16:187–97. doi: 10.1016/j.smrv.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Centurion C, Gerashchenko D, Salin-Pascual RJ, Shiromani PJ. Effects of hypocretin2-saporin and antidopamine-beta-hydroxylase-saporin neurotoxic lesions of the dorsolateral pons on sleep and muscle tone. Eur J Neurosci. 2004;19:2741–52. doi: 10.1111/j.1460-9568.2004.03366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury MJ, Dement WC, Edgar DM. Effects of adrenalectomy and subsequent corticosterone replacement on rat sleep state and EEG power spectra. Am J Physiol. 1998;275:R555–65. doi: 10.1152/ajpregu.1998.275.2.R555. [DOI] [PubMed] [Google Scholar]
- Brock JW, Farooqui SM, Ross KD, Payne S, Prasad C. Stress-related behavior and central norepinephrine concentrations in the REM sleep-deprived rat. Physiol Behav. 1994;55:997–1003. doi: 10.1016/0031-9384(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Cannon W. The wisdom of the body. Physiol Rev. 1929;9:399–431. [Google Scholar]
- Carskadon MA, Dement WC. Cumulative effects of sleep restriction on daytime sleepiness. Psychophysiology. 1981;18:107–13. doi: 10.1111/j.1469-8986.1981.tb02921.x. [DOI] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–33. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Thakkar MM, Winston S, Bolortuya Y, Basheer R, McCarley RW. REM sleep changes in rats induced by siRNA-mediated orexin knockdown. Eur J Neurosci. 2006;24:2039–48. doi: 10.1111/j.1460-9568.2006.05058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Huber R, Gopalakrishnan A, Southard TL, Tononi G. Locus ceruleus control of slow-wave homeostasis. J Neurosci. 2005;25:4503–11. doi: 10.1523/JNEUROSCI.4845-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci U S A. 2003;100:11696–701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deurveilher S, Rusak B, Semba K. Time-of-Day Modulation of Homeostatic and Allostatic Sleep Responses to Chronic Sleep Restriction in Rats. Am J Physiol Regul Integr Comp Physiol. 2012 doi: 10.1152/ajpregu.00678.2011. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Rogers NL, Baynard MD. Chronic sleep deprivation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Elsevier Saunders; Philadelphia: 2005. pp. 67–76. Vol. [Google Scholar]
- Gompf HS, Mathai C, Fuller PM, Wood DA, Pedersen NP, Saper CB, Lu J. Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment. J Neurosci. 2010;30:14543–51. doi: 10.1523/JNEUROSCI.3037-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady E, Bohm S, McConalogue K, Garland A, Ansel J, Olerud J, Bunnett N. Mechanisms attenuating cellular responses to neuropeptides: extracellular degradation of ligands and desensitization of receptors. J Investig Dermatol Symp Proc. 1997;2:69–75. doi: 10.1038/jidsymp.1997.14. [DOI] [PubMed] [Google Scholar]
- Harik SI, Duckrow RB, LaManna JC, Rosenthal M, Sharma VK, Banerjee SP. Cerebral compensation for chronic noradrenergic denervation induced by locus ceruleus lesion: recovery of receptor binding, isoproterenol-induced adenylate cyclase activity, and oxidative metabolism. J Neurosci. 1981;1:641–9. doi: 10.1523/JNEUROSCI.01-06-00641.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–86. doi: 10.1016/j.tips.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kalen P, Rosegren E, Lindvall O, Bjorklund A. Hippocampal Noradrenaline and Serotonin Release over 24 Hours as Measured by the Dialysis Technique in Freely Moving Rats: Correlation to Behavioural Activity State, Effect of Handling and Tail-Pinch. Eur J Neurosci. 1989;1:181–188. doi: 10.1111/j.1460-9568.1989.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Kim Y, Laposky AD, Bergmann BM, Turek FW. Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proc Natl Acad Sci U S A. 2007;104:10697–702. doi: 10.1073/pnas.0610351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Bolortuya Y, Chen L, Basheer R, McCarley RW, Strecker RE. Decoupling of Sleepiness from Sleep Time and Intensity during Chronic Sleep Restriction: Evidence for a Role of the Adenosine System. Sleep. 2012;35:861–9. doi: 10.5665/sleep.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar VM, Vetrivelan R, Mallick HN. Alpha-1 adrenergic receptors in the medial preoptic area are involved in the induction of sleep. Neurochem Res. 2006;31:1095–102. doi: 10.1007/s11064-006-9109-8. [DOI] [PubMed] [Google Scholar]
- Lancel M, Kerkhof GA. Effects of repeated sleep deprivation in the dark- or light-period on sleep in rats. Physiol Behav. 1989;45:289–97. doi: 10.1016/0031-9384(89)90130-3. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. Springer; New York, NY: 1984. Vol. [Google Scholar]
- Leemburg S, Vyazovskiy VV, Olcese U, Bassetti CL, Tononi G, Cirelli C. Sleep homeostasis in the rat is preserved during chronic sleep restriction. Proc Natl Acad Sci U S A. 2010;107:15939–44. doi: 10.1073/pnas.1002570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Manns ID, Lee MG, Modirrousta M, Hou YP, Jones BE. Alpha 2 adrenergic receptors on GABAergic, putative sleep-promoting basal forebrain neurons. Eur J Neurosci. 2003;18:723–7. doi: 10.1046/j.1460-9568.2003.02788.x. [DOI] [PubMed] [Google Scholar]
- McCoy JG, Christie MA, Kim Y, Brennan R, Poeta DL, McCarley RW, Strecker RE. Chronic sleep restriction impairs spatial memory in rats. Neuroreport. 2013;24:91–5. doi: 10.1097/WNR.0b013e32835cd97a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism. 2006;55:S20–3. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Mistlberger RE, Jacobs BL, Heller HC, McGinty D. New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med Rev. 2009;13:187–94. doi: 10.1016/j.smrv.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezick EJ, Matthews KA, Hall M, Kamarck TW, Buysse DJ, Owens JF, Reis SE. Intra-individual variability in sleep duration and fragmentation: associations with stress. Psychoneuroendocrinology. 2009;34:1346–54. doi: 10.1016/j.psyneuen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogilnicka E. REM sleep deprivation changes behavioral response to catecholaminergic and serotonergic receptor activation in rats. Pharmacol Biochem Behav. 1981;15:149–51. doi: 10.1016/0091-3057(81)90355-5. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Hernandez SA, Pradervand S, Dorsaz S, Curie T, Hagiwara G, Gip P, Heller HC, Franken P. Separating the contribution of glucocorticoids and wakefulness to the molecular and electrophysiological correlates of sleep homeostasis. Sleep. 33:1147–57. doi: 10.1093/sleep/33.9.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–24. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Pare WP. Stress ulcer susceptibility and depression in Wistar Kyoto (WKY) rats. Physiol Behav. 1989;46:993–8. doi: 10.1016/0031-9384(89)90203-5. [DOI] [PubMed] [Google Scholar]
- Pare WP, Redei E. Depressive behavior and stress ulcer in Wistar Kyoto rats. J Physiol Paris. 1993;87:229–38. doi: 10.1016/0928-4257(93)90010-q. [DOI] [PubMed] [Google Scholar]
- Pawlyk AC, Morrison AR, Ross RJ, Brennan FX. Stress-induced changes in sleep in rodents: models and mechanisms. Neurosci Biobehav Rev. 2008;32:99–117. doi: 10.1016/j.neubiorev.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Smith SE, Taira T, Urban JH, Levine JE, Turek FW, Stenberg D. Noradrenergic activity in rat brain during rapid eye movement sleep deprivation and rebound sleep. Am J Physiol. 1995;268:R1456–63. doi: 10.1152/ajpregu.1995.268.6.R1456. [DOI] [PubMed] [Google Scholar]
- Radulovacki M, Micovic N. Effects of REM sleep deprivation and desipramine on beta-adrenergic binding sites in rat brain. Brain Res. 1982;235:393–6. doi: 10.1016/0006-8993(82)91019-8. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Bergmann BM, Gilliland MA, Bauer K. Effects of method, duration, and sleep stage on rebounds from sleep deprivation in the rat. Sleep. 1999;22:11–31. doi: 10.1093/sleep/22.1.11. [DOI] [PubMed] [Google Scholar]
- Redei E, Pare WP, Aird F, Kluczynski J. Strain differences in hypothalamic-pituitary-adrenal activity and stress ulcer. Am J Physiol. 1994;266:R353–60. doi: 10.1152/ajpregu.1994.266.2.R353. [DOI] [PubMed] [Google Scholar]
- Roman V, Hagewoud R, Luiten PG, Meerlo P. Differential effects of chronic partial sleep deprivation and stress on serotonin-1A and muscarinic acetylcholine receptor sensitivity. J Sleep Res. 2006;15:386–94. doi: 10.1111/j.1365-2869.2006.00555.x. [DOI] [PubMed] [Google Scholar]
- Shouse MN, Staba RJ, Saquib SF, Farber PR. Monoamines and sleep: microdialysis findings in pons and amygdala. Brain Res. 2000;860:181–9. doi: 10.1016/s0006-8993(00)02013-8. [DOI] [PubMed] [Google Scholar]
- Sibley DR, Lefkowitz RJ. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985;317:124–9. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–30. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of life stress, cognition and health. John Wiley; New York: 1988. pp. 629–49. Vol. [Google Scholar]
- Takahashi K, Kayama Y, Lin JS, Sakai K. Locus coeruleus neuronal activity during the sleep-waking cycle in mice. Neuroscience. 2010;169:1115–26. doi: 10.1016/j.neuroscience.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Tsai LL, Bergmann BM, Perry BD, Rechtschaffen A. Effects of chronic total sleep deprivation on central noradrenergic receptors in rat brain. Brain Res. 1993;602:221–7. doi: 10.1016/0006-8993(93)90686-h. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Zielinski MR, Davis JM, Fadel JR, Youngstedt SD. Influence of chronic moderate sleep restriction and exercise on inflammation and carcinogenesis in mice. Brain Behav Immun. 2012;26:672–9. doi: 10.1016/j.bbi.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]