SYNOPSIS
Cytomegalovirus (CMV) is the most common congenital viral infection in the developed world, with an overall birth prevalence of approximately 0.6%. Approximately 10% of congenitally infected infants have signs and symptoms of disease at birth, and these symptomatic infants have a high risk for demonstration of subsequent neurologic sequelae, including sensorineural hearing loss (SNHL), mental retardation, microcephaly, development delay, seizure disorders, and cerebral palsy. Antiviral therapy of children with symptomatic central nervous system (CNS) congenital CMV infection is effective at reducing the risk of long-term disabilities and should be offered to families with affected newborns. An effective pre-conceptual vaccine against CMV could, by preventing congenital infection, protect against long-term neurological sequelae and other disabilities. A variety of active and passive immunization strategies are in clinical trials and are likely to be licensed in the next few years. Until a vaccine is licensed, preventive strategies aimed at reducing transmission should be emphasized and public awareness increased, particularly among women of child-bearing age.
Keywords: Congenital cytomegalovirus (CMV) infection, Antiviral therapy, Newborn screening, Sensorineural hearing loss, CMV vaccines
INTRODUCTION
Cytomegalovirus (CMV) is a ubiquitous herpesvirus spread by close interpersonal contact through saliva, blood, genital secretions, urine, or breast milk that infects up to 90% of the US population by the 8th decade of life and establishes lifelong latency in monocytes and granulocytes.1-3 Maternal transmission to the fetus of a new or reactivated latent infection may occur at any gestation, leading to congenital CMV. About 20,000-40,000 infants per year in the United States are born with congenital CMV infection, with a corresponding incidence of 0.6 - 0.7% of all deliveries of the developed world, making CMV the most common congenital viral infection.4-7 CMV is mostly asymptomatic or mildly symptomatic in infants, children, and adults, however can be devastating to immunocompromised hosts including infected newborns, and results in the greatest long-term neurodevelopment morbidity of all the perinatally acquired viral infections. The most frequent sequel is sensorineural hearing loss (SNHL), of which CMV is the leading nonhereditary cause overall.8, 9
Despite its frequency and substantial consequences, congenital CMV is less known to the general population compared to other conditions with lesser prevalence such as Down Syndrome, fetal alcohol syndrome, and spina bifida. This lack of awareness is problematic given that the only way currently to prevent fetal infection is by prevention, with good hygeine practices with hand washing and avoidance of potential sources of CMV. This review summarizes the current state of knowledge regarding the epidemiology, pathogenesis, diagnosis, treatment, and prognosis of congenital CMV infection. New and emerging strategies for prevention and therapy are emphasized, including vaccines currently in clinical trials. Key principles of management, including appropriate use of consultants, are summarized. Finally, potential resources for parents raising a child with symptomatic congenital CMV infection are provided.
EPIDEMIOLOGY
Cytomegalovirus is a ubiquitous infection and most individuals are eventually exposed to this agent. There is no seasonality to infection. Patient populations with an increased incidence of primary infection include breastfeeding infants, toddlers and care providers in group daycare, and sexually active adolescents._ENREF_1010-16 CMV infections are generally asymptomatic in immunocompetent individuals, but may produce a heterophile-negative mononucleosis syndrome in approximately 10% of primary infections in older children and adults.17 CMV seroprevalence demonstrates striking geographic and racial variation, and tends to be highest in South America, Africa and Asia and lowest in Western Europe and United States. Seroprevalence is higher among non-whites and among individuals of lower socioeconomic status.18, 19 The factors responsible for geographic and racial variation in seroprevalence remain incompletely understood.
The biggest risk factor for CMV transmission in women of reproductive age is exposure to urine and saliva of young children, and mothers of children who are shedding CMV are ten times more likely to seroconvert than women in other comparison groups.12, 20, 21 Children in group daycare represent a particularly important reservoir of CMV. It is postulated that maternal CMV infection could be prevented during pregnancy through education and behavioral changes, however many women have not heard of CMV, obstetricians may not discuss CMV prevention with their patients, and these opportunities are missed.20 A lack of public awareness of CMV is a major barrier to disease control. A recent study, using a national mail survey designed to be similar to the US population, showed that awareness of CMV was very low.20 Only 7% of men and 13% of women had heard of congenital CMV. Awareness of other congenital infections, such as rubella and toxoplasmosis, was slightly greater despite their lesser prevalence. This lack of awareness is particularly troubling given that CMV is fairly easy to inactivate through simple hand-washing interventions.22
CONGENITAL AND PERINATAL CMV INFECTIONS: CLINICAL PRESENTATION
CMV infection may be acquired in the newborn via congenital, intrapartum and antenatal routes of infection. Infection of the newborn may occur secondary to exposure to CMV-infected cervical secretions during vaginal delivery or via ingestion of CMV-infected breast milk, but these types of infections rarely result in significant symptoms or sequelae in term babies.23 Post-natal acquisition of CMV has little significance, is not associated with long-term disability, and rarely causes clinical signs of any illness in term infants. The exception is low-birth weight premature infants. Premature babies appear to be at particularly high risk for CMV-associated disease. These infants may additionally have symptoms of worsening respiratory status, neutropenia, or septic appearance (with apnea, bradycardia, pallor, and bowel distention) at the onset of infection, regardless of whether the virus was acquired postnatally from human milk or transfusions.24, 25 For premature infants acquiring infection postnatally, CMV’s ability to cause long-term sequelae independent from prematurity remains unclear, though minor effects on motor development have been suggested.25-27 A recent study suggested that postnatal CMV infection of preterm infants did not result in an increased risk of SNHL.28
Congenital CMV occurs transplacentally and may result in symptomatic or asymptomatic infection in the neonate. The likelihood of fetal transmission and symptomatic disease is much greater during a primary maternal CMV infection. It is estimated that 1-4% of CMV seronegative mothers will become infected during pregnancy, and 30-40% of these infected women will transmit virus to the fetus. Non-primary maternal CMV infections can also result in fetal transmission. These infections may represent reactivated latent infection or reinfection with a new strain in seropositive women. Currently it is estimated that 10-30% of women with preconception immunity become re-infected, and 1-3% will transmit to the fetus.5, 6, 19, 29, 30 Symptoms of disease in the newborn and long-term neurodevelopmental sequelae can occur after transmission in the setting of primary or recurrent infection. Symptoms occur in 11% - 12.7% of all neonates with congenital CMV according to 2 recent meta-analyses.4, 5 Clinical findings include IUGR, hydrops, generalized petechiae, purpura, thrombocytopenia, jaundice, hepatosplenomegaly, pneumonitis, microcephaly, periventricular calcifications, seizures, chorioretinitis, sensorineural hearing loss, bone abnormalities, abnormal dentition, and hypocalcified enamel. Table 1 summarizes the frequencies of these findings as noted in a review of 106 infants with symptomatic congenital CMV infection, as well as the most common associated laboratory abnormalities.31
Table 1.
Clinical and laboratory abnormalities in symptomatic congenital CMV infection. Data from: Boppana SB, Pass RF, Britt WJ, et al. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J 1992;11:93.
| Clinical and Laboratory Abnormalities in Symptomatic Congenital CMV Infection | |
|---|---|
| Clinical | Finding Frequency (%) |
| Petechiae | 76 |
| Neurologic- one or more of the following: | 68 |
| Microcephaly | 53 |
| Lethargy/hopotonia | 27 |
| Poor suck | 19 |
| Seizures | 7 |
| Jaundice | 67 |
| Hepatosplenomegaly | 60 |
| Small for Gestational Age (Weight <10 percentile) | 50 |
| Prematurity (<38 weeks gestation) | 34 |
| Clinical Finding | Frequency (%) |
| Elevated ALT (>80 units/L) | 83 |
| Thrombocytopenia: | |
| <100 × 10 3/mm 3 | 77 |
| <50 × 10 3/mm 3 | 53 |
| Conjugated hyperbilirubinemia: | |
| Direct bilirubin >2 mg/dL | 81 |
| Direct bilirubin >4 mg/dL | 69 |
| Hemolysis | 51 |
| Increased CSF protein (>120 mg/dL) | 46 |
The differential diagnosis of congenital CMV includes other congenital viral infections, toxoplasmosis, and syphilis, given that many of the presenting symptoms are nonspecific. For example, rubella may also present with petechiae, bony defects, and sensorineural hearing loss.32 Neonatal enteroviral infections, particularly infections with the recently described parechoviruses, can be associated with fetal brain injury and long-term sequalae.33 Neonatal HSV infection may present with seizures, parvovirus B19 with hepatomegaly and anemia, and lymphocytic choriomeningitis virus with microcephaly, chorioretinitis and intracranial calcifications.34
Long-term sequelae occur following both symptomatic and asymptomatic congenital infections, with the more frequent and severe sequelae occurring in symptomatic infants. It has been estimated that 40-58% of infants who are symptomatic at birth go on to develop sequelae4, and these may include sensorineural hearing loss, vision loss, mental retardation, seizure disorder, cerebral palsy, visual deficits, or developmental delay.31, 35, 36 Approximately 13.5% of the asymptomatic neonates may still go on to develop neurodevelopment injury, which is most commonly manifest as hearing loss.4 Hearing loss is most common when CMV infection occurs in the first or second trimester.37, 38 Sensorineural hearing loss following symptomatic or asymptomatic congenital infection is often progressive, can be unilateral or bilateral, and may be absent at birth, only to become clinically manifest later in childhood.39-43 About 21% of all hearing loss at birth and 25% of hearing loss at 4 years of age is attributable to congenial CMV infection; therefore, these children require regular hearing evaluations and early intervention.44
DIAGNOSTIC EVALUATION
There is no universal screening for CMV in mothers or newborns. Pregnant mothers can be diagnosed by seroconversion from IgG -negative to IgG-positive status, or by positive IgM if confirmed with low-avidity IgG (IgM may remain positive for 6-9 months after the end of acute phase infection).30 Fetal infection is diagnosed by positive viral culture or PCR from amniotic fluid. Diagnosis in the neonate is made by viral detection in body fluids via PCR, culture, or antigen testing (pp65 antigen) within the first 3 weeks of life.45 The finding of CMV antibodies or viral DNA after this point makes congenital versus postnatally acquired infection difficult to distinguish. Antibody titers cannot reliably make the diagnosis as maternal CMV IgG crosses the placenta, and neonates mount weak IgM responses. The preferred specimens are saliva and urine as newborns shed high levels of the virus from these fluids. Saliva samples may be more easily obtained and have been shown to be as reliable as urine samples in diagnosing CMV, so some propose that saliva PCR should be considered the investigation of choice. 46-48
For the primary care clinician, having an appropriate index of suspicion is key. In addition to the signs, symptoms and laboratory abnormalities noted in Table 1, CMV diagnostic studies should be considered in infants with more subtle potential manifestations of illness, such as subtle growth retardation, or a failed newborn hearing screen. Once the diagnosis is confirmed, further laboratory tests, imaging, and eye and hearing assessments are indicated. CBC and LFTs may reveal pancytopenia and hepatitis, and coagulation studies bay be abnormal in the setting of hepatitis. Renal function is checked as a baseline prior to beginning treatment with ganciclovir (see below). Neuroimaging assessment: available techniques include cerebral ultrasound, CT, and MRI for suspected or proven congenital infection. Lesions that occur before 19 weeks post menstrual age include lissencephaly with a thin cortex, cerebellar hypoplasia, ventriculomegaly, periventricular calcification and delayed myelination. At 18-24 weeks, migrational abnormalities may occur such as polymicrogyria, occasionally schizencephaly, periventricular cysts and cerebellar hypoplasia. CNS lesions may include delayed myelination, dysmyelination and white matter disease. In all cases, calcification is a common finding. A range of seizure disorders, including infantile spasm, have been described, and ongoing consultation with a pediatric neurologist may be necessary.49 Cranial ultrasound is a good screening tool, with subsequent MRI being recommended for definitive evaluation (Figure 1).50, 51 Ophthalmologic assessment should be performed on all infants with congenital CMV infection. Ophthalmological signs are seen in a large percentage of symptomatic infants and include chorioretinitis, optic atrophy, and cortical visual impairment.52 Strabmismus is also a common long-term ophthalmologic complication.53 Audiological assessment should be performed on all infants with congenital CMV infection: as noted, SNHL may be absent at birth, and progressive in nature, and frequent evaluations are required throughout childhood to evaluate for the possibility of hearing deterioration.39 At a minimum, audiological assessment should be performed every 6 months for the first three years of life, and annually thereafter. For children with severe-to-profound hearing loss caused by congenital CMV, cochlear implantation is a successful intervention.54, 55 Hypoplasia and hypocalcification of tooth enamel is common in children with congenital CMV infection56, and regular dental visits are an important component of the long-term care of these infants. Children with evidence of cerebral palsy may require consultative care from a clinician expert in the management of this disorder. A number of suggested diagnostic studies and potential specialty referrals are noted in Table 2; however, it should be noted that the range of management issues for any given child may be quite variable, and not all children with congenital CMV will require all of these services.
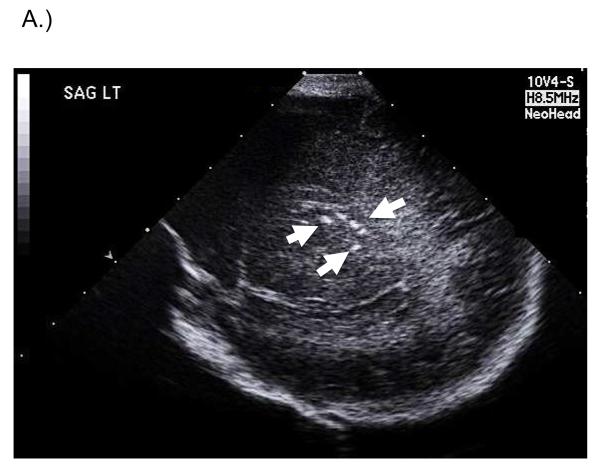
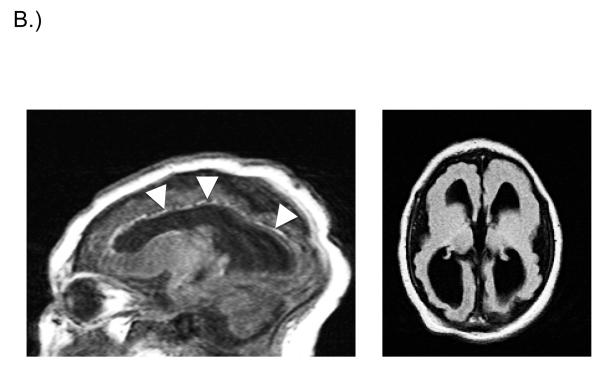
Fig. 1.
A.) Head ultrasound (saggital view) from newborn infant with symptomatic congenital CMV infection. Intracranial and periventricular calcifications are noted (arrows). This infant went on to have severe bilateral SNHL and other developmental disabilities. B.) Brain MRI of infant with congenital CMV infection. Fetal CMV infection was first identified in utero and immune globulin therapy commenced. Saggital (left panel) and axial (right panel) views are demonstrated. 1.5 Tesla, T1-weighted images demonstrate ventriculomegaly, periventricular calcifications, marked loss of brain volume with reduced white matter, pachygyria and lissencephaly on the surface, and very thin cortex. Saggital view demonstrates calcifications most clearly (arrowheads). This infant went on to manifest a seizure disorder and moderate bilateral SNHL.
Table 2.
Potential diagnostic studies and subspecialty consultations in the management of congenital CMV infection. Not all children with congenital CMV will require all of these studies. The need for specific studies will be guided by the clinical picture in an individual child.
| Diagnostic Assessment and Subspecialty Consultations in Infants with Suspected Congenital CMV Infection |
|---|
|
|
| Potential Diagnostic Studies |
| • Diagnostic virology |
| • PCR and/or culture of infant urine, blood and saliva |
| • Specimens must be obtained prior to day 21 of life to confirm congenital infection (versus post-natal acquisition) |
| • Neurodiagnostic imaging |
| • Head ultrasound good screening exam in neonatal period |
| • MRI of brain more definitive for symptomatic/affected infants |
| • Ophthalmological evaluation |
| • Audiological evaluation |
| • Newborn hearing screening in nursery |
| • Definitive auditory evoked response (ABR) on follow-up evaluation |
| • CBC, platelet counts, transaminases, bilirubin for symptomatic infants |
| • EEG if seizures clinically evident or suspected |
|
|
| Potential Consultants |
|
|
| • Audiology |
| • Otolaryngology |
| • Pediatric Infectious Disease |
| • Neurology |
| • Physical Medicine/rehabilitation |
| • Orthopedics |
| • Developmental Pediatrics |
| • Pediatric Ophthalmology |
| • Pediatric Dentistry |
TREATMENT
Treatment of congenital CMV infection with antivirals should be instituted in infants with evidence of central nervous system (CNS) involvement, including SNHL, and should be considered in infants with serious end-organ disease (hepatitis, pneumonia, thrombocytopenia). The cornerstone of antiviral therapy is ganciclovir, which was the first compound licensed specifically for treatment of CMV infections. Ganciclovir is a synthetic acyclic nucleoside analog, structurally similar to guanine. Its structure is similar to that of acyclovir, and, like acyclovir, it requires phosphorylation for antiviral activity. Following phosphorylation by a viral protein known as UL97, cellular enzymes phosphorylate the monophosphate form to di- and tri-phosphate metabolites; the ganciclovir triphosphate metabolite then exerts its antiviral effect in the CMV-infected cell. The first reports of the use of ganciclovir therapy for congenital CMV infection date to the late 1980s57. In subsequent reports,58, 59 ganciclovir has been shown to be generally safe and well-tolerated when used in newborns, and has appeared to be useful in the management of severe, focal, end-organ disease in infants. It is important to note that no sustained effect on CMV shedding at mucosal sites can be expected: once therapy is completed, infants resume excreting of CMV in urine and saliva.
It has become increasingly clear that ganciclovir also provides long-term neurodevelopmental benefit for some infants with congenital CMV infection, as demonstrated in a phase III randomized double-blind study of parenteral ganciclovir in neonates with symptomatic congenital CMV infection.60 This study indicated that 84% of 25 ganciclovir recipients either had improved hearing, or maintained normal hearing between baseline and 6 months. In contrast, only 59% of 17 control patients had improved or stable hearing (p=.06). Results were even more encouraging when the study and control groups were compared for subsequent maintenance of normal hearing. None (0%) of 25 ganciclovir recipients had worsening in hearing between baseline and 6 month follow-up, compared to 7 (41%) of 17 control patients (p<.01). The study further examined whether a therapeutic benefit was noted after 12 months of follow-up. Among 43 patients who had a BSER at both baseline and at 1 year or beyond, five (21%) of 24 ganciclovir recipients had worsening of hearing, versus 13 (68%) of 19 control patients (p<.01). A subsequent report compared long-term neurodevelopmental outcomes in infants treated with GCV and untreated infants, using the Denver Developmental Screening Test.61 This study indicated that receiving intravenous ganciclovir therapy had fewer developmental delays at 6 and 12 months compared with untreated infants.
Based on these data as well as the data regarding ganciclovir treatment and hearing outcomes, 6 weeks of intravenous ganciclovir therapy is recommended in the management of babies with symptomatic congenital CMV disease involving the CNS (Figure 2). Treatment should be initiated within the first month of life. Infants need to be monitored closely for toxicity, especially neutropenia, which may be observed in up to 60% of infants on long-term therapy.62 Care must be taken, and dosage adjustments made, when treating infants with impaired renal function.63 Treatment of infants with ganciclovir should be approached with realistic expectations, and should be undertaken with the assistance of an expert familiar with the use of this medication in infants. The risk of toxicity needs to be explained to parents, and it must be stressed that ganciclovir will not reverse established CNS injury. If neutropenia occurs on therapy, human granulocyte colony-stimulating factor therapy can be administered, and is usually effective in restoring an adequate neutrophil count, such that therapy may be continued. Ganciclovir or valganciclovir (if able to take enteral medication, see below) may also be considered in neonates with symptomatic end-organ disease other than CNS disease (hepatitis, pneumonia, thrombocytopenia), but the efficacy of treatment for non-CNS symptomatic congenital CMV infection has not been assessed large multi-center studies (Figure 2).
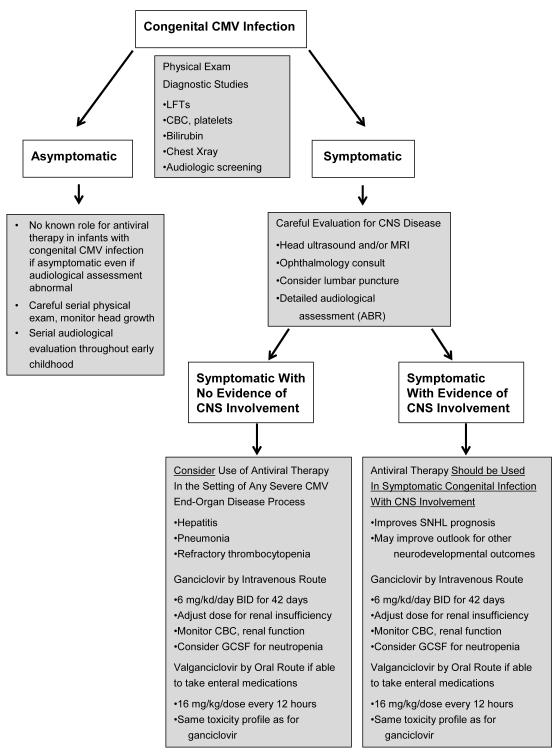
Fig. 2.
Management strategies for congenital CMV infection. Congenital CMV infection may be either asymptomatic or symptomatic at birth. Asymptomatic congenital infection is rarely recognized (since there is seldom any clinical impetus to evaluate an asymptomatic newborn), but may be diagnosed as in incidental finding, or because of concern regarding a primary maternal infection during pregnancy. It is possible that more asymptomatic congenital infections will be recognized in the future because of ongoing programs evaluating the potential value of performing universal CMV screening on all newborns.46 Such infants require frequent audiological screening, but are not known to benefit from antiviral therapy. Infants with symptomatic congenital CMV infection should be evaluated for CNS involved (by ophthalmological evaluation, CNS imaging studies, audiological evaluation, and when feasible lumbar puncture). If CNS involvement is noted, six weeks of therapy with ganciclovir is known to improve hearing, and possibly neurodevelopmental, outcomes. Oral valganciclovir is an alternative. Infants with symptomatic infection without CNS involvement are not known to benefit from antiviral therapy.
An alternative to intravenous ganciclovir for neonates who can take enteral medication is the use of its oral prodrug, valganciclovir. This approach is attractive insofar as it obviates the need for placement of a central venous catheter for six weeks of intravenous therapy. Valganciclovir is very well absorbed following oral administration. It is rapidly metabolized following oral dosing into ganciclovir. A suspension formulation is licensed and available, and although not licensed for the treatment of congenital CMV, its use can be considered as an alternative to intravenous therapy. Studies in neonates have demonstrated stable drug levels following oral dosing.63, 64 Currently, data from a clinical trial of 6 weeks versus 6 months of valganciclovir performed by the Collaborative Antiviral Study Group is being analyzed, toward the goal of determining whether long-term therapy confers additional neurodevelopmental benefits to infants.62 Although anecdotal reports from uncontrolled studies suggest that long-term oral therapy is well tolerated and possibly effective65, 66, there is insufficient evidence at this point to recommend a long-term (6 months) course of therapy of infants with congenital CMV infection. Similarly, it remains unclear if treatment initiated beyond the neonatal period provides any benefit with respect to neurodevelopmental outcomes, although additional studies of this question are warranted.
Consensus guidelines for antiviral management of congenital CMV have not been formulated in the US, though some recommended guidelines from Europe have been published with proposed treatment algorithms (Figure 2).48, 67 Other antiviral agents are available for CMV infection, including drugs such as foscarnet and cidofovir68, but there is very little experience with the use of these agents in infants, and at the current time their usefulness is limited to exceptional circumstances, such as the emergence of antiviral resistance to ganciclovir and/or in the treatment of immunocompromised infants with serious CMV end-organ disease.69
PROSPECTS FOR PREVENTION: ACTIVE AND PASSIVE IMMUNIZATION
Development of a CMV vaccine is the most promising strategy for addressing the problem of congenital CMV. An effective vaccine could, by preventing neurological sequelae and other disabilities, provide a newborn with a lifetime of benefit. A report from the Institute of Medicine of the National Academy of Sciences placed CMV in its highest priority category for vaccine development, concluding that a vaccine would be strongly cost saving.70 Several CMV vaccines are currently being evaluated in a number of clinical trials. A live, attenuated strain of CMV, the Towne strain, has been evaluated as a potential vaccine in a number of studies, including several studies in immunocompromised solid organ transplant patients at risk for CMV disease.71-73 Although Towne vaccine has elicited humoral and cellular immune responses in these studies and demonstrated an effect on CMV disease, its potential as a vaccine against congenital CMV infection was called into question in a study of young women with children attending group day care. In this study, Towne vaccine showed no reduction in the infection rate of Towne-vaccinated mothers compared with placebo-inoculated mothers.74 An approach to improve the immunogenicity of a live virus CMV vaccine has recently been undertaken by engineering recombinant ‘chimeras’ of the attenuated Towne strain and the less attenuated, low-passage Toledo strain, and these vaccines are also in clinical trials.75 In addition to live virus vaccines, purified protein and DNA subunit vaccines are also in clinical trials.76 These vaccines focus on the virally-encoded proteins that are the key targets of both the humoral and cellular immune response to CMV. A vaccine based on the immunodominant CMV envelope glycoprotein, gB, was recently studied in adolescent and young adult women, with the primary end-point reported in this study being time to primary CMV infection. Primary CMV infection was confirmed in 8% in the vaccine group, compared to 14% in the placebo group, an overall efficacy of 50%.77 Additional evidence of the efficacy of the gB subunit vaccine was demonstrated in a placebo-controlled phase II study in solid organ transplant recipients.78 Ongoing and future clinical trials will hopefully lead to the licensure of a CMV vaccine in the not-to-distant future.
In addition to active immunization strategies, passive immunization, based on administration of anti-CMV immune globulin to women at risk of transmitting CMV to the fetus, is currently an intensely active area of clinical research. In a study of pregnant women with a primary CMV infection, whose amniotic fluid contained either CMV or CMV DNA, subjects were offered intravenous CMV hyperimmune globulin (HIG), in two different dose regimens, a “therapy” regimen or a “prevention” regimen.79 In the therapy group, only 1/31 women gave birth to an infant with CMV disease (defined as an infant who was symptomatic at birth and handicapped at two or more years of age), compared with 7 of 14 women in an untreated control group. Similar benefits were noted in the prevention group. The administration of HIG to women in the primary infection group was associated with significant reductions in placental pathology, and with regression of cerebral structural abnormalities in some infants.80, 81 Another retrospective, observational study of HIG reported a trend toward reduced intrauterine transmission of CMV.82 The use of HIG during pregnancy has also been reported to be associated with improved neurodevelopmental outcomes in infants in the first year of life.83 Randomized controlled trials of HIG are warranted in high-risk pregnancies, to validate the protective effect of passive immunization.
SUMMARY
Congenital CMV infection is common and under-recognized. Pediatricians and primary care physicians should be familiar with maternal risk factors and clinical clues in newborns that might suggest the diagnosis of congenital infection. Increased public awareness is needed, particularly among women of child-bearing age. Therapeutic options are available, both for women who have transmitted CMV to the developing fetus, as well as for symptomatic newborns. Children with congenital CMV infection are at risk for adverse neurodevelopmental outcomes, particularly SNHL. The pediatrician plays an essential role in the long-term anticipatory management of children with congenital CMV infection. Progress toward development of a CMV vaccine has accelerated. Eventual licensure of a vaccine coupled with increased recognition of the importance of this common and disabling disease will make inroads into reducing the impact of this virus on the health and well-being of children. Parents of children with congenital CMV have formed support groups and created web sites for fostering knowledge and awareness (e.g., www.buckbuck.org; www.congenitalcmvfoundation.org; www.averysjourney.com; www.stopcmv.org) and these organizations can be a useful source of information for parents and clinicians alike who are seeking answers regarding this major public health problem.
KEY POINTS.
In the developed world, CMV is the most common congenital viral infection, with an overall birth prevalence of approximately 0.6%.
Approximately 10% of congenitally infected infants have signs and symptoms of disease at birth, and these symptomatic infants have a high risk for demonstration of subsequent neurologic sequelae, including sensorineural hearing loss (SNHL), mental retardation, microcephaly, development delay, seizure disorders, and cerebral palsy.
The public health impact of congenital CMV infection is substantial and under-recognized.
Antiviral therapy of children with symptomatic central nervous system (CNS) congenital CMV infection is effective at reducing the risk of long-term disabilities and should be offered to families with affected newborns.
An effective pre-conceptual vaccine against CMV could, by preventing congenital infection, protect against long-term neurological sequelae and other disabilities. A variety of active and passive immunization strategies are in clinical trials and are likely to be licensed in the next few years. Until a vaccine is licensed, preventive strategies aimed at reducing transmission should be emphasized and public awareness increased, particularly among women of childbearing age.
Acknowledgments
SUPPORT Support from NIH HD044864, HD038416 is acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Staras SA, Dollard SC, Radford KW, et al. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2006;43(9):1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 2.Kondo K, Xu J, Mocarski ES. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. Proc Natl Acad Sci U S A. 1996;93(20):11137–11142. doi: 10.1073/pnas.93.20.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hargett D, Shenk TE. Experimental human cytomegalovirus latency in CD14+ monocytes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(46):20039–20044. doi: 10.1073/pnas.1014509107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17(5):355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 5.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 6.Nyholm JL, Schleiss MR. Prevention of maternal cytomegalovirus infection: current status and future prospects. Int J Womens Health. 2010;2:23–35. doi: 10.2147/ijwh.s5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung AK, Sauve RS, Davies HD. Congenital cytomegalovirus infection. J Natl Med Assoc. 2003;95(3):213–218. [PMC free article] [PubMed] [Google Scholar]
- 8.Nance WE, Lim BG, Dodson KM. Importance of congenital cytomegalovirus infections as a cause for pre-lingual hearing loss. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2006;35(2):221–225. doi: 10.1016/j.jcv.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Fowler KB, Boppana SB. Congenital cytomegalovirus (CMV) infection and hearing deficit. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2006;35(2):226–231. doi: 10.1016/j.jcv.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Stagno S, Reynolds DW, Pass RF, et al. Breast milk and the risk of cytomegalovirus infection. N Engl J Med. 1980;302(19):1073–1076. doi: 10.1056/NEJM198005083021908. [DOI] [PubMed] [Google Scholar]
- 11.Pass RF, Hutto C, Ricks R, et al. Increased rate of cytomegalovirus infection among parents of children attending day-care centers. N Engl J Med. 1986;314(22):1414–1418. doi: 10.1056/NEJM198605293142204. [DOI] [PubMed] [Google Scholar]
- 12.Hyde TB, Schmid DS, Cannon MJ. Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMV. Rev Med Virol. 2010;20(5):311–326. doi: 10.1002/rmv.659. [DOI] [PubMed] [Google Scholar]
- 13.Marshall BC, Adler SP. The frequency of pregnancy and exposure to cytomegalovirus infections among women with a young child in day care. American journal of obstetrics and gynecology. 2009;200(2):163, e161–165. doi: 10.1016/j.ajog.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohn YM, Oh MK, Balcarek KB, et al. Cytomegalovirus infection in sexually active adolescents. The Journal of Infectious Diseases. 1991;163(3):460–463. doi: 10.1093/infdis/163.3.460. [DOI] [PubMed] [Google Scholar]
- 15.Stadler LP, Bernstein DI, Callahan ST, et al. Seroprevalence of cytomegalovirus (CMV) and risk factors for infection in adolescent males. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;51(10):e76–81. doi: 10.1086/656918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staras SA, Flanders WD, Dollard SC, et al. Influence of sexual activity on cytomegalovirus seroprevalence in the United States, 1988-1994. Sexually transmitted diseases. 2008;35(5):472–479. doi: 10.1097/OLQ.0b013e3181644b70. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz CA, Henle W, Henle G, et al. Clinical and laboratory evaluation of cytomegalovirus-induced mononucleosis in previously healthy individuals. Report of 82 cases. Medicine (Baltimore) 1986;65(2):124–134. doi: 10.1097/00005792-198603000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50(11):1439–1447. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20(4):202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 20.Cannon MJ, Westbrook K, Levis D, et al. Awareness of and behaviors related to child-to-mother transmission of cytomegalovirus. Prev Med. 2012;54(5):351–357. doi: 10.1016/j.ypmed.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Revello MG, Campanini G, Piralla A, et al. Molecular epidemiology of primary human cytomegalovirus infection in pregnant women and their families. Journal of medical virology. 2008;80(8):1415–1425. doi: 10.1002/jmv.21243. [DOI] [PubMed] [Google Scholar]
- 22.Cannon MJ, Davis KF. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health. 2005;5:70. doi: 10.1186/1471-2458-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schleiss MR. Acquisition of human cytomegalovirus infection in infants via breast milk: natural immunization or cause for concern? Rev Med Virol. 2006;16(2):73–82. doi: 10.1002/rmv.484. [DOI] [PubMed] [Google Scholar]
- 24.Buxmann H, Miljak A, Fischer D, et al. Incidence and clinical outcome of cytomegalovirus transmission via breast milk in preterm infants </=31 weeks. Acta Paediatr. 2009;98(2):270–276. doi: 10.1111/j.1651-2227.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- 25.Kurath S, Halwachs-Baumann G, Muller W, et al. Transmission of cytomegalovirus via breast milk to the prematurely born infant: a systematic review. Clin Microbiol Infect. 2010;16(8):1172–1178. doi: 10.1111/j.1469-0691.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- 26.Hamprecht K, Maschmann J, Jahn G, et al. Cytomegalovirus transmission to preterm infants during lactation. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2008;41(3):198–205. doi: 10.1016/j.jcv.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Bryant P, Morley C, Garland S, et al. Cytomegalovirus transmission from breast milk in premature babies: does it matter? Arch Dis Child Fetal Neonatal Ed. 2002;87(2):F75–77. doi: 10.1136/fn.87.2.F75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nijman J, van Zanten BG, de Waard AK, et al. Hearing in Preterm Infants with Postnatally Acquired Cytomegalovirus Infection. Pediatr Infect Dis J. 2012 doi: 10.1097/INF.0b013e31825eb3e5. [DOI] [PubMed] [Google Scholar]
- 29.Boppana SB, Rivera LB, Fowler KB, et al. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med. 2001;344(18):1366–1371. doi: 10.1056/NEJM200105033441804. [DOI] [PubMed] [Google Scholar]
- 30.Stagno S, Pass RF, Cloud G, et al. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. Jama. 1986;256(14):1904–1908. [PubMed] [Google Scholar]
- 31.Boppana SB, Pass RF, Britt WJ, et al. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11(2):93–99. doi: 10.1097/00006454-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Freij BJ, South MA, Sever JL. Maternal rubella and the congenital rubella syndrome. Clin Perinatol. 1988;15(2):247–257. [PubMed] [Google Scholar]
- 33.Verboon-Maciolek MA, Krediet TG, Gerards LJ, et al. Severe neonatal parechovirus infection and similarity with enterovirus infection. Pediatr Infect Dis J. 2008;27(3):241–245. doi: 10.1097/INF.0b013e31815c1b07. [DOI] [PubMed] [Google Scholar]
- 34.Bale JF, Jr., Murph JR. Congenital infections and the nervous system. Pediatr Clin North Am. 1992;39(4):669–690. doi: 10.1016/s0031-3955(16)38370-5. [DOI] [PubMed] [Google Scholar]
- 35.Conboy TJ, Pass RF, Stagno S, et al. Early clinical manifestations and intellectual outcome in children with symptomatic congenital cytomegalovirus infection. J Pediatr. 1987;111(3):343–348. doi: 10.1016/s0022-3476(87)80451-1. [DOI] [PubMed] [Google Scholar]
- 36.Pass RF, Stagno S, Myers GJ, et al. Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics. 1980;66(5):758–762. [PubMed] [Google Scholar]
- 37.Foulon I, Naessens A, Foulon W, et al. Hearing loss in children with congenital cytomegalovirus infection in relation to the maternal trimester in which the maternal primary infection occurred. Pediatrics. 2008;122(6):e1123–1127. doi: 10.1542/peds.2008-0770. [DOI] [PubMed] [Google Scholar]
- 38.Pass RF, Fowler KB, Boppana SB, et al. Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2006;35(2):216–220. doi: 10.1016/j.jcv.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Dahle AJ, Fowler KB, Wright JD, et al. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J Am Acad Audiol. 2000;11(5):283–290. [PubMed] [Google Scholar]
- 40.Fowler KB, Dahle AJ, Boppana SB, et al. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J Pediatr. 1999;135(1):60–64. doi: 10.1016/s0022-3476(99)70328-8. [DOI] [PubMed] [Google Scholar]
- 41.Dollard SC, Schleiss MR, Grosse SD. Public health and laboratory considerations regarding newborn screening for congenital cytomegalovirus. J Inherit Metab Dis. 2010;33(Suppl 2):S249–254. doi: 10.1007/s10545-010-9125-3. [DOI] [PubMed] [Google Scholar]
- 42.Grosse SD, Ross DS, Dollard SC. Congenital cytomegalovirus (CMV) infection as a cause of permanent bilateral hearing loss: a quantitative assessment. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2008;41(2):57–62. doi: 10.1016/j.jcv.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Foulon I, Naessens A, Foulon W, et al. A 10-year prospective study of sensorineural hearing loss in children with congenital cytomegalovirus infection. J Pediatr. 2008;153(1):84–88. doi: 10.1016/j.jpeds.2007.12.049. [DOI] [PubMed] [Google Scholar]
- 44.Morton CC, Nance WE. Newborn hearing screening--a silent revolution. N Engl J Med. 2006;354(20):2151–2164. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- 45.Bhatia P, Narang A, Minz RW. Neonatal cytomegalovirus infection: diagnostic modalities available for early disease detection. Indian J Pediatr. 2010;77(1):77–79. doi: 10.1007/s12098-009-0255-2. [DOI] [PubMed] [Google Scholar]
- 46.Boppana SB, Ross SA, Shimamura M, et al. Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N Engl J Med. 2011;364(22):2111–2118. doi: 10.1056/NEJMoa1006561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto AY, Mussi-Pinhata MM, Marin LJ, et al. Is saliva as reliable as urine for detection of cytomegalovirus DNA for neonatal screening of congenital CMV infection? Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2006;36(3):228–230. doi: 10.1016/j.jcv.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 48.Kadambari S, Williams EJ, Luck S, et al. Evidence based management guidelines for the detection and treatment of congenital CMV. Early Hum Dev. 2011;87(11):723–728. doi: 10.1016/j.earlhumdev.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 49.Cheeran MC, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev. 2009;22(1):99–126. doi: 10.1128/CMR.00023-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanari M, Capretti MG, Lazzarotto T. Neuroimaging examination of newborns in vertically acquired infections. J Matern Fetal Neona. 2011;24(Suppl 1):117–119. doi: 10.3109/14767058.2011.607585. [DOI] [PubMed] [Google Scholar]
- 51.Lanari M, Capretti MG, Lazzarotto T, et al. Neuroimaging in CMV congenital infected neonates: how and when. Early Hum Dev. 2012;88(Suppl 2):S3–5. doi: 10.1016/S0378-3782(12)70003-8. [DOI] [PubMed] [Google Scholar]
- 52.Ghekiere S, Allegaert K, Cossey V, et al. Ophthalmological findings in congenital cytomegalovirus infection: when to screen, when to treat? J Pediatr Ophthalmol Strabismus. 2012;49(5):274–282. doi: 10.3928/01913913-20120710-03. [DOI] [PubMed] [Google Scholar]
- 53.Coats DK, Demmler GJ, Paysse EA, et al. Ophthalmologic findings in children with congenital cytomegalovirus infection. J Aapos. 2000;4(2):110–116. doi: 10.1067/mpa.2000.103870. [DOI] [PubMed] [Google Scholar]
- 54.Shin JJ, Keamy DG, Jr., Steinberg EA. Medical and surgical interventions for hearing loss associated with congenital cytomegalovirus: a systematic review. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2011;144(5):662–675. doi: 10.1177/0194599811399241. [DOI] [PubMed] [Google Scholar]
- 55.Lee DJ, Lustig L, Sampson M, et al. Effects of cytomegalovirus (CMV) related deafness on pediatric cochlear implant outcomes. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2005;133(6):900–905. doi: 10.1016/j.otohns.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 56.Stagno S, Pass RF, Thomas JP, et al. Defects of tooth structure in congenital cytomegalovirus infection. Pediatrics. 1982;69(5):646–648. [PubMed] [Google Scholar]
- 57.Fan-Havard P, Nahata MC, Brady MT. Ganciclovir--a review of pharmacology, therapeutic efficacy and potential use for treatment of congenital cytomegalovirus infections. J Clin Pharm Ther. 1989;14(5):329–340. doi: 10.1111/j.1365-2710.1989.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 58.Vallejo JG, Englund JA, Garcia-Prats JA, et al. Ganciclovir treatment of steroid-associated cytomegalovirus disease in a congenitally infected neonate. The Pediatric infectious disease journal. 1994;13(3):239–241. doi: 10.1097/00006454-199403000-00019. [DOI] [PubMed] [Google Scholar]
- 59.Nigro G, Scholz H, Bartmann U. Ganciclovir therapy for symptomatic congenital cytomegalovirus infection in infants: a two-regimen experience. The Journal of pediatrics. 1994;124(2):318–322. doi: 10.1016/s0022-3476(94)70327-2. [DOI] [PubMed] [Google Scholar]
- 60.Kimberlin DW, Lin CY, Sanchez PJ, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. The Journal of pediatrics. 2003;143(1):16–25. doi: 10.1016/s0022-3476(03)00192-6. [DOI] [PubMed] [Google Scholar]
- 61.Oliver SE, Cloud GA, Sanchez PJ, et al. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2009;46(Suppl 4):S22–26. doi: 10.1016/j.jcv.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nassetta L, Kimberlin D, Whitley R. Treatment of congenital cytomegalovirus infection: implications for future therapeutic strategies. J Antimicrob Chemother. 2009;63(5):862–867. doi: 10.1093/jac/dkp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marshall BC, Koch WC. Antivirals for cytomegalovirus infection in neonates and infants: focus on pharmacokinetics, formulations, dosing, and adverse events. Paediatr Drugs. 2009;11(5):309–321. doi: 10.2165/11316080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 64.Stronati M, Lombardi G, Garofoli F, et al. Pharmacokinetics, pharmacodynamics and clinical use of valganciclovir in newborns with symptomatic congenital Cytomegalovirus infection. Curr Drug Metab. 2012 [PubMed] [Google Scholar]
- 65.Amir J, Wolf DG, Levy I. Treatment of symptomatic congenital cytomegalovirus infection with intravenous ganciclovir followed by long-term oral valganciclovir. Eur J Pediatr. 2010;169(9):1061–1067. doi: 10.1007/s00431-010-1176-9. [DOI] [PubMed] [Google Scholar]
- 66.del Rosal T, Baquero-Artigao F, Blazquez D, et al. Treatment of symptomatic congenital cytomegalovirus infection beyond the neonatal period. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2012;55(1):72–74. doi: 10.1016/j.jcv.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 67.Coll O, Benoist G, Ville Y, et al. Guidelines on CMV congenital infection. J Perinat Med. 2009;37(5):433–445. doi: 10.1515/JPM.2009.127. [DOI] [PubMed] [Google Scholar]
- 68.Razonable RR. Strategies for managing cytomegalovirus in transplant recipients. Expert Opin Pharmacother. 2010;11(12):1983–1997. doi: 10.1517/14656566.2010.492395. [DOI] [PubMed] [Google Scholar]
- 69.Blackman SC, Lurain NS, Witte DP, et al. Emergence and compartmentalization of fatal multi-drug-resistant cytomegalovirus infection in a patient with autosomal-recessive severe combined immune deficiency. Journal of pediatric hematology/oncology : official journal of the American Society of Pediatric Hematology/Oncology. 2004;26(9):601–605. doi: 10.1097/01.mph.0000135283.77668.6a. [DOI] [PubMed] [Google Scholar]
- 70.Arvin AM, Fast P, Myers M, et al. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;39(2):233–239. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- 71.Plotkin SA, Smiley ML, Friedman HM, et al. Towne-vaccine-induced prevention of cytomegalovirus disease after renal transplants. Lancet. 1984;1(8376):528–530. doi: 10.1016/s0140-6736(84)90930-9. [DOI] [PubMed] [Google Scholar]
- 72.Plotkin SA, Smiley ML, Friedman HM, et al. Prevention of cytomegalovirus disease by Towne strain live attenuated vaccine. Birth defects original article series. 1984;20(1):271–287. [PubMed] [Google Scholar]
- 73.Plotkin SA, Starr SE, Friedman HM, et al. Effect of Towne live virus vaccine on cytomegalovirus disease after renal transplant. A controlled trial. Ann Intern Med. 1991;114(7):525–531. doi: 10.7326/0003-4819-114-7-525. [DOI] [PubMed] [Google Scholar]
- 74.Adler SP, Starr SE, Plotkin SA, et al. Immunity induced by primary human cytomegalovirus infection protects against secondary infection among women of childbearing age. The Journal of infectious diseases. 1995;171(1):26–32. doi: 10.1093/infdis/171.1.26. [DOI] [PubMed] [Google Scholar]
- 75.Heineman TC, Schleiss M, Bernstein DI, et al. A phase 1 study of 4 live, recombinant human cytomegalovirus Towne/Toledo chimeric vaccines. The Journal of infectious diseases. 2006;193(10):1350–1360. doi: 10.1086/503365. [DOI] [PubMed] [Google Scholar]
- 76.Sung H, Schleiss MR. Update on the current status of cytomegalovirus vaccines. Expert Rev Vaccines. 2010;9(11):1303–1314. doi: 10.1586/erv.10.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360(12):1191–1199. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Griffiths PD, Stanton A, McCarrell E, et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet. 2011;377(9773):1256–1263. doi: 10.1016/S0140-6736(11)60136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nigro G, Adler SP, La Torre R, et al. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353(13):1350–1362. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 80.La Torre R, Nigro G, Mazzocco M, et al. Placental enlargement in women with primary maternal cytomegalovirus infection is associated with fetal and neonatal disease. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2006;43(8):994–1000. doi: 10.1086/507634. [DOI] [PubMed] [Google Scholar]
- 81.Nigro G, Torre RL, Pentimalli H, et al. Regression of fetal cerebral abnormalities by primary cytomegalovirus infection following hyperimmunoglobulin therapy. Prenat Diagn. 2008;28(6):512–517. doi: 10.1002/pd.2013. [DOI] [PubMed] [Google Scholar]
- 82.Buxmann H, Stackelberg OM, Schlosser RL, et al. Use of cytomegalovirus hyperimmunoglobulin for prevention of congenital cytomegalovirus disease: a retrospective analysis. J Perinat Med. 2012;40(4):439–446. doi: 10.1515/jpm-2011-0257. [DOI] [PubMed] [Google Scholar]
- 83.Visentin S, Manara R, Milanese L, et al. Early primary cytomegalovirus infection in pregnancy: maternal hyperimmunoglobulin therapy improves outcomes among infants at 1 year of age. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55(4):497–503. doi: 10.1093/cid/cis423. [DOI] [PubMed] [Google Scholar]