Summary
Biofilms promote attachment of Vibrio cholerae in aquatic ecosystems and aid in transmission. Intracellular c-di-GMP levels that control biofilm development positively correlate with expression of Qrr sRNAs, which are transcribed when quorum sensing (QS) autoinducer levels are low. The Qrr sRNA base-pair with and repress translation of hapR encoding the QS “master regulator”, hence increased c-di-GMP and biofilm development at low density were believed to be solely a consequence of Qrr/hapR pairing. We show that Qrr sRNAs also base-pair with and activate translation of the mRNA of a diguanylate cyclase (DGC), Vca0939; relieving an inhibitory structure in vca0939 that occludes the ribosome binding site. A nucleotide substitution in vca0939 disrupted sRNA/mRNA base-pairing and prevented vca0939 translation, while a compensating Qrr sRNA substitution restored pairing and Vca0939 levels. Qrr-dependent DGC activation led to c-di-GMP accumulation and biofilm development in V. cholerae. This represents the first description of 1) a DGC post-transcriptionally activated by direct pairing with an Hfq-dependent sRNA, and 2) control of a V. cholerae QS phenotype, independent of HapR. Thus, direct interactions of the same sRNAs with two mRNAs promote c-di-GMP-dependent biofilm formation by complementary mechanisms in V. cholerae; by negatively regulating HapR, and positively regulating the DGC Vca0939.
Keywords: V. cholerae, Qrr sRNA, vca0939, diguanylate cyclase, c-di-GMP, biofilm
Introduction
Quorum sensing (QS) is a process that bacteria use to communicate with one another by producing and then detecting small extracellular signal molecules called autoinducers (AIs), which accumulate in proportion to bacterial density. Binding of the AIs to cellular receptors initiates a signal transduction cascade that alters gene expression. QS coordinates a variety of genes for group behaviors that include bioluminescence, biofilm formation, sporulation, antibiotic production, virulence factor expression, and competence for DNA uptake (Ng & Bassler, 2009). Vibrio cholerae produces two AIs, CAI-1 and AI-2 (Chen et al., 2002) (Higgins et al., 2007). At low cell density (LCD), in the absence of AIs, their cognate membrane-bound sensors CqsS and LuxP/Q act as kinases, shuttling phosphate through the phosphotransferase LuxU to the response regulator LuxO (Ng & Bassler, 2009). Together with σ54-loaded RNA polymerase, phosphorylated LuxO activates the transcription of genes encoding four non-coding small RNAs (sRNAs) called Qrr1–4 (quorum regulatory RNAs) (Lenz et al., 2004). Recently, it was shown that the Qrr sRNAs, with the assistance of the RNA chaperone Hfq, negatively regulate hapR mRNA by direct base-pairing with the 5’ untranslated region (5’ UTR) of hapR (Bardill et al., 2011). Specifically the site to which the Qrr sRNAs bind overlaps the ribosome binding site (RBS). HapR has been termed the “master regulator” of QS, since all QS-mediated phenotypes described to date require HapR for regulation.
At high cell density (HCD), when the concentrations of AIs are high, binding of each AI to its cognate receptor switches the receptors from kinases to phosphatases. Phosphate flow in the signal transduction pathway is reversed, resulting in dephosphorylation and inactivation of LuxO (Ng & Bassler, 2009). Therefore, the Qrr sRNAs are not transcribed, hapR mRNA is stabilized, and HapR protein is produced (Lenz et al., 2004). HapR acts as both an activator and a repressor of gene expression. Specifically, AI-stimulated production of HapR activates the transcription of hapA, encoding the secreted hemagglutinin/protease (Finkelstein et al., 1992, Jobling & Holmes, 1997), and comEA, which is required for natural competence of V. cholerae (Meibom et al., 2005, Antonova & Hammer, 2011). HapR also represses transcription of the aphA gene, which encodes an activator of the ToxT regulon that includes the virulence factors cholera toxin (CT) and the toxin co-regulated pilus (TCP) (Zhu et al., 2002, Kovacikova et al., 2004). HapR also indirectly represses transcription of the vpsL-Q exopolysaccharide biosynthesis operon by binding directly to the promoter of the biofilm transcription factor vpsT (Zhu & Mekalanos, 2003) (Hammer & Bassler, 2003) (Waters et al., 2008).
The Qrr sRNAs also alter translation of several mRNA targets in addition to hapR mRNA. Specifically, the Qrr sRNAs negatively regulate the mRNA of luxO, establishing a feedback loop to control Qrr production (Svenningsen et al., 2009), and activate production of aphA (Rutherford et al., 2011). Finally, the Qrr sRNAs also positively regulate the mRNA of vca0939, annotated as a “GGDEF family protein”, which harbor a GGDEF or GGEEF motif, and act as diguanylate cyclases (DGCs) to synthesize the intracellular second messenger bis-(3'−5')-cyclic dimeric guanosine monophosphate (c-di-GMP) (Hammer & Bassler, 2007). While genetic evidence supports a model that Qrr sRNAs regulate these genes post-transcriptionally, direct binding of the Qrr sRNAs to luxO, aphA, or vca0939 mRNA has not been documented experimentally.
Many bacteria use intracellular c-di-GMP as a signaling molecule to facilitate transition between a sedentary and planktonic lifestyle (Galperin, 2004, Hengge, 2009). C-di-GMP synthesized from two molecules of GTP by DGCs is broken down into 5-phosphoguanylyl-(3'−5')-guanosine (pGpG) or GMP by specific phosphodiesterases (PDEs) that contain either an EAL or HD-GYP motif, respectively (Ryjenkov et al., 2005, Schmidt et al., 2005, Tal et al., 1998). In general, c-di-GMP promotes the biosynthesis of attachment factors and exopolysaccharide matrix in biofilms and inhibits motility by a variety of mechanisms; however numerous fundamental bacterial behaviors such as cell cycle progression, antibiotic production, pilin synthesis, type III secretion, RNA modulation, stress response, bacterial predation, and virulence are also c-di-GMP controlled (reviewed in (Hengge, 2009)).
In V. cholerae, it has been proposed that QS cooperates with c-di-GMP signaling pathways to regulate the transition between a motile, virulent state within the host and a sessile, biofilm state in aquatic environmental reservoirs (Srivastava & Waters, 2012). Clinical Vibrio cholerae isolate, C6706, used here and in many other QS studies (Ng & Bassler, 2009) (Bardill et al., 2011), encodes 40 DGC domains, 20 EAL domains, and 9 HD-GYP domains (Galperin, 2004). HapR directly represses vpsT transcription by binding to its promoter (Waters et al., 2008) (Casper-Lindley & Yildiz, 2004), but also decreases intracellular c-di-GMP levels by controlling the transcription of 14 GGDEFs and EALs (Waters et al., 2008), and four HD-GYPs (Hammer & Bassler, 2009). This reduction of c-di-GMP levels also reduces vpsT transcription by reducing the activity of VpsR (Srivastava et al., 2011). Moreover, because direct interaction of c-di-GMP to VpsT drives its dimerization and binding to the vps biosynthesis gene promoters (Krasteva et al., 2010), the reduction of c-di-GMP by HapR also indirectly represses VpsT activity. The consequence of this regulation is that the intracellular concentration of c-di-GMP, and thus biofilm formation, is elevated at LCD (Fig. 1) and reduced upon reaching HCD (Waters et al., 2008, Hammer & Bassler, 2009).
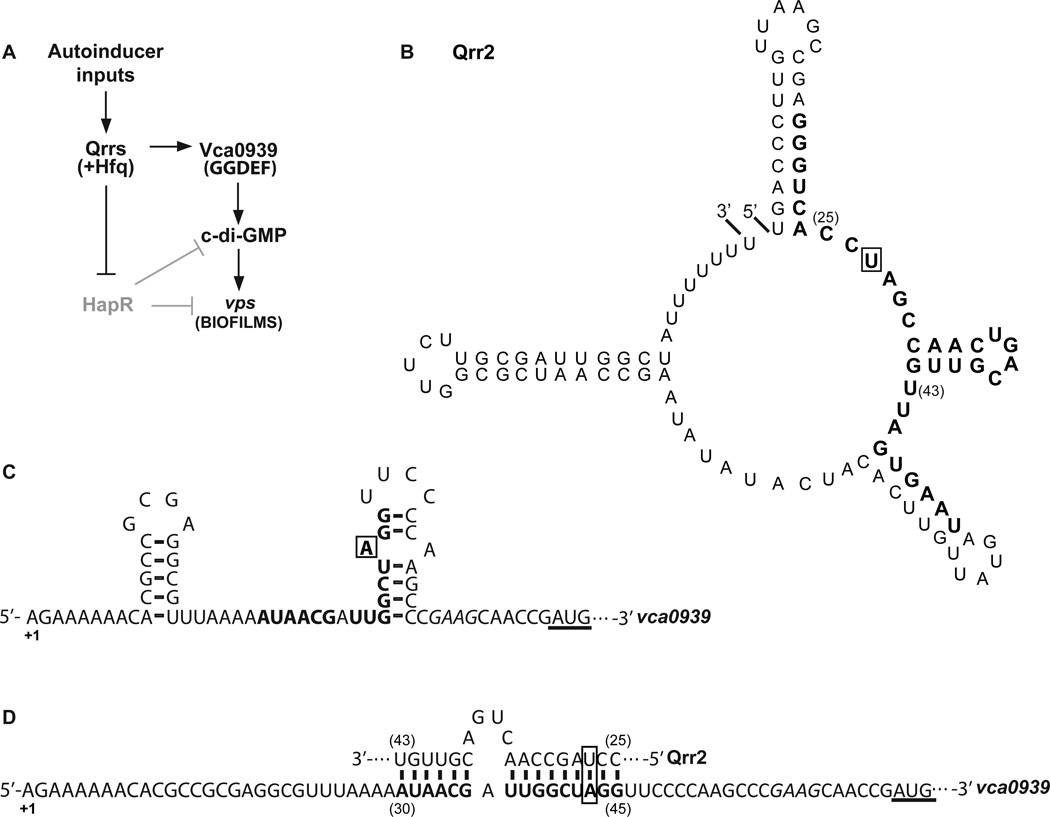
Figure 1.
Model of biofilm regulation by the quorum sensing-controlled Qrr sRNAs in V. cholerae. A. Due to the lack of autoinducers, Qrr sRNAs are expressed that in turn repress hapR translation. The absence of HapR results in high c-di-GMP levels and derepression of vps genes for biofilm formation. Qrr sRNAs also activate translation of vca0939, a GGDEF family protein, which produces c-di-GMP to promote vps gene expression and biofilm formation. B. Predicted secondary structure of Qrr2 sRNA, and C. the putative secondary structure of the 5’ UTR of vca0939 mRNA by Mfold (Zuker, 2003). D. Predicted pairing of the conserved region of Qrr sRNAs to the 5’ UTR of vca0939 as in (Hammer & Bassler, 2007). Nucleotide positions are indicated relative to the +1 of transcription of each RNA. Nucleotides substituted in this study are boxed. The italicized sequence is the predicted vca0939 ribosome binding site. The underlined sequence is the annotated translation start site. The thirty two consecutive nucleotides conserved in Qrr sRNAs of pathogenic Vibrios (Lenz et al., 2004) are in bold in B, and the vca0939 nucleotides predicted to base-pair with the Qrr sRNAs are in bold in C and D.
Previously, genetic data and computational predictions suggested that in the absence of the Qrr sRNAs, the 5’ UTR of vca0939 mRNA forms an inhibitory stem-loop structure containing a portion of a putative sRNA binding site adjacent to the RBS (Hammer & Bassler, 2007). It was hypothesized that with assistance of the RNA chaperone Hfq, Qrr sRNAs form an RNA duplex with the 5’ stem of the putative inhibitory structure (Fig. 1), analogous to sRNAs in other bacteria, such as RprA and DsrA in Escherichia coli that positively control rpoS by an “anti-antisense” mechanism (Majdalani et al., 1998, Majdalani et al., 2002, Frohlich & Vogel, 2009). Induction of Vca0939 by the Qrr sRNAs was proposed to contribute to elevated c-di-GMP levels and biofilm (vps) gene expression at LCD; and while a Δvca0939 is not impaired in biofilm formation (Hammer & Bassler, 2007), overexpression of the coding region of Vca0939 enhances biofilms consistent with this model (Massie et al., 2012). It remains unknown whether Vca0939 is indeed a DGC as the active site GGEEF domain has not been studied, nor has it been determined whether Qrr-dependent activation of vca0939 translation is sufficient to increase c-di-GMP levels and enhance biofilm development.
Here we demonstrate for the first time post-transcriptional activation of a GGDEF protein, Vca0939, which results in synthesis of c-di-GMP and development of biofilms in V. cholerae. Base-pairing of QS-controlled Qrr sRNAs to the 5’ UTR of vca0939 is enhanced by Hfq and required for translation of Vca0939 protein. A single nucleotide substitution within a V. cholerae Qrr sRNA prevents activation of vca0939 translation and as a consequence, hinders c-di-GMP production and biofilm formation. Qrr/vca0939 base-pairing and biofilms are restored by a compensatory single nucleotide mutation in the 5’ UTR of vca0939. Thus, the QS sRNAs control biofilm formation by two mechanisms; by negatively regulating HapR as shown prior (Bardill et al., 2011, Lenz et al., 2004), and positively regulating Vca0939 as shown here.
Results
Activation of Vca0939 by the Qrr sRNAs promotes biofilm formation
V. cholerae QS-dependent Qrr sRNAs positively regulate vca0939, which is predicted to encode a GGDEF family protein with diguanylate cyclase activity for synthesizing cyclic-di-GMP (c-di-GMP) (Hammer & Bassler, 2007) (Fig. 1). Accumulation of c-di-GMP can promote biofilm formation, but a V. cholerae Δvca0939 mutant was not reduced for biofilm formation, and it was speculated this may be due to redundancy among the 40 GGDEF proteins in V. cholerae, as described elsewhere (Srivastava & Waters, 2012, Hammer & Bassler, 2007). However, we recently demonstrated that overexpression of the vca0939 coding region independent of the native 5’ UTR enhanced biofilms in V. cholerae (Massie et al., 2012). Therefore, to test whether Qrr-dependent activation of Vca0939 was sufficient to enhance biofilm formation, we constructed on a plasmid a flag-tagged version of vca0939 (pVca0939), which remained under control of the native promoter and 5’ UTR. An additional plasmid was designed to express Qrr2 (Fig. 1B) under control of the Ptac promoter (pQrr2), similar to plasmids used prior to document Qrr-based control of several target genes, including vca0939 (Bardill et al., 2011, Tu et al., 2010, Svenningsen et al., 2009, Rutherford et al., 2011, Hammer & Bassler, 2007). This simplified Qrr expression by uncoupling it from the feedback loop that controls transcription of each qrr from its native promoter (Svenningsen et al., 2009). Plasmids lacking the entire Qrr2 sequence (pKK) or the entire vca0939 sequence (pEVS141) served as negative controls in V. cholerae.
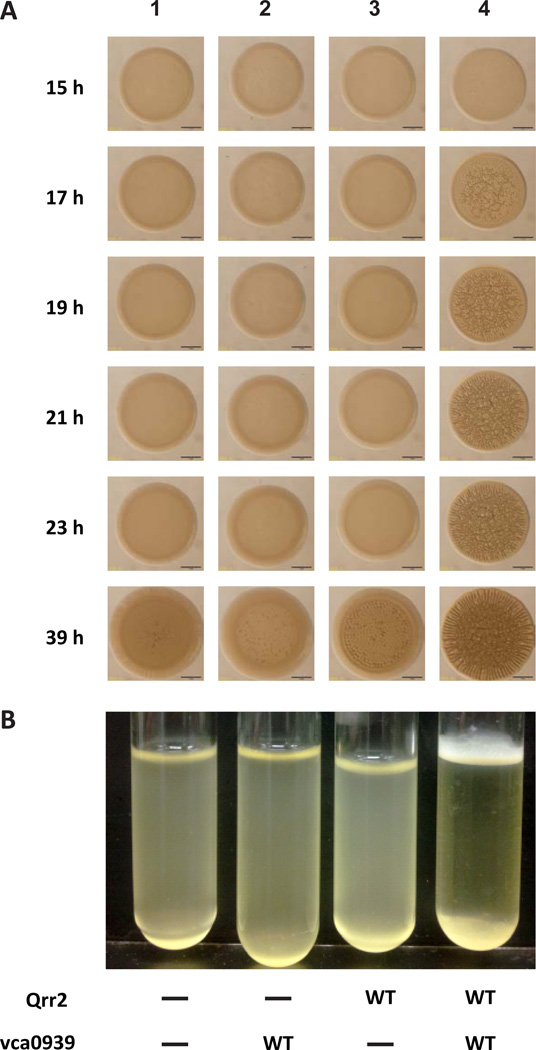
Because V. cholerae only expresses the Qrr sRNAs at low cell density (LCD), we introduced both plasmids into a V. cholerae luxOD47E Δqrr1–4 strain described prior (Hammer & Bassler, 2007). This mutant carries an allele of luxO that “locks” the strain in a LCD mode, but lacks the qrr genes, which allowed us to measure contributions of the plasmid-borne Qrr2 sRNAs to biofilm formation on solid growth medium and in liquid medium. Aliquots of a diluted overnight culture were spotted onto Luria Broth (LB) agar to document biofilm development, as described elsewhere (Morris et al., 2011) (Ray et al., 2012). Images of each colony formed were captured at multiple intervals over ∼ 40 h growth. Consistent with prior studies of Qrr-deficient strains (Hammer & Bassler, 2003, Zhu et al., 2002), V. cholerae carrying both vectors controls (pKK and pEVS141) displayed a smooth colony morphology; similar to the strain carrying vector control pKK and pVca0939 (Fig. 2A, panels 1 and 2). V. cholerae carrying pQrr2 and the pEVS141 vector control appeared smooth until late time points (39 h) when we observed a modest wrinkly (rugose) morphology indicative of vps transcription (Yildiz & Schoolnik, 1999) (Fig. 2A, panel 3). This late biofilm development likely resulted from repression of the hapR gene on the chromosome by the Ptac-controlled sRNA on pQrr2 (Fig. 1, (Hammer & Bassler, 2003), and Zhao & Hammer, unpublished). In contrast, when both pQrr2 and pVca0939 were present, rugose colony morphology was detected by 17 h (Fig. 2A, panel 4). Biofilm formation of each strain in liquid culture, observed as the appearance of a pellicle at the air-broth interface, was consistent with the colony morphology observed; pellicle formation was only detected in the strain carrying both pQrr2 and pVca0939 (Fig. 2B). Similar biofilm results were obtained with a plasmid expressing a Ptac-controlled Qrr1 (Fig. S1), which was used prior to demonstrate Qrr-based control of both hapR (Lenz et al., 2004) and vca0939 (Hammer & Bassler, 2007). These results demonstrate that activation of vca0939 by Qrr sRNAs promotes biofilm formation in V. cholerae.
Figure 2.
Activation of Vca0939 by Qrr sRNAs promotes biofilm formation in V. cholerae. A. Biofilm formation was observed at the time points indicated during 40 h growth on solid medium (A), and at 12 h in liquid medium (B). The V. cholerae Qrr-deficient strain carried: 1) pKK (-) and pEVS141 (-); 2) pKK (-) and pVca0939 (WT); 3) pQrr2 (WT) and pEVS141 (-); and 4) pQrr2 (WT) and pVca0939 (WT). Shown is one representative of three experiments performed.
Vca0939 is a diguanylate cyclase, which promotes early biofilm formation in V. cholerae
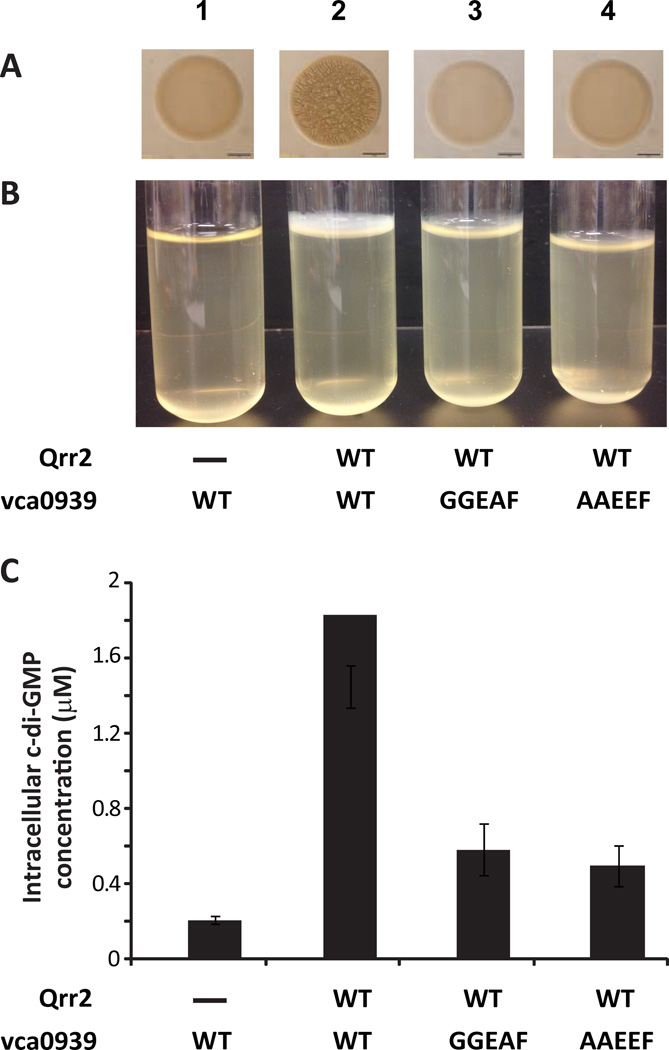
In V. cholerae c-di-GMP binds to VpsR, which is required for vpsT transcription and directly interacts to the vpsT promoter. C-di-GMP also binds to VpsT, and both VpsR and VpsT are thought to activate the transcription of the vps biosynthesis genes required for biofilm development (Srivastava et al., 2011, Krasteva et al., 2010). Vca0939 is annotated as a “GGDEF family protein” due to a conserved amino acid motif (Tal et al., 1998), and thus predicted to act as a DGC capable of c-di-GMP synthesis. Indeed, Vca0939 has a GGEEF domain, which is a common variant of the GGDEF signature motif, and is typically enzymatically active (Kulasakara et al., 2006). To test whether the V. cholerae biofilm phenotypes observed (Fig. 2) were due to the DGC activity of Vca0939, we altered the GGEEF motif, to AAEEF or GGEAF (Waters et al., 2008) (and Waters unpublished data) on the plasmid expressing Vca0939-FLAG (pVca0939). In the V. cholerae Qrr-deficient strain described in Fig. 2, levels of Vca0939AAEEF -FLAG and Vca0939GGEAF -FLAG protein were comparable to wild type (WT) Vca0939-FLAG by western blot (data not shown). In control strains, where V. cholerae expressed no Qrr sRNAs, so that vca0939 was not activated, colonies appeared smooth and no pellicle was observed, as expected (Fig. 3A and 3B, panel 1). In contrast, V. cholerae carrying pQrr2 and pVca0939, formed rugose colonies and a thick pellicle, presumably due to the DGC activity of Vca0939 (Fig. 3A and 3B, panel 2). However, when V. cholerae carried pQrr2, and either pVca0939GGEAF or pVca0939AAEEF, no rugosity was observed and only a thin pellicle detected (Fig. 3A and 3B, panels 3 and 4), consistent with the hypothesis that the GGEEF motif of Vca0939 is responsible for synthesis of c-di-GMP.
Figure 3.
The active site of Vca0939 is required for biofilm formation and enhanced c-di-GMP levels in V. cholerae. Biofilm formation on solid medium (A) and in liquid medium (B) was observed in V. cholerae carrying; Panel 1) pKK (-) and pVca0939 (WT); Panel 2) pQrr2 (WT) and pVca0939 (WT); Panel 3) pQrr2 (WT) and pVca0939GGEAF (GGEAF); and Panel 4) pQrr2 (WT) and pVca0939AAEEF (AAEEF). Shown is one representative of three experiments performed. C. C-di-GMP concentrations of the strains shown in A and B. Data shown are mean values +/− standard deviation for triplicate cultures from one representative experiment of three performed.
To confirm that the defect in biofilm formation observed with the mutants in Fig. 3A was due to the loss of DGC activity for each protein, we also quantified c-di-GMP levels by LS/MS-MS (Massie et al., 2012) for each strain. The V. cholerae strain lacking Qrr sRNAs showed low levels of c-di-GMP (Fig. 3C bar 1), consistent with the smooth biofilm phenotype and lack of pellicle observed. Levels of c-di-GMP in the strain that carried both pQrr2 and pVca0939 were > 10-fold higher, consistent with enhanced biofilms (Fig. 3C, panel 2). However, each biofilm-defective strain that carried a putative GGEEF active site mutation produced lower levels of c-di-GMP consistent with the smooth colonies and modest pellicles observed (Fig. 3C, bars 3 and 4), confirming that Vca0939 is a DGC for c-di-GMP synthesis.
Activation of Vca0939 translation requires Qrr sRNA base-pairing in V. cholerae and E. coli
Direct base-pairing of Qrr sRNA to vca0939 mRNA was predicted to positively control translation of Vca0939 protein (Hammer & Bassler, 2007). Therefore, to determine whether biofilm observations (Fig. 2) reflected changes in Vca0939 translation, we measured levels of Vca0939-FLAG protein by western blot in the Qrr-deficient V. cholerae strain. No Vca0939 protein was observed when only Qrr2 sRNA was expressed along with a vector control (Fig. 4, lane 1). Likewise, Vca0939 was not detected in the strain carrying pVca0939 and a vector control (Fig. 4, lane 2). However, Vca0939 protein was observed in V. cholerae that carried both pQrr2 and pVca0939 (Fig 4, lane 5), consistent with the model that translation of vca0939 mRNA requires activation by direct Qrr sRNA interaction. Similar results were obtained in E. coli carrying the two plasmids that expressed Qrr2 and Vca0939-FLAG (Fig. S2, panel E).
Figure 4.
The effect of QrrU27G mutation on the expression of Vca0939-Flag in V. cholerae. V. cholerae strains expressing the indicated plasmid-borne qrr2 and vca0939-flag alleles were grown and analyzed for protein production using western blot. Liquid cultures of the strains indicated were pelleted, resuspended in sample buffer, subjected to SDS-PAGE, and transferred to PVDF membrane. Blots were probed with an antibody to the Flag tag and visualized. Shown is one representative of three experiments performed.
Qrr sRNAs interact with the 5’ UTR of hapR mRNA that is predicted to be relatively unstructured at the Qrr binding site (Fig. S2, panel A). Base-pairing of Qrr sRNAs at the ribosome binding site (RBS) represses hapR translation, while the absence of Qrr sRNAs results in constitutive HapR translation (Lenz et al., 2004, Bardill et al., 2011). It has been proposed that Qrr sRNAs interact with the 5’ UTR of vca0939 to activate translation by disrupting an inhibitory stem loop structure adjacent to the RBS, which prevents Vca0939 translation in the absence of Qrr sRNAs (Fig. 1C) (Zuker, 2003, Hammer & Bassler, 2007). To test the importance of the putative inhibitory structure, deletions were introduced into the pVca0939 plasmid to eliminate the DNA sequence 5’ of the putative RBS. Consistent with the Qrr-activation model, deletion of nucleotide (nt) sequence comprising the left stem (LS) or right stem (RS) of the inhibitory structure of vca0939, as well as deletion of the sequence (LS+RS) for the entire stem loop, resulted in Vca0939-FLAG production independent of Qrr2 sRNA expression in E. coli (Fig. S2, panels B and C). Single nucleotide mutations (C41G and G44C) in pVca0939 predicted to disrupt the inhibitory structure also resulted in Qrr2-independent Vca0939-FLAG production, while a mutation (G35C) in pVca0939 predicted to maintain the inhibitory structure was not expressed in E. coli, as expected (Fig. S2, panels B and D). These results confirmed that the sequence comprising the putative structure indeed inhibits translation, and also that the RBS of vca0939 is not contained within the sequence of the putative inhibitory structure.
Previously, we used single nucleotide (nt) substitutions to validate that interaction of Qrr sRNAs with hapR mRNA repressed hapR translation (Bardill et al., 2011). Thus, to determine whether Qrr sRNAs interactions with vca0939 also activate its translation in V. cholerae, we engineered into the vca0939-flag expression plasmid a mutation in a nt within the 5’ UTR (pVca0939A43C) that was not expected to be involved in pairing within the inhibitory stem loop structure (Fig. 1C), but was still predicted to interact with the Qrr RNA (Fig. 1D). In the absence of Qrr2 sRNA expression, Vca0939A43C was not expressed (Fig. 4, lane 3), like wild type (WT) Vca0939, consistent with an intact inhibitory structure. Although the presence of WT Qrr2 sRNA activated translation of WT vca0939 (Fig. 4, lane 5), it was unable to activate translation of vca0939A43C (Fig. 4, lane 6); presumably due to disruption of the Qrr/vca0939 interaction. To demonstrate that loss of Qrr-dependent vca0939 activation was caused by impairment of the Qrr/vca0939 interaction, we engineered a compensatory mutation (Qrr2U27G) in the plasmid-borne qrr2 and expressed it in V. cholerae carrying either WT vca0939 or vca0939A43C. No Vca0939 protein was observed when Qrr2U27G was expressed alone or with WT vca0939 (Fig. 4, lanes 4 and 7). However, expression of Qrr2U27G was able to effectively induce Vca0939A43C production (Fig. 4, lane 8), presumably because the mutations in each RNA restored the Qrr/vca0939 interaction. These V. cholerae results, and similar results in E. coli (Fig. S2, panel E), suggested that Qrr-dependent activation of vca0939 translation requires base-pairing between Qrr27 and vca093943 (Fig 1D).
Qrr sRNA binds to vca0939 mRNA in vitro
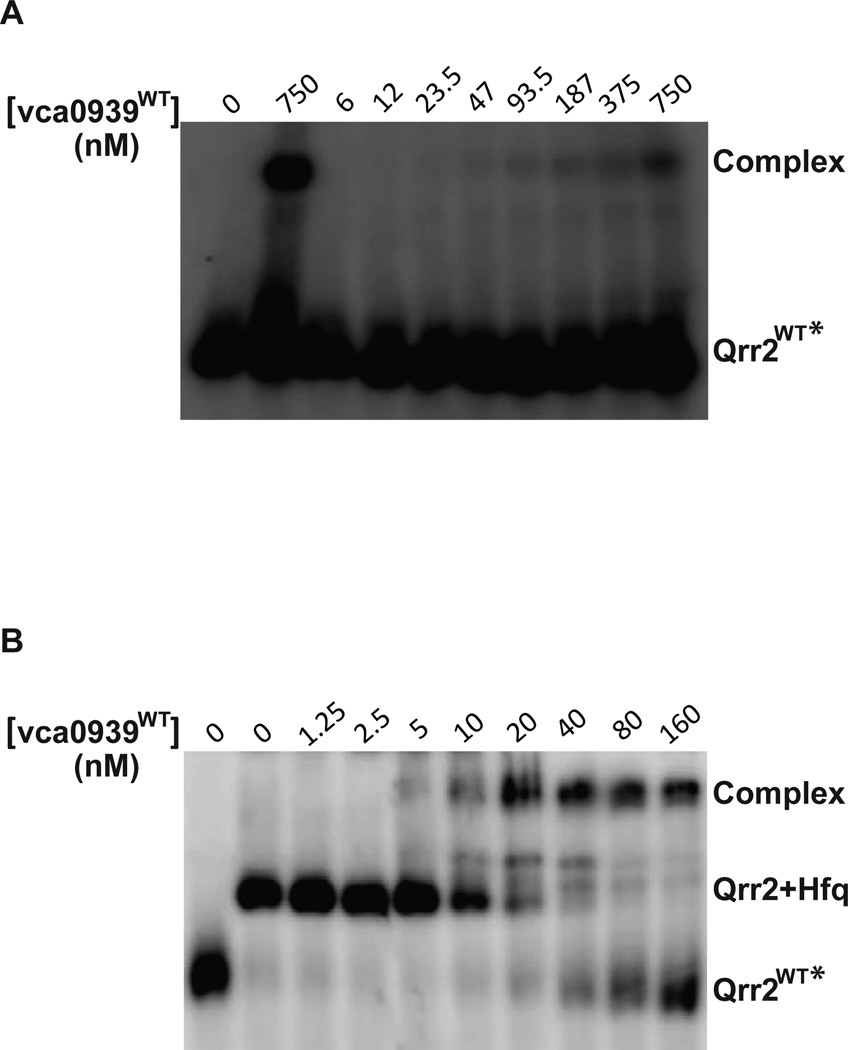
Previous genetic studies in V. cholerae showed that deletion of the hfq gene or all four qrr genes severely impaired vca0939 expression, consistent with both Hfq and Qrr sRNA involvement in vca0939 activation (Fig. 1) (Hammer & Bassler, 2007). We sought to determine whether vca0939 activation by Qrr sRNA was indeed due to formation of a sRNA/mRNA duplex and whether Hfq facilitates this process. Electrophoretic mobility shift assays (EMSA) were used to measure binding of purified, 32P-radiolabelled Qrr2 RNA and unlabeled vca0939 RNA, both in the presence and absence of Hfq. Full length Qrr2 RNA was prepared as described (Bardill et al., 2011). 5' RACE identified the +1 of transcription and verified that the length of the 5’ UTR of vca0939 was 67 nt (Fig. 1); and a 270 nt truncated vca0939 RNA was transcribed in vitro to allow detection of a shift on a native polyacrylamide gel that preserves macromolecular complexes.
In the absence of Hfq, 0.4 nM 32P-radiolabelled Qrr2 RNA was detected. When incubated at 70 °C with 750 nM vca0939 RNA to eliminate RNA structure and then slowly cooled to 25 °C to maximize pairing, a shift was detected that indicated a Qrr/vca0939 complex (Fig 5A, compare first two lanes). When the 32P-radiolabelled Qrr RNA and vca0939 RNA were separately heated and then cooled on ice prior to incubation together as described prior in (Bardill et al., 2011, Updegrove et al., 2008), increasing vca0939 RNA only resulted in a band shift with high vca0939 RNA concentrations (750 nM) that was of low intensity and only faintly observed when the contrast of the image was increased, indicative of weak binding (Fig. 5A). In the presence of purified V. cholerae Hfq (150 nM), 32P-radiolabelled Qrr2 RNA displayed a shift in mobility (Fig. 5B, lane 2), and then with increasing amount of unlabeled vca0939 RNA, resulted in a supershift consistent with the formation of a complex that may contain both RNAs and Hfq (Fig. 5B, lane 3–10). The apparent Kd of Qrr2/vca0939 pairing with Hfq was calculated to be ∼23 nM, showing a slightly weaker binding that than for Qrr2/hapR pairing (7 nM), as described (Bardill et al., 2011). These results are consistent with computational predictions (Gruber et al., 2008) that the energy required for the Qrr sRNAs to disrupt the inhibitory stem loop structure of the vca0939 5’ UTR and bind is likely greater than the energy required for Qrr sRNAs to bind to the less structured hapR 5’ UTR (Fig. S2, panels A and B).
Figure 5.
Binding of Qrr2 RNA to vca0939 RNA in the presence or absence of V. cholerae Hfq. 0.4 nM of radiolabeled Qrr2 was incubated with increasing amounts of vca0939 mRNA without (A) or with 150 nM Hfq (B) as described in Experimental procedures. Concentration of vca0939 mRNA (in nM) is indicated above each gel. Complex refers to the Qrr2/vca0939 complex that may contain Hfq. As a control, Qrr2 RNA and vca0939 RNA were mixed together, heated to 70 °C, and then cooled slowly without Hfq to document duplex formation as described in text (panel A, lane 2).
Mutation of a single nucleotide abolishes binding of Qrr to vca0939 RNA in vitro
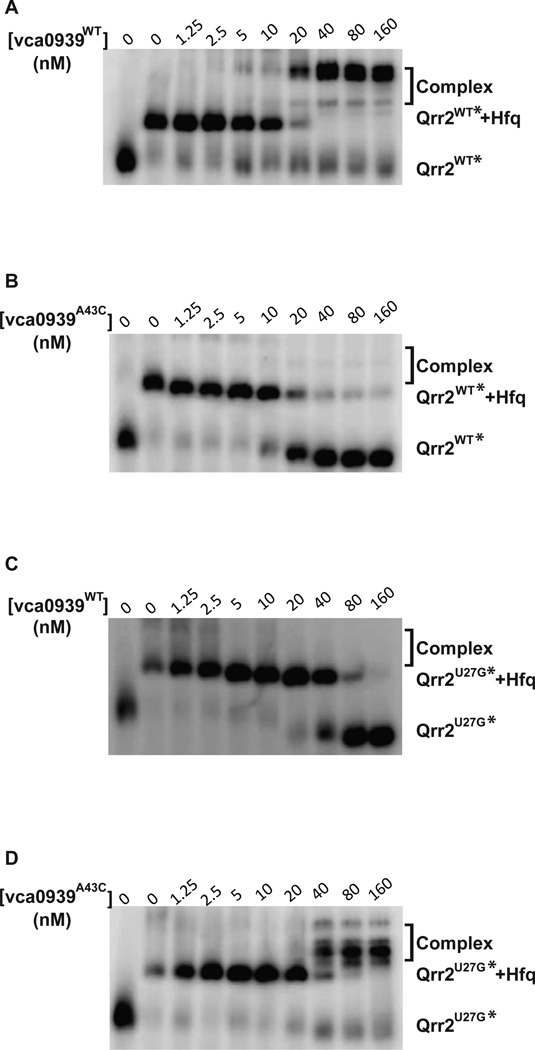
In V. cholerae and E. coli, Vca0939A43C was not translated in the presence of WT Qrr2, nor was WT Vca0939 translated in the presence of Qrr2U27G (Figs. 4 and S2). To test whether this loss of activation in vivo was a consequence of RNA binding defects, we purified and radiolabelled Qrr2U27G RNA, and also purified vca0939A43C RNA for EMSA. As in Fig. 5B, WT vca0939 RNA binds WT Qrr2 RNA in the presence of Hfq, resulting in a supershift with ∼20 nM vca0939 (Fig. 6A). However, no supershift was observed with addition of vca0939A43C RNA to WT Qrr2 RNA (Fig. 6B), or addition of WT vca0939 RNA to Qrr2U27G RNA (Fig. 6C). When unlabeled vca0939 RNA exceeded ∼20 nM, the band representing the Qrr2 RNA bound to Hfq (Qrr+Hfq) diminished while a presumptive unbound Qrr band increased in intensity (Fig. 6B and 6C), consistent with titration of Hfq away from radiolabelled Qrr2 RNA by excessive unlabelled vca0939 RNA. Importantly, Qrr2U27G and vca0939A43C that reestablished translational activation in vivo (Figs. 4 and S2), also showed a restoration of base-pairing and formation of a supershift complex by EMSA (Fig 6D, see Discussion for details).
Figure 6.
The Effect of Qrr2U27G on vca0939 binding in the presence of Vibrio cholerae Hfq. 0.4 nM of each radiolabeled Qrr2 RNA was incubated with increasing amount of vca0939 RNA. Concentration of vca0939 RNA (in nM) is indicated above each gel. Reactions were run on a 8% native polyacrylamide gel and radiolabeled Qrr2 RNA was visualized using a phosphorimager. Complexes refers to the Qrr2/vca0939 complex that may contain Hfq. A. Radiolabeled WT Qrr2 RNA incubated with WT vca0939 RNA. B. Radiolabeled WT Qrr2 incubated with vca0939A43C RNA. C. Radiolabeled Qrr2U27G RNA incubated with WT vca0939 RNA. D. Radiolabeled Qrr2U27G RNA incubated with vca0939A43C RNA.
Base-pairing between Qrr and vca0939 promotes V. cholerae biofilm formation
Since we confirmed that vca0939 translation is activated by Qrr2 sRNAs via base-pairing interaction (Fig. 4 and Fig. 6), we sought to examine whether the single nucleotide mutations in Qrr2 sRNAs and vca0939 mRNA that disrupt pairing were sufficient to alter biofilm formation and c-di-GMP production. We constructed the Qrr2U27G and vca0939A43C alleles in both plasmids used in Fig. 2, and introduced each into V. cholerae to measure rugose colony formation at 21 h, pellicle formation, and c-di-GMP levels. The control V. cholerae strains that do not express Qrr sRNA exhibit a smooth colony morphology and produce no pellicle (Fig. 7A and 7B, panels 1 and 2). The strains carrying pQrr2U27G and pVca0939, and also carrying pQrr2 with pVca0939A43C, exhibit a similar smooth colony morphology and thin pellicle (Fig. 7A and 7B, panels 4 and 5). Similar to the positive control rugose strain that expresses both WT Qrr2 sRNA and WT vca0939, the strain carrying pQrr2U27G and pVca0939A43C was restored for rugose colony morphology and pellicle formation (compare Fig. 7A and 7B, panels 3 and 6). These results are consistent with both the western blot and in vitro EMSA data (Fig. 4 and 6). Indeed, the levels of c-di-GMP also followed the pattern observed with the biofilm phenotypes; strains with a rugose colony morphology and thick pellicles had high c-di-GMP levels, strains with a smooth colony morphology and a thin pellicle showed c-di-GMP near background levels, while the two strains that appeared smooth on plates and lacked pellicles also produced c-di-GMP at background levels (Compare Fig. 7B and 7C). Taken together, these data confirm that base-pairing of Qrr sRNAs and the mRNA of vca0939 activates DGC activity that promotes biofilm formation.
Figure 7.
Qrr2/vca0939 base pairing regulates c-di-GMP production and biofilm formation. A. Biofilm formation on solid medium (A) and in liquid medium (B) was measured in the V. cholerae Qrr-deficient strain carrying: Pane1 1) pKK and pVca0939; Panel 2) pKK and pVca0939A43C; Panel 3) pQrr2 and pVca0939; Panel 4) pQrr2U27G and pVca0939; Panel 5) pQrr2 and pVca0939A43C; and Panel 6) pQrr2U27G and pVca0939A43C. Shown is one representative of three experiments performed. C. C-di-GMP concentrations of the strains in A and B. Data shown are mean values +/- standard deviation for triplicate cultures from one representative experiment of three performed.
Finally, because V. cholerae rugose colony morphology often occurs due to the effect of elevated c-di-GMP levels on expression of two vps vibrio polysaccharide biosynthesis operons, vpsA-K and the vpsL-Q (Hammer & Bassler, 2003, Yildiz & Schoolnik, 1999), we compared transcript abundance of vpsA and vpsL by quantitative RT-PCR in the V. cholerae strains carrying pQrr2 with pEVS141 or pVca0939. As expected, higher levels of vpsA and vpsL transcription (4.3-fold and 5-fold, respectively) were observed in the V. cholerae strain that carried pQrr2 and pVca0939, compared to the strain that carried pQrr2 and pEVS141 (Fig. 2, panels 3 and 4). As a control we confirmed that similar higher levels of 2-fold and 3-fold were also observed for vpsA and vpsL mRNA, respectively, in a rugose V. cholerae ΔhapR strain relative to WT V. cholerae, as described prior (Hammer & Bassler, 2003). Thus the DGC Vca0939, when post-transcriptionally activated by Qrr sRNA expression, produces c-di-GMP that upregulates vps genes for biofilm formation.
Discussion
Vibrio cholerae populations sense and respond to environmental cues to control important developmental processes such as biofilm formation (Srivastava & Waters, 2012). Specifically, V. cholerae utilize QS as one of several signaling systems to control transcription of the vps (Vibrio polysaccharide) genes required for biofilm development (Hammer & Bassler, 2003, Casper-Lindley & Yildiz, 2004, Tischler & Camilli, 2004, Hammer & Bassler, 2009, Martinez-Wilson et al., 2008). Much research has focused on the contribution of the HapR “QS master regulator” to vps-dependent biofilms, as HapR abundance is inversely related to that of c-di-GMP. At low cell density when Qrr sRNA accumulation prevents HapR expression, ci-di-GMP levels are high and biofilms form; and at high cell density when the lack of Qrr sRNAs allows HapR production, c-di-GMP levels are low and biofilm formation is halted (Waters et al., 2008, Hammer & Bassler, 2009). Prior studies identified vca0939 as a gene that is positively regulated by the Qrr sRNAs independent of HapR and that was predicted to behave as a DGC capable of synthesizing c-di-GMP and promoting biofilms (Hammer & Bassler, 2007, Hammer & Bassler, 2009). We recently demonstrated that overexpression of the vca0939 coding region independent of the native 5’ UTR enhanced biofilms in V. cholerae (Massie et al., 2012). In this paper, we have defined the regulation and consequences of vca0939 expression in V. cholerae. Specifically we showed that vca0939 translation is positively controlled by base-pairing of Qrr2 sRNA to its native 5’ UTR, and that Vca0939 requires DGC activity to increase c-di-GMP levels and to enhance biofilm formation in V. cholerae. To our knowledge, E. coli ydaM, which is negatively controlled by direct pairing with the RprA sRNA(Mika et al., 2012), is the only other GGDEF protein shown to be post-transcriptionally regulated as a result of direct pairing with an Hfq-dependent sRNA.
The four Qrr sRNAs of V. cholerae C6706 function redundantly to post transcriptionally regulate hapR mRNA, because a mutant with any three qrr genes deleted behaves like the WT strain for hapR expression (Lenz et al., 2004). This redundancy is due to a “dosage compensation” mechanism, whereby HapR indirectly activates transcription from the native promoter of the remaining qrr gene via a feedback loop (Svenningsen et al., 2008) (Svenningsen et al., 2009). We showed prior, by expressing Qrr2 from a non-native Ptac promoter and bypassing this feedback loop, that Qrr/hapR base pairing via a region of the Qrr sRNAs 100% conserved among pathogenic Vibrios controls the HapR-mediated QS response (Bardill et al., 2011). Our results here support a model that the Qrr sRNAs also act redundantly on vca0939. Firstly, the 100% conserved region in Qrrs 1–4 (Fig. S3) was predicted to facilitate pairing with not only hapR, but also vca0939 (and luxO) (Lenz et al., 2004) (Hammer & Bassler, 2007) (Svenningsen et al., 2009). Secondly, we show here that Qrr/vca0939 pairing indeed occurs via this conserved region (Fig. 6). Thirdly, ectopic expression from Ptac of both Qrr1 and Qrr2 activates vca0939 expression and resulting biofilm formation (Fig. S1, and (Hammer & Bassler, 2007)). In contrast Qrr2–4, but not Qrr1, control aphA expression, because aphA interaction requires additional Qrr sequence distinct from the 100% conserved region, which is present in Qrr2–4 but absent from Qrr1 (Fig. S3 and (Rutherford et al., 2011)). A comparative analysis of Qrr control of the two positively regulated targets (vca0939 and aphA) and two negatively regulated targets (hapR and luxO) remains to be described.
The Qrr sRNA “anti-antisense” mechanism described here is similar to how DsrA and RprA sRNAs positively control translation of the rpoS mRNA in E. coli; by preventing formation of an inhibitory secondary structure in the 5’UTR of rpoS that prevents translation in the absence of sRNAs (Majdalani et al., 1998, Majdalani et al., 2002). By binding to the left stem of the putative inhibitory stem loop in vca0939, Qrr sRNAs appear capable of competing with the right stem, thereby allowing access of translational machinery to the RBS (Fig. S2). Deletion of the left, right or entire sequence of the inhibitory structure, and several single nucleotide sustitutions in the left arm enabled Qrr-independent vca0939 expression (Fig. S2, panels B, C and D). In contrast, single nucleotide substitutions in the right arm prevented translation despite preserving the putative structure predicted by Mfold (data not shown). This is not due to disruption of the RBS, since deletion of the entire right arm still allowed for Vca0939 production (Fig. S2, panel C). It is interesting that the Qrr binding site in the 5’ UTR of hapR is predicted to be primarily single stranded, while nearly half of the Qrr binding site within vca0939 is predicted to be contained in the inhibitory structure, with the reminder accessible for Qrr pairing (Fig. S2) (Bardill et al., 2011). RNAup (Gruber et al., 2008) also predicts that Qrr pairing to vca0939 requires additional “opening energy” to disrupt the inhibitory structure that is not required for Qrr pairing to hapR (Fig. S2). These predictions are consistent with the observation that the apparent Kd of Qrr/vca0939 pairing (23 nM) shows weaker binding than that for Qrr/hapR pairing (7 nM). It will be interesting to determine the kinetics of Qrr binding for vca0939 and hapR (Bardill et al., 2011), as well as the remaining mRNAs predicted to be under sRNA control in V. cholerae (Rutherford et al., 2011, Svenningsen et al., 2009, Bardill & Hammer, 2012) .
We demonstrate in vitro that Hfq facilitates the transition from the inhibitory vca0939 structure to the activated form, in which WT Qrr RNA is paired to the sequence corresponding to the left stem of WT vca0939 (Fig. 1 and Fig. S2). We noted that the QrrWT/vca0939WT complex appeared to supershift to a location that may be distinct from the supershift observed for the QrrU3G/vca0939A43C complex (Fig. 6). There are several possible explanations for this apparent change in the supershift complex. Firstly, there may be conformational changes in the vca0939 mRNA not observable by computational methods, as Mfold predicted maintenance of the putative inhibitory structure despite single nt changes in the right stem that prevented translation (data not shown). Secondly, how Hfq contributes to Qrr/vca0939 pairing is poorly understood. Indeed, whether Hfq remains associated with an E. coli RprA/rpoS duplex or rapidly dissociates following RNA pairing is still not entirely clear, evident by two complexes by EMSA; one containing both RNAs and Hfq, the other containing both RNAs but lacking Hfq (Fender et al., 2010, Soper et al., 2011, Hwang et al., 2011). Because the only AU-rich region of the 67 nt 5’ UTR of vca0939 to which Hfq likely binds extends into the Qrr-binding site (Fig. S2, panel B), Hfq may also dissociate following Qrr/vca0939 duplex formation. Thirdly, single nucleotide changes that restore RybB/ompD pairing in E. coli alter mRNA structure and the position at which the sRNA binds (Balbontin et al., 2010). Similar structural complexity may exist in the 5’ UTR of vca0939 that might account for varied EMSA binding patterns (Fig. 5A and D). Analysis of vca0939 RNA structure could begin to address whether additional complexity exists during activation of vca0939 by the QS sRNAs.
Accumulation of c-di-GMP in V. cholerae has also been shown to not only enhance biofilm formation, but inhibit motility, by hampering transcription of flagellar genes including flrB and flrC (Beyhan et al., 2006, Liu et al., 2010, Krasteva et al., 2010). However, we observed no defect in motility and no significant change in transcript abundance of flrB and flrC by RT-PCR of V. cholerae luxOD47E, Δqrr1–4 carrying pQrr2 and either pEVS141 or pVca0939 (data not shown). It remains possible that levels of Vca0939, when induced by Qrr sRNA expression, although sufficient to enhance biofilms, are insufficient to repress motility under the conditions tested. It is also reasonable that Vca0939 may show “high specificity signaling” and regulates only a subset of dedicated targets involved in biofilms but not motility (Massie et al., 2012). Further investigation is warranted.
V. cholerae C6706 encodes 40 DGCs, as well as 29 PDEs that include 20 EAL domain proteins and 9 HD-GYP domain proteins (Galperin, 2004). DGC enzymes are often modular proteins encoding a variety of signal reception domains in the N terminus, and it has been proposed that certain DGCs may be activated in response to particular signals and tailored to control a defined set of c-di-GMP-controlled processes (Massie et al., 2012). Like other DGCs described, Vca0939 is predicted to carry an N-terminal PAS domain, which may be used to respond to oxygen or redox conditions (Qi et al., 2009, Chang et al., 2001). As such it is possible that the activity of Vca0939 to regulate biofilm formation integrates two distinct environmental inputs: QS control of translation, as well as signal activation of enzyme activity via modulation of its N-terminal PAS domain (Hengge, 2009).
The vca0939 gene is one of four genes shown to be under Qrr control by genetic analysis. Each of the other three genes (hapR, luxO, aphA) participates in a feedback loop with other components of the V. cholerae QS pathway (Svenningsen et al., 2008) (Svenningsen et al., 2009) (Rutherford et al., 2011). Although not yet shown to participate in QS feedback, Vca0939 and HapR do participate together in regulation of c-di-GMP levels critical for biofilm development (Fig. 1), indicating that QS utilizes multiple Qrr-dependent pathways to control attachment. Because the Qrr sRNAs negatively regulate HapR and both factors jointly control expression of the vps biofilm genes (Fig. 1), this regulatory network has the potential to function as a coherent feed-forward loop (FFL) (Mangan & Alon, 2003). However, a Δvca0939 mutant, unlike a ΔhapR mutant, is unaltered for biofilm formation under the conditions tested (Hammer & Bassler, 2003) (Hammer & Bassler, 2007), suggesting that this sRNA-based network, which includes an intracellular second messenger (c-di-GMP), does not behave as a FFL. It also remains possible that additional target genes under direct Qrr sRNA control remain to be identified in V. cholerae, as numerous sRNAs in E. coli have now been shown to control multiple mRNA targets (Soper et al., 2010, De Lay & Gottesman, 2012). The regulatory inputs controlling sRNA expression and the mRNA target genes under their control in V. cholerae will undoubtedly reveal a complex post-transcriptional network controlling many behaviors in this pathogen. Vca0939-controlled biofilm development is the first QS-mediated phenotype described to date in V. cholerae that is independent of the “master QS regulator” HapR.
Experimental procedures
Strains, plasmids and culture conditions
Standard microbiological techniques were used for growth of V. cholerae and E. coli. Liquid cultures in Luria broth (LB) were incubated at 37 °C with shaking unless noted otherwise. Antibiotics were added as appropriate to maintain plasmid selection. The genotypes of strains used in this study are in Table 1. V. cholerae strains are derived from strain C6706str2 (Thelin & Taylor, 1996). E. coli strains DH5α, S17λ-pir, DH10B, and EC100D (Epicentre) were used for cloning and expression. All plasmids for expressing RNA by in vitro transcription were constructed using pUC18. Plasmids for expressing Qrr2 sRNA and vca0939 were constructed in pEVS141 and pKK, which both lack the lacI gene, as indicated in Table 1. Thus, Qrr2 ectopically expressed from the Ptac promoter is constitutively transcribed in the pKK vector in the V. cholerae experiments, and in the pEVS141 in the E. coli experiments without addition of IPTG (Lenz et al., 2004, Miller et al., 2002). The plasmid for expression and purification of V. cholerae Hfq was described prior (Bardill et al., 2011). Single nucleotide substitutions were introduced by PCR-based methods or using a QuikChange XL kit (Stratagene). All constructs were sequenced to ensure that they did not contain sequence errors. Primer sequences used for PCR amplification and additional cloning details are available on request.
Table 1.
Strains and plasmids used in this study
| Strain | Description | Reference |
|---|---|---|
| BH2126 | V. choleraeΔqrr1–4, luxOD47E | Hammer & Bassler, 2007 |
| BH2130 | E. coli for 5’RACE | Hammer & Bassler, 2007 |
| Plasmids used in E. coli | Description | Reference |
| pEVS141 | vector control for pQrr2Ec | (Dunn et al., 2006) |
| pQrr2Ec | Ectopic WT Qrr2 expression | Bardill et al., 2011 |
| pQrr2U27CEc | Ectopic Qrr2U27C expression | This study |
| pKK | vector control for pVca0939Ec | Lenz et al., 2004 |
| pVca0939Ec | Vca0939-FLAG reporter | This study |
| pVca0939A43CEc | Vca0939A43C-FLAG reporter | This study |
| pVca0939ΔLSEc | Vca0939ΔLS -FLAG reporter | This study |
| pVca0939ΔRSEc | Vca0939ΔRS -FLAG reporter | This study |
| pVca0939ΔLS+RSEc | Vca0939ΔLS+RS -FLAG reporter | This study |
| pVca0939C41GEc | Vca0939C41G-FLAG reporter | This study |
| pVca0939G44CEc | Vca0939G44C-FLAG reporter | This study |
| pVca0939G35CEc | Vca0939G35C-FLAG reporter | This study |
| Plasmids used in V. cholerae | Description | Reference |
| pKK | vector control for pQrr2 | Lenz et al., 2004 |
| pQrr2 | Ectopic WT Qrr2 expression | This study |
| pQrr2U27C | Ectopic Qrr2U27C expression | This study |
| pEVS141 | vector control for pVca0939 | Dunn et al., 2006 |
| pVca0939 | Vca0939-FLAG reporter | This study |
| pVca0939A43C | Vca0939A43C-FLAG reporter | This study |
| pVca0939AAEEF | Vca0939AAEEF-FLAG reporter | This study |
| pVca0939GGEAF | Vca0939GGEAF-FLAG reporter | This study |
| Other Plasmids | Description | Reference |
| pPB004 | Qrr2 in vitro transcription | Bardill et al., 2011 |
| pEZ637 | Vca0939 in vitro transcription | This study |
| pEZ731 | Qrr2U27Gin vitro transcription | This study |
| pEZ774 |
Vca0939A43C in vitro transcription |
This study |
| pPB007 | Hfq expression plasmid | Bardill et al., 2011 |
Western Blotting
Bacterial cultures were grown to mid-logarithmic phase, normalized to cell density, pelleted by centrifugation and resuspended in Laemmli sample buffer. These samples were subjected to SDS-PAGE and transferred to PVDF membrane. Protein loading was verified using Ponceau S stain. Membranes were blocked in a 5% milk Tris-buffered saline solution and probed with a monoclonal anti-FLAG antibody (Sigma) followed by peroxidase conjugated secondary antibody (Sigma). A chemiluminescent detection kit (Thermo-Fisher) was used to visualize the proteins on a ChemiDoc XRS HQ system (Bio-Rad).
Hfq and RNA purification and labeling
V. cholerae Hfq protein was purified as previously described (Bardill et al., 2011) using the IMPACT affinity tag intein kit (New England Biolabs). Protein quality was assessed by SDS-PAGE and concentration was determined spectrophotometrically. Qrr RNA and vca0939 RNA were generated by run-off transcription using a MEGAscript kit (Ambion). Following transcription, the RNAs were precipitated with LiCl and resuspended in RNase free water as described in (Bardill et al., 2011). Both denaturing and non-denaturing PAGE were used to assess RNA quality following staining with SYBR Gold. RNA concentrations were determined spectrophotometrically. Dephosphorylated RNA was labeled using [γ-32P] ATP and T4 polynucleotide kinase (Ambion). Labeled RNA was purified with NucAway spin columns (Ambion).
Electrophoretic mobility shift assays
Binding assays were conducted as described previously (Bardill et al., 2011). Briefly labeled Qrr RNA was mixed with varying concentrations of vca0939 RNA in the presence or absence of V. cholerae Hfq. Final concentration of Qrr RNA used was 0.4 nM; Hfq concentration was 150 nM. Before mixing, the RNAs were denatured by incubation at 70 °C and quick-cooled on ice. After mixing, the reactions were incubated at 25 °C for 1 hr and subjected to non-denaturing PAGE at 4 °C. As a control, Qrr and vca0939 RNAs were mixed together, heated at 70 °C and cooled slowly without Hfq to document duplex formation. Gels were exposed a storage phosphor screen that was scanned and analyzed with a Typhoon Phosphorimager (GE). All reactions were carried out in buffer containing 20 mM Tris-HCL pH 8, 100 mM NH4Cl, 50 nM NaCl, 50 nM KCl and 5% glycerol.
Biofilm development assays
Analyses of rugose colony morphology development were conducted as described previously (Fong et al., 2010, Ray et al., 2012). Cultures grown overnight at 30 °C with shaking (250 rpm) were serially diluted with LB medium and a 10 µl aliquot of each diluted culture was spotted onto LB agar medium with ampicillin and kanamycin. Spot development was followed over a course of 40 h at 30 °C. Images of the spotted cultures were acquired at the indicated time points using a dissecting microscope (Olympus SZX16).
Determination of the Intracellular Concentration of c-di-GMP
To quantify c-di-GMP, an overnight culture of each strain of interest was back diluted 1:100 with three biological replicates, and the 1.5 mL cultures were grown to mid-exponential phase. Cells were then pelleted by centrifugation and resuspended in 100 µL cold extraction buffer (40% Acetonitrile, 40% Methanol, 0.1 N Formic Acid). The supernatant was concentrated using a centrifugal evaporator and the product was resuspended in 100 µL water. Quantification of c-di-GMP was performed as previously described (Massie 2012) using an Acquity Ultra Performance liquid chromatography system coupled with a Quattro Premier XE mass spectrometer. The concentration of c-di-GMP was determined by quantifying an 8-point standard curve of chemically synthesized c-di-GMP (Biolog) ranging from 250 nM to 1.9 nM.
5 ’ rapid amplification of cDNA ends (5 ′-RACE)
Total RNA from E. coli strain BH 2130 (Hammer & Bassler, 2007) was isolated using the mirVana RNA isolation kit (Ambion) as recommended by the manufacturer. The obtained full length cDNA was amplified by PCR and nested PCR reactions using the 5' RACE System for Rapid Amplification of cDNA Ends, Version 2.0 (Invitrogen). Sequencing determined the transcriptional start site using a primer specific to vca0939 (available upon request).
RNA extraction and real-time quantitative RT-PCR analysis
Total RNA was extracted from cells using the mirVana RNA isolation kit (Ambion) according to the manufacturer’s instructions. cDNA synthesis and the mRNA expression was performed using a Power SYBR® Green RNA-to-CT™ 1-Step Kit (Applied Biosystems) on an Applied Biosystems Step One Plus Sequence Detector (Applied Biosystems). Thermal cycling conditions included reverse transcription at 48°C for 30 min, activation of AmpliTaq Gold DNA polymerase at 95 °C for 10 min followed by 40 cycles at 95 °C for 15 s and 60°C for 1 min. Primers are available upon request.
Supplementary Material
Acknowledgements
The authors thank Dr. Roger Wartell and Dr. Taylor Updegrove for assistance with the EMSA experiments and the MSU mass spectrometry facility for technical assistance. The authors would also like to thank Dr. Patrick Bardill for advice regarding the manuscript. This study was supported by a National Science Foundation grant (MCB-0919821) to Dr. Brian K. Hammer and a National Institutes of Health grant 5U19AI090872-03 to Dr. Christopher M. Waters.
References
- Antonova ES, Hammer BK. Quorum-sensing autoinducer molecules produced by members of a multispecies biofilm promote horizontal gene transfer to Vibrio cholerae. FEMS microbiology letters. 2011;322:68–76. doi: 10.1111/j.1574-6968.2011.02328.x. [DOI] [PubMed] [Google Scholar]
- Balbontin R, Fiorini F, Figueroa-Bossi N, Casadesus J, Bossi L. Recognition of heptameric seed sequence underlies multi-target regulation by RybB small RNA in Salmonella enterica. Molecular microbiology. 2010;78:380–394. doi: 10.1111/j.1365-2958.2010.07342.x. [DOI] [PubMed] [Google Scholar]
- Bardill JP, Hammer BK. Non-coding sRNAs regulate virulence in the bacterial pathogen Vibrio cholerae. RNA biology. 2012;9:392–401. doi: 10.4161/rna.19975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardill JP, Zhao X, Hammer BK. The Vibrio cholerae quorum sensing response is mediated by Hfq-dependent sRNA/mRNA base pairing interactions. Molecular microbiology. 2011;80:1381–1394. doi: 10.1111/j.1365-2958.2011.07655.x. [DOI] [PubMed] [Google Scholar]
- Beyhan S, Tischler AD, Camilli A, Yildiz FH. Transcriptome and phenotypic responses of Vibrio cholerae to increased cyclic di-GMP level. Journal of bacteriology. 2006;188:3600–3613. doi: 10.1128/JB.188.10.3600-3613.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper-Lindley C, Yildiz FH. VpsT is a transcriptional regulator required for expression of vps biosynthesis genes and the development of rugose colonial morphology in Vibrio cholerae O1 El Tor. Journal of bacteriology. 2004;186:1574–1578. doi: 10.1128/JB.186.5.1574-1578.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AL, Tuckerman JR, Gonzalez G, Mayer R, Weinhouse H, Volman G, Amikam D, Benziman M, Gilles-Gonzalez MA. Phosphodiesterase A1, a regulator of cellulose synthesis in Acetobacter xylinum, is a heme-based sensor. Biochemistry. 2001;40:3420–3426. doi: 10.1021/bi0100236. [DOI] [PubMed] [Google Scholar]
- Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler BL, Hughson FM. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- De Lay N, Gottesman S. A complex network of small non-coding RNAs regulate motility in Escherichia coli. Molecular microbiology. 2012;86:524–538. doi: 10.1111/j.1365-2958.2012.08209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AK, Millikan DS, Adin DM, Bose JL, Stabb EV. New rfp- and pES213-derived tools for analyzing symbiotic Vibrio fischeri reveal patterns of infection and lux expression in situ. Applied and environmental microbiology. 2006;72:802–810. doi: 10.1128/AEM.72.1.802-810.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fender A, Elf J, Hampel K, Zimmermann B, Wagner EG. RNAs actively cycle on the Sm-like protein Hfq. Genes & development. 2010;24:2621–2626. doi: 10.1101/gad.591310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RA, Boesman-Finkelstein M, Chang Y, Hase CC. Vibrio cholerae hemagglutinin/protease, colonial variation, virulence, and detachment. Infection and immunity. 1992;60:472–478. doi: 10.1128/iai.60.2.472-478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong JC, Syed KA, Klose KE, Yildiz FH. Role of Vibrio polysaccharide (vps) genes in VPS production, biofilm formation and Vibrio cholerae pathogenesis. Microbiology. 2010;156:2757–2769. doi: 10.1099/mic.0.040196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich KS, Vogel J. Activation of gene expression by small RNA. Current opinion in microbiology. 2009;12:674–682. doi: 10.1016/j.mib.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Galperin MY. Bacterial signal transduction network in a genomic perspective. Environmental microbiology. 2004;6:552–567. doi: 10.1111/j.1462-2920.2004.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AR, Lorenz R, Bernhart SH, Neubock R, Hofacker IL. The Vienna RNA websuite. Nucleic acids research. 2008;36:W70–W74. doi: 10.1093/nar/gkn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer BK, Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae. Molecular microbiology. 2003;50:101–104. doi: 10.1046/j.1365-2958.2003.03688.x. [DOI] [PubMed] [Google Scholar]
- Hammer BK, Bassler BL. Regulatory small RNAs circumvent the conventional quorum sensing pathway in pandemic Vibrio cholerae. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11145–11149. doi: 10.1073/pnas.0703860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer BK, Bassler BL. Distinct sensory pathways in Vibrio cholerae El Tor and classical biotypes modulate cyclic dimeric GMP levels to control biofilm formation. Journal of bacteriology. 2009;191:169–177. doi: 10.1128/JB.01307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge R. Principles of c-di-GMP signalling in bacteria. Nature reviews Microbiology. 2009;7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- Higgins DA, Pomianek ME, Kraml CM, Taylor RK, Semmelhack MF, Bassler BL. The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature. 2007;450:883–886. doi: 10.1038/nature06284. [DOI] [PubMed] [Google Scholar]
- Hwang W, Arluison V, Hohng S. Dynamic competition of DsrA and rpoS fragments for the proximal binding site of Hfq as a means for efficient annealing. Nucleic acids research. 2011;39:5131–5139. doi: 10.1093/nar/gkr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling MG, Holmes RK. Characterization of hapR, a positive regulator of the Vibrio cholerae HA/protease gene hap, and its identification as a functional homologue of the Vibrio harveyi luxR gene. Molecular microbiology. 1997;26:1023–1034. doi: 10.1046/j.1365-2958.1997.6402011.x. [DOI] [PubMed] [Google Scholar]
- Kovacikova G, Lin W, Skorupski K. Vibrio cholerae AphA uses a novel mechanism for virulence gene activation that involves interaction with the LysR-type regulator AphB at the tcpPH promoter. Molecular microbiology. 2004;53:129–142. doi: 10.1111/j.1365-2958.2004.04121.x. [DOI] [PubMed] [Google Scholar]
- Krasteva PV, Fong JC, Shikuma NJ, Beyhan S, Navarro MV, Yildiz FH, Sondermann H. Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science. 2010;327:866–868. doi: 10.1126/science.1181185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger J, Rehmsmeier M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic acids research. 2006;34:W451–W454. doi: 10.1093/nar/gkl243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulasakara H, Lee V, Brencic A, Liberati N, Urbach J, Miyata S, Lee DG, Neely AN, Hyodo M, Hayakawa Y, Ausubel FM, Lory S. Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3’−5’)-cyclic-GMP in virulence. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2839–2844. doi: 10.1073/pnas.0511090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS, Bassler BL. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell. 2004;118:69–82. doi: 10.1016/j.cell.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Liu X, Beyhan S, Lim B, Linington RG, Yildiz FH. Identification and characterization of a phosphodiesterase that inversely regulates motility and biofilm formation in Vibrio cholerae. Journal of bacteriology. 2010;192:4541–4552. doi: 10.1128/JB.00209-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalani N, Cunning C, Sledjeski D, Elliott T, Gottesman S. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:12462–12467. doi: 10.1073/pnas.95.21.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdalani N, Hernandez D, Gottesman S. Regulation and mode of action of the second small RNA activator of RpoS translation, RprA. Molecular microbiology. 2002;46:813–826. doi: 10.1046/j.1365-2958.2002.03203.x. [DOI] [PubMed] [Google Scholar]
- Mangan S, Alon U. Structure and function of the feed-forward loop network motif. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11980–11985. doi: 10.1073/pnas.2133841100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Wilson HF, Tamayo R, Tischler AD, Lazinski DW, Camilli A. The vibrio cholerae hybrid sensor kinase VieS contributes to motility and biofilm regulation by altering the cyclic diguanylate level. Journal of bacteriology. 2008;190:6439–6447. doi: 10.1128/JB.00541-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie JP, Reynolds EL, Koestler BJ, Cong JP, Agostoni M, Waters CM. Quantification of high-specificity cyclic diguanylate signaling. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12746–12751. doi: 10.1073/pnas.1115663109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meibom KL, Blokesch M, Dolganov NA, Wu CY, Schoolnik GK. Chitin induces natural competence in Vibrio cholerae. Science. 2005;310:1824–1827. doi: 10.1126/science.1120096. [DOI] [PubMed] [Google Scholar]
- Mika F, Busse S, Possling A, Berkholz J, Tschowri N, Sommerfeldt N, Pruteanu M, Hengge R. Targeting of csgD by the small regulatory RNA RprA links stationary phase, biofilm formation and cell envelope stress in Escherichia coli. Molecular microbiology. 2012;84:51–65. doi: 10.1111/j.1365-2958.2012.08002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell. 2002;110:303–314. doi: 10.1016/s0092-8674(02)00829-2. [DOI] [PubMed] [Google Scholar]
- Morris AR, Darnell CL, Visick KL. Inactivation of a novel response regulator is necessary for biofilm formation and host colonization by Vibrio fischeri. Molecular microbiology. 2011;82:114–130. doi: 10.1111/j.1365-2958.2011.07800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng WL, Bassler BL. Bacterial quorum-sensing network architectures. Annual review of genetics. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Rao F, Luo Z, Liang ZX. A flavin cofactor-binding PAS domain regulates c-di-GMP synthesis in AxDGC2 from Acetobacter xylinum. Biochemistry. 2009;48:10275–10285. doi: 10.1021/bi901121w. [DOI] [PubMed] [Google Scholar]
- Ray VA, Morris AR, Visick KL. A semi-quantitative approach to assess biofilm formation using wrinkled colony development. Journal of visualized experiments : JoVE. 2012:e4035. doi: 10.3791/4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford ST, van Kessel JC, Shao Y, Bassler BL. AphA and LuxR/HapR reciprocally control quorum sensing in vibrios. Genes & development. 2011;25:397–408. doi: 10.1101/gad.2015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryjenkov DA, Tarutina M, Moskvin OV, Gomelsky M. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. Journal of bacteriology. 2005;187:1792–1798. doi: 10.1128/JB.187.5.1792-1798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AJ, Ryjenkov DA, Gomelsky M. The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. Journal of bacteriology. 2005;187:4774–4781. doi: 10.1128/JB.187.14.4774-4781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper T, Mandin P, Majdalani N, Gottesman S, Woodson SA. Positive regulation by small RNAs and the role of Hfq. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9602–9607. doi: 10.1073/pnas.1004435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper TJ, Doxzen K, Woodson SA. Major role for mRNA binding and restructuring in sRNA recruitment by Hfq. Rna. 2011;17:1544–1550. doi: 10.1261/rna.2767211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D, Harris RC, Waters CM. Integration of cyclic di-GMP and quorum sensing in the control of vpsT and aphA in Vibrio cholerae. Journal of bacteriology. 2011;193:6331–6341. doi: 10.1128/JB.05167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D, Waters CM. A tangled web: regulatory connections between quorum sensing and cyclic Di-GMP. Journal of bacteriology. 2012;194:4485–4493. doi: 10.1128/JB.00379-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsen SL, Tu KC, Bassler BL. Gene dosage compensation calibrates four regulatory RNAs to control Vibrio cholerae quorum sensing. The EMBO journal. 2009;28:429–439. doi: 10.1038/emboj.2008.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsen SL, Waters CM, Bassler BL. A negative feedback loop involving small RNAs accelerates Vibrio cholerae’s transition out of quorum-sensing mode. Genes & development. 2008;22:226–238. doi: 10.1101/gad.1629908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal R, Wong HC, Calhoon R, Gelfand D, Fear AL, Volman G, Mayer R, Ross P, Amikam D, Weinhouse H, Cohen A, Sapir S, Ohana P, Benziman M. Three cdg operons control cellular turnover of cyclic di-GMP in Acetobacter xylinum: genetic organization and occurrence of conserved domains in isoenzymes. Journal of bacteriology. 1998;180:4416–4425. doi: 10.1128/jb.180.17.4416-4425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelin KH, Taylor RK. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect Immun. 1996;64:2853–2856. doi: 10.1128/iai.64.7.2853-2856.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler AD, Camilli A. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Molecular microbiology. 2004;53:857–869. doi: 10.1111/j.1365-2958.2004.04155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu KC, Long T, Svenningsen SL, Wingreen NS, Bassler BL. Negative feedback loops involving small regulatory RNAs precisely control the Vibrio harveyi quorum-sensing response. Molecular cell. 2010;37:567–579. doi: 10.1016/j.molcel.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegrove T, Wilf N, Sun X, Wartell RM. Effect of Hfq on RprA-rpoS mRNA pairing: Hfq-RNA binding and the influence of the 5’ rpoS mRNA leader region. Biochemistry. 2008;47:11184–11195. doi: 10.1021/bi800479p. [DOI] [PubMed] [Google Scholar]
- Waters CM, Lu W, Rabinowitz JD, Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. Journal of bacteriology. 2008;190:2527–2536. doi: 10.1128/JB.01756-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz FH, Schoolnik GK. Vibrio cholerae O1 El Tor: identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4028–4033. doi: 10.1073/pnas.96.7.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Mekalanos JJ. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Developmental cell. 2003;5:647–656. doi: 10.1016/s1534-5807(03)00295-8. [DOI] [PubMed] [Google Scholar]
- Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3129–3134. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic acids research. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.