Summary
Background
Most ADAMTS13 assays use non-physiological conditions (low ionic strength, low pH, barium chloride), are subject to interference from plasma proteins, hemoglobin and bilirubin, and have limited sensitivity, especially for inhibitors.
Objectives
We addressed these constraints by designing a substrate that can be used in undiluted plasma.
Methods
A polypeptide was expressed in E. coli that corresponds to von Willebrand factor Gln1599-Arg1668, with mutations N1610C and K1617R and an N-terminal Gly. Substrate FRETS-rVWF71 was prepared by modifying Cys1610 with DyLight 633 (abs 638 nm, em 658 nm) and the N-terminus with IRDye QC-1 (abs 500-800 nm). Assays were performed at pH 7.4 in 150 mM NaCl, 10 mM CaCl2.
Results
Serum and plasma anticoagulated with citrate or heparin had equivalent ADAMTS13 activity with FRETS-rVWF71. Neither bilirubin (≤20 mg/dL) nor hemoglobin (≤20 g/L) interfered with product detection. Assays with FRETS-rVWF71 and FRETS-VWF73 gave similar results (R2 = 0.95) for plasma from 80 subjects with thrombotic microangiopathy, 22 subjects with other causes of thrombocytopenia, and 20 healthy controls. The limit of detection with FRETS-rVWF71 for ADAMTS13 activity was ≤0.3%. Inhibitor assays with FRETS-rVWF71 gave titers ~2.5-fold higher than with FRETS-VWF73 and clearly distinguished patients with and without inhibitors.
Conclusions
FRETS-rVWF71 is suitable for ADAMTS13 assays in minimally diluted plasma or serum without interference from proteins, bilirubin or free hemoglobin in plasma. Optimized detection of ADAMTS13 inhibitors will facilitate the monitoring of antibody responses during the treatment of thrombotic thrombocytopenic purpura.
Keywords: ADAMTS13 protein, human; Purpura, thrombotic thrombocytopenic; von Willebrand factor; Recombinant Fusion Proteins; Kinetics; Substrate Specificity
Introduction
Thrombotic thrombocytopenic purpura (TTP) is characterized by microangiopathic hemolytic anemia, thrombocytopenia, and tissue damage caused by microvascular thrombosis. Congenital TTP is caused by inherited deficiency of ADAMTS13, a metalloprotease that cleaves von Willebrand factor (VWF) multimers at the Tyr1605-Met1606 bond in the A2 domain. Idiopathic TTP in adults is usually caused by autoantibodies that inhibit ADAMTS13 activity or promote ADAMTS13 clearance from plasma [1, 2]. Idiopathic TTP is usually fatal without treatment, but plasma exchange therapy has reduced the morality rate to <20% [3].
The ability to assay plasma ADAMTS13 was a crucial advance in understanding the pathogenesis of TTP. The first generation of assays used VWF as the substrate with urea or guanidine hydrochloride to expose the cleavage site in the VWF A2 domain [4-6]. These assays were groundbreaking, but technically challenging and difficult to automate.
Many of these problems were circumvented by the development of FRETS-VWF73 [7], a fluorogenic substrate that corresponds to VWF Asp1596-Arg1668 (73 residues). Results with FRETS-VWF73 have been congruent with assays based on VWF substrates, with increased sensitivity and precision [7-10].
Despite these attributes, FRETS-VWF73 has some limitations. The nonphysiological assay conditions (pH 6.0, no NaCl) [7] can alter the interaction of ADAMTS13 with substrates or inhibitors [11]. In addition, its N-methyl anthranilate moiety absorbs at 340 nm and emits at 450 nm, which makes assays susceptible to interference from autofluorescence, absorbance and quenching by plasma proteins, hemoglobin and bilirubin [12]. To avoid this interference, plasma is diluted ≥1:20, which limits assay sensitivity to ~3-5% of normal ADAMTS13 levels and prevents the detection of some inhibitors [9, 10, 13]. Also, dissociation of ADAMTS13-inhibitor complexes during the assay can lead to overestimation of ADAMTS13 activity for patients with low titer inhibitors [13].
We addressed these issues by designing the fluorogenic substrate FRETS-rVWF71, which is similar to FRETS-VWF73 but with a recombinant peptide backbone and brighter dyes that absorb at wavelengths where plasma is almost transparent. This substrate is compatible with minimally diluted plasma under physiological conditions of pH and ionic strength, which permits highly sensitive assays for ADAMTS13 activity and inhibitors.
Materials and Methods
Plasmid Constructs
DNA encoding VWF Gln1599-Arg1668 was amplified from pSVHvWF1 [14] using primers:
Forward, GGTATTGAGGGTCGCGAGAACCTTTATTTCCAGGGCCAGGCGCCC
Reverse, AGAGGAGAGTTAGAGCCTCACCTCTGCAGCACCAGGTC
The PCR product was inserted [15] into pET-32 Xa/LIC (Novagen). Mutations N1610C and K1617R were introduced to yield pET32XaTEVvWF71, which encodes thioredoxin, a His-tag, a TEV cleavage site, a Gly residue, and VWF Gln1599-Arg1668.
Recombinant Peptide Preparation
E. coli BL21 (DE3) transformed with pET32XaTEVvWF71 was grown at 37 °C in LB medium, 50 μg/mL ampicillin. Log-phase cultures were induced with 1 mM isopropyl β-D-1-thiogalactopyranoside for 4 hours. After centrifugation, cells were lysed in 10 mL/g of B-PER Protein Extraction Reagent (Pierce), 250 U/mL benzonase (Novagen), 10 μL/mL Halt Protease Inhibitor Cocktail (Pierce) and 1 mM phenylmethylsulfonyl fluoride for 15 min at room temperature. After centrifugation (20,000 × g, 15 min, 4 °C) the supernatant was diluted 1:1 with His-buffer (20 mM sodium phosphate, pH 7.4, 500 mM NaCl, 10 mM imidazole), adsorbed on 5 ml Ni-NTA agarose (Agarose Beads Technologies, Spain), and eluted with 300 mM imidazole in His-buffer. The eluate was concentrated to 10 mL by ultrafiltration and dialyzed (Slide-A-Lyzer MWCO 7,000, Pierce) against 50 mM Tris-HCl, pH 8.0, 1 mM dithiothreitol, and 0.5 mM EDTA.
His-tagged TEV protease [16] was added (100 μg/10 mg of protein) for 16-20 h at room temperature. The solution was dialyzed against His-buffer (Slide-A-Lyzer, MWCO 3,000). TEV protease was removed by adsorption on Ni-NTA agarose. Dithiothreitol (10 mM) was added and VWF71 was purified by HPLC on a C18 column (300 Å, 5 μm, 150 × 10 mm, GraceVydac) in buffer A (50 mM triethylammonium acetate (TEAA), pH 6.0) at 2 mL/min followed by a 35 min gradient of 35%-75% buffer B (60% acetonitrile/40% 50 mM TEAA, pH 6.0). Alternatively, the column was equilibrated with 0.1% trifluoroacetic acid (TFA) and developed with a gradient of 20%-90% buffer C (0.092% TFA, 90% acetonitrile). Fractions containing VWF71 were lyophilized, dissolved in 100 mM sodium phosphate, pH 7.1, and desalted on PD-10 (GE Healthcare).
Fluorescent Dye Labeling
DyLight 633 maleimide (abs 638 nm, em 658 nm, ε 170,000 M−1 cm−1, Thermo Scientific) 1 mg/100 μL in dimethyl sulfoxide (DMSO) was added to VWF71 (10 mg) in ≤2 mL of 100 mM sodium phosphate, pH 7.1, in the dark, and stirred overnight at room temperature. DyLight 633-rVWF71 was purified by HPLC with TEAA/acetonitrile, lyophilized and desalted on PD-10 in ≤2 mL of 100 mM sodium phosphate, pH 7.9. IRDye QC-1 N-hydroxysuccinimide ester (abs 737 nm, ε 96,000 M−1 cm−1, LI-COR) 0.5 mg in 100 μL DMSO was added with stirring overnight in the dark. Product FRETS-rVWF71 was purified by HPLC, lyophilized, dissolved in ≤0.5 mL water and applied onto a column (7 × 230 mm) of Amberlite IR120 sodium form (Dow Chemical Company). FRETS-rVWF71 was eluted with water and concentrated to ≥250 μM as determined by amino acid analysis (The Protein Chemistry Laboratory, Texas A&M University) and stored at −20°C.
FRETS-rVW71 ADAMTS13 Activity Assays
For routine assays, up to 100 μL of test sample (plasma or serum) and 10 μL protease inhibitor solution composed of 2 μL of 10 mM phenylmethylsulfonyl fluoride, 3 μL of Halt inhibitor cocktail and 5 μL assay buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 10 mM CaCl2, and 0.05% Tween-20) to make 110 μL total volume were added to 96 well white microplates (Optiplate-96, PerkinElmer) at 30 °C. Plasma samples were usually assayed using 25 μL and 100 μL plasma. A standard curve was constructed with 0, 5, 10, 25, 50, 75 and 100 μL pooled normal Li+-heparin plasma (PNP), with assay buffer added to make 110 μL total volume. Assays were initiated by adding 90 μL of 2.2 μM FRETS-rVWF71 in assay buffer for a final assay volume of 200 μL. Negative control assays were performed similarly with 10 mM EDTA. Cleavage was detected as an increase in fluorescence using a Victor2V Multilabel Counter (PerkinElmer) or Synergy H1 Hybrid Multi-Mode Microplate Reader (Biotek) with 635 ± 10 nm excitation and 660 ± 10 nm emission filters. Initial velocities were determined by fitting progress curves to ΔF(t) = A + Bt + Ct2, where ΔF(t) is the change in fluorescence at time t, A is the y-intercept, B is the initial velocity (slope), and the term Ct2 accounts for any decline in velocity due to substrate consumption or photobleaching. The PNP standard curve was fit to B(v) = D + Ev + Fv2, where v is the volume of PNP added. Values for test samples were interpolated.
FRETS-rVWF71 assays with citrated plasma samples contained 10 U/mL heparin to prevent clotting. ADAMTS13 activity was assayed with 25 μL citrated plasma and for samples with ADAMTS13 activity <3% assays were repeated with 100 μL plasma for highest sensitivity.
FRETS-rVWF71 ADAMTS13 Inhibitor Assay
For standard inhibitor assays, Li+-heparin plasma samples with ADAMTS13 activity <20% are serially diluted (without heat treatment) in assay buffer. 50 μL PNP is mixed with 50 μL test plasma dilutions in 96 well white microplates. We have tested heat inactivation and it makes almost no difference in the inhibitor titers for samples with ADAMTS13 activity <20%. Citrated plasma samples were assayed similarly except that 10 U/mL heparin was added. Microplates were sealed with adhesive film and incubated at 37 °C for 1 h. Reaction was initiated by adding 90 μL of assay buffer containing 2.2 μM FRETS-rVWF71. The inhibitor titer was determined by fitting initial reaction rates to a sigmoidal dose response equation by nonlinear regression:
Rate(D) is the initial rate with test plasma at dilution D, Max is the rate without test plasma, Min is the rate with no enzyme, H is the Hill slope, and T is the inhibitor titer in “Bethesda-like” units (U). 1 U reduces ADAMTS13 activity 50%. Titers are reported for a decrease in ADAMTS13 of ≥30% (inhibitor ≥0.4 U).
FRETS-VWF73 Assays
FRETS-VWF73 activity assays were performed as described [7] but with 10 μL of citrated plasma per 500 μL total assay volume. Inhibitor assays were performed for samples with ADAMTS13 activity <5%, according to BloodCenter of Wisconsin standard procedures. Test plasma was incubated at 56 °C for 30 minutes, then mixed with an equal volume of citrated PNP for 1 hour at room temperature before assay.
Hemoglobin was prepared by hypotonic lysis of red blood cells and quantified with Drabkin’s reagent (Sigma).
FRETS-rVWF71 kinetics
Cleavage by plasma ADAMTS13 (50 μL) was assessed in 200 μL reactions containing assay buffer and 0.25-12 μM FRETS-rVWF71. ADAMTS13 in pooled normal plasma (PNP) is ~1.03 μg/ml [17] or ~6 nM. The fluorescence yield of product was determined by cleaving FRETS-rVWF71 to completion with recombinant ADAMTS13 MDTCS [18]. Initial velocities were fitted to the Michaelis-Menten equation by nonlinear regression analysis (Prism, GraphPad).
Healthy Controls and Subjects with TTP
Serum was prepared by clotting whole blood at room temperature for 30-60 minutes and centrifugation at 2500 × g for 10 minutes at 4 °C. Matched samples of serum and plasma anticoagulated with sodium citrate (3.2%, diluted 1:9, 10.9 mM final concentration), Li+-heparin and Na+-heparin (15 U/ml final heparin concentration) were obtained from healthy donors, and Li+-heparin samples were obtained from patients with TTP with informed consent according to a protocol approved by the Washington University Institutional Review Board.
Li+-heparin plasma samples were obtained from 96 healthy controls (Biological Specialty Corp., Colmar, PA). Li+-heparin PNP standard was prepared from ≥35 donors. 25 μL and 100 μL plasma samples were assayed in duplicate for ADAMTS13 activity. For cross-validation, ≥3 samples from each batch were reanalyzed with the subsequent batch.
Citrated plasma samples from the clinical laboratory of the BloodCenter of Wisconsin were deidentified and coded according to a protocol approved by the Institutional Review Board.
Results
Preparation of fluorogenic FRETS-rVWF71 substrate
The substrate includes VWF Gln1599-Arg1668. The mutation N1610C introduces a unique Cys, and the mutation K1617R removes a primary amine that would compete with modification of the N-terminus. Chimeric thioredoxin-rVWF71 was purified (Fig. S1) with a yield of >50 mg/L of bacterial culture. Removal of thioredoxin with TEV protease gave product rVWF71, which has an extra Gly before VWF Gln1599-Arg1668. The product was purified to yield >10 mg rVWF71/L of culture medium.
The rVWF71 was modified with DyLight 633 maleimide in ~70% yield. The N-terminal Gly of DyLight 633-rVWF71 was modified with IRDye QC-1 N-hydroxy-succinimidyl ester in ~90% yield. Purified FRETS-rVWF71 (Fig. S2) was stable at −20 °C (data not shown) and soluble in water at >250 μM.
FRETS-rVWF71 had absorbance maxima at 627 nm and 819 nm, consistent with the presence of both dyes. Based on amino acid analysis, the extinction coefficient at 627 nm is 246,000 M−1cm−1. Upon excitation at 635 nm, fluorescence emission at 660 nm of cleaved or uncleaved FRETS-rVWF71 varied <7% between pH 5 and pH 10. The fluorescence emission of FRETS-rVWF71 increased ~400-fold upon cleavage, indicating that internal quenching is efficient.
Optimization of FRETS-rVW71 cleavage by plasma ADAMTS13
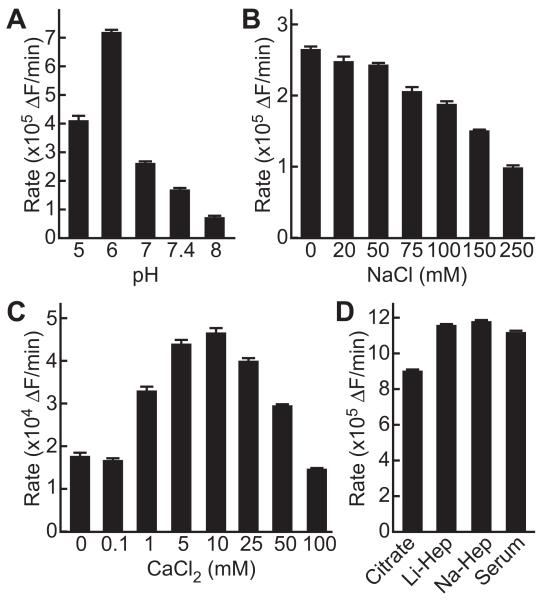
Optimal conditions for ADAMTS13 cleavage of FRETS-rVWF71 were similar to those for other substrates [5, 7, 19]. Activity was maximal at pH 6, low ionic strength, and 5-10 mM CaCl2 (Fig. 1). At pH 7.4 and 150 mM NaCl, the rate of reaction decreased ~75%; these conditions were selected for standard assays to optimize compatibility with plasma.
Fig. 1.
ADAMTS13 activity toward FRETS-rVWF71 under different conditions. (A) Dependence on pH. Reactions (200 μL) contained PNP (10 μL), 150 mM NaCl, 10 mM CaCl2, 0.05% Tween-20, 1 μM FRETS-rVWF71 and 20 mM sodium acetate (pH 5), Bis-Tris (pH 6), HEPES (pH 7, 7.4), or Tris-HCl (pH 8). (B,C) Dependence on ionic strength and calcium ions. Reactions (200 μL) in standard assay buffer contained PNP (10 μL) and variable concentrations of (B) NaCl or (C) CaCl2. (D) Dependence on sample preparation. Reactions (200 μL) contained 1 μM FRETS-rVWF71, 100 μL standard assay buffer, and 100 μL of serum, citrated plasma, Li+-heparin plasma, or Na+-heparin plasma. Values plotted are the mean ± SE (n=2)
FRETS-rVWF71 assays of serum or plasma anticoagulated with citrate, Li+-heparin or Na+-heparin gave comparable results. Using <75 μL plasma per assay, the values for citrated plasma were decreased ~10% compared to heparinized plasma because of dilution by the citrate anticoagulant (Fig. S3). However, assays with 100 μL citrated plasma (Fig. 1) gave values that were decreased an additional ~18% because the higher citrate concentration (5.5 mM) reduces the calcium ion concentration below the optimum for ADAMTS13 activity. This effect can be countered by adding proportionally more calcium chloride (Fig. S3). Plasma anticoagulated with heparin did not exhibit this behavior and required no adjustments to buffer conditions across the working range of the assay. Li+-heparin plasma is commonly used for clinical chemistry assays and was selected for ADAMTS13 assay development.
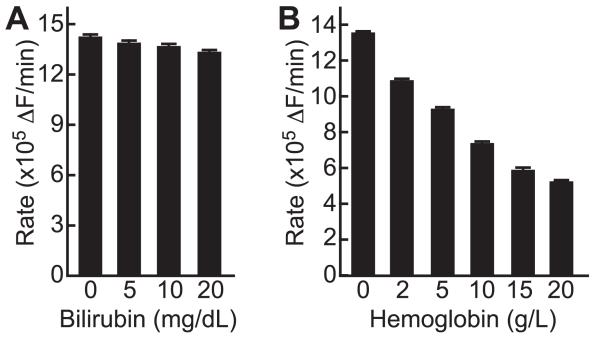
Bilirubin interferes with ADAMTS13 assays with FRETS-VWF73 [12]. However, the spectrum of bilirubin does not overlap with DyLight 633 and IRDye QC-1, and bilirubin (≤20 mg/dL) did not inhibit ADAMTS13 activity assays with FRETS-rVWF71 (Fig. 2). Hemoglobin interferes with FRETS-VWF73 assays and inhibits ADAMTS13 regardless of the assay method [20]. As expected, hemoglobin ≤20 g/L added to assays did not affect the detection of FRETS-rVWF71 cleavage products but inhibited ADAMTS13 with an IC50 of 10-15 g/L (Fig. 2).
Fig. 2.
Effect of bilirubin and hemoglobin. PNP (100 μL) was assayed in the presence of bilirubin (A) or hemoglobin (B). Values plotted are the mean ± SE (n=2).
Kinetics of FRETS-rVWF71 cleavage
Plasma ADAMTS13 cleaved FRETS-rVWF71 with Km of 1.8 μM and kcat of 6.8 min−1 at 30 °C (Fig. S4). ADAMTS13 cleaves FRETS-VWF73 in the same buffer with a Km of 3.2 μM and a kcat of 58 min−1 at 37 °C [19]. Therefore, the larger dyes of FRETS-rVWF71 decrease the catalytic efficiency of substrate cleavage ~5-fold.
FRETS-rVWF71 assay performance
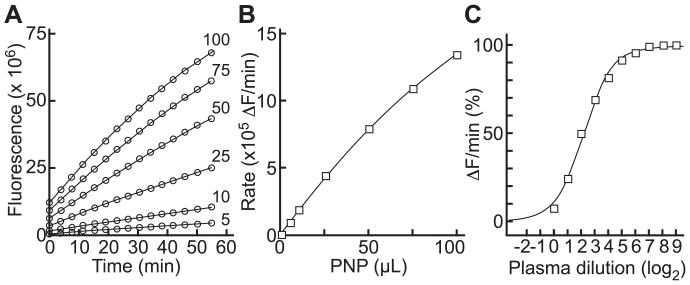
Progress curves for cleavage of FRETS-rVWF71 by plasma ADAMTS13 were approximately linear for at least 60 minutes (Fig. 3A) and reaction rate increased approximately linearly with the volume of added plasma (Fig. 3B). Results with FRETS-rVWF71 and FRETS-VWF73 assayed under standard conditions [7] correlated well with an inter-assay CV of 3.8%.
Fig. 3.
FRETS-rVWF71 assays. (A) Change in fluorescence with the indicated volumes of PNP. (B) Dependence of initial rate on PNP. Lines were fitted by nonlinear regression. (C) ADAMTS13 inhibitor assay for plasma with an inhibitor titer of 4.5 U (95% confidence interval 4.1 to 5.0 U).
The FRETS-rVWF71 assay was adapted to measure autoantibody inhibitors of ADAMTS13 in a manner analogous to the measurement of factor VIII inhibitors in “Bethesda-like” units (Fig. 3C). This assay design allows the detection of inhibitors with a titer ≥0.4 U.
ADAMTS13 assays in Healthy Donors and patients with TTP
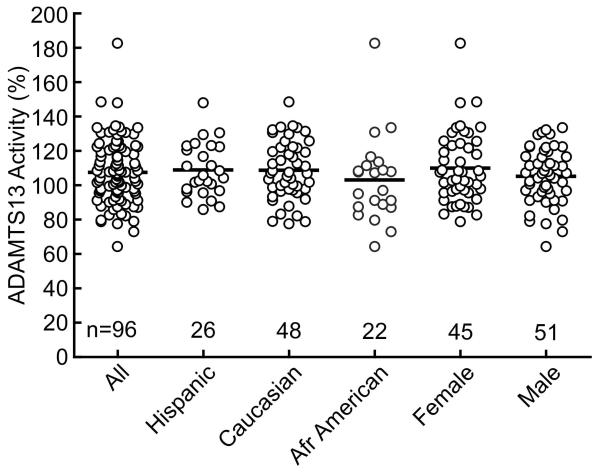
For 96 healthy controls, the mean ADAMTS13 activity was 107 ± 18% (SD) (Fig. 4). Mean ADAMTS13 activity was not significantly different based on gender, ethnicity (Fig. 4), or age (data not shown). The intra-assay coefficient of variation (CV) was 1.8% and the inter-assay CV was 1.7%.
Fig. 4.
Plasma ADAMTS13 activity of healthy controls. Mean ADAMTS13 activity of 96 individuals was 107.1 ± 18% (SD) and did not depend on gender (male 104.9 ± 16%; female 109.6 ± 16%) or ethnicity (African American 102.7 ± 24%; Caucasian 108.2 ± 17%; Hispanic 108.4 ± 15%). Bars indicate mean values.
In separate assays of PNP samples with mean ADAMTS13 activity of 2.4% and 26%, the intra-assay CV was <2% in each case. The limit of detection was determined from 60 assays of PNP containing 10 mM EDTA that have no ADAMTS13 activity. The SD was 0.086% ADAMTS13 activity, which indicates a limit of detection of ~0.3% (3.29 × SD) [21].
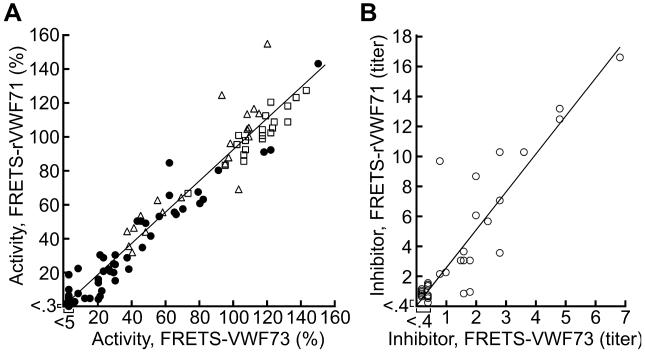
ADAMTS13 assays with FRETS-rVWF71 and FRETS-VWF73 were compared using citrated plasma samples submitted to BloodCenter of Wisconsin for clinical laboratory testing from 80 subjects with thrombotic microangiopathy, 22 with other causes of thrombocytopenia, and 20 healthy controls. Agreement between methods was excellent (R2 = 0.95) (Fig. 5A). Assays with FRETS-rVWF71 were more sensitive with a limit of detection at least 15-fold lower than assays with FRETS-VWF73. The slope of the linear regression line for the results is 0.92, which indicates that values with FRETS-rVWF71 are lower because a slightly more active Li+-heparin PNP calibrator was used for the FRETS-rVWF71 assays. A few plasma samples gave discordant results that were confirmed upon retesting. Five samples had ADAMTS13 activity <10% with FRETS-rVWF71, but values between 12% and 22% with FRETS-VWF73 (Table S1). Two samples (subjects 50 and 54) had ADAMTS13 activity of 18% and 22% with FRETS-rVWF71, and <5% and 8%, respectively, with FRETS-VWF73. Additional data are not available for these subjects and the clinical significance of this variation cannot be assessed. Excellent agreement between the methods was also observed for Li+-heparin plasma samples from patients with TTP (Table S2).
Fig. 5.
Comparison of ADAMTS13 assays with FRETS-VWF73 and FRETS-rVWF71. (A) Citrated plasma from subjects with thrombotic microangiopathy (closed circles), other causes of thrombocytopenia (open triangles) and healthy controls (open squares) were assayed with FRETS-VWF73 and FRETS-rVWF71. Linear regression (solid line) gave a slope of 0.92 and R2 of 0.95. (B) ADAMTS13 inhibitors in samples from 34 subjects assayed with FRETS-VWF73 or FRETS-rVWF71. Linear regression (solid line) gave a slope of 2.51 and R2 of 0.80.
ADAMTS13 inhibitors were detected for 34 subjects, of whom 26 had inhibitors when assayed with both FRETS-VWF73 and FRETS-rVWF71 (Fig. 5B). Correlation between the two assays was excellent (R2 = 0.80). The regression line slope of 2.5 indicates that inhibitor assays with FRETS-rVWF71 are more sensitive. This result is consistent with the 1:20 dilution of plasma samples in FRETS-VWF73 assays compared to 1:1 dilution in FRETS-rVWF71 assays.
For 7 patients, ADAMTS13 inhibitors were detected with FRETS-rVWF71 and not with FRETS-VWF73. Another 7 patients had inhibitor titers of 0.6 U to 1.4 U with FRETS-rVWF71 but borderline detectable inhibitors of 0.4 U with FRETS-VWF73. Conversely, only one sample (subject 34) had an inhibitor titer of 0.4 U with FRETS-VWF73 that was not detected with FRETS-rVWF71.
These results indicate that assays with FRETS-rVWF71 were more sensitive for both ADAMTS13 activity and inhibitors. This increased sensitivity supports a correlation between the degree of ADAMTS13 deficiency and the prevalence of ADAMTS13 inhibitors (Table 1).
Table 1.
Relationship between ADAMTS13 activity and inhibitors
| Inhibitor | No inhibitor | Total | |
|---|---|---|---|
| ADAMTS13 <0.3% | 16 | 2 | 18 |
| ADAMTS13 0.3-5% | 13 | 9 | 22 |
| ADAMTS13 5-20% | 4 | 9 | 13 |
| Total | 33 | 20 | 53 |
ADAMTS13 activity and inhibitors were assayed with FRETS-rVWF71 (Table S1). The likelihood of detecting an inhibitor increases with the severity of ADAMTS13 deficiency, χ2 = 11, P = 0.004
Discussion
The dyes used for FRETS-rVWF71 have spectral properties that permit ADAMTS13 assays to be performed in ≤95% plasma with no significant interference from plasma proteins, bilirubin or hemoglobin. As expected from studies of other ADAMTS13 substrates, the rate of FRETS-rVWF71 cleavage under plasma-like conditions (pH 7.4, 150 mM NaCl) is only ~25% of the rate under the optimal conditions of pH 6.0 and low ionic strength [7, 11]. Despite substantially lower ADAMTS13 activity under plasma-like conditions, the combination of increased fluorescence and compatibility with plasma allows FRETS-rVWF71 assays to be completed in 1 hour with excellent reproducibility and a limit of detection of ~0.3 %.
Assaying plasma from patients is challenging because of variability in the type and concentration of interfering substances. For example, leukocyte proteases can cleave FRETS-VWF73 [22] and may degrade VWF during disseminated intravascular coagulation or sepsis. We find that some plasma specimens cleave peptide substrates even when ADAMTS13 is inhibited with EDTA, possibly because of contamination with leukocyte-derived proteases. This calcium-independent protease activity is blocked completely by phenylmethanesulfonyl fluoride and other broad-spectrum inhibitors that target serine and cysteine proteases, thereby allowing the specific measurement of ADAMTS13 activity.
ADAMTS13 levels were similar in serum and plasma as expected [4], and we observed no significant difference in results for several methods of plasma anticoagulation. Citrated plasma has been the standard enzyme source for other ADAMTS13 assays [4-8, 10, 23] and can be used with FRETS-rVWF71 if another anticoagulant is included to prevent clotting after recalcification and additional calcium chloride is added to counteract the reduction in free calcium ion concentration caused by citrate.
When FRETS-rVWF71 assays were compared to FRETS-VWF73 assays on plasma samples submitted to the BloodCenter of Wisconsin for ADAMTS13 testing, the two methods agreed well and FRETS-rVWF71 assays were more sensitive for both ADAMTS13 activity and inhibitors. Highly sensitive ADAMTS13 assays may prove to be useful for the management of patients with TTP. For example, in congenital TTP the level of residual ADAMTS13 activity has been correlated with the severity of the clinical phenotype [24]. In acquired TTP, severe ADAMTS13 deficiency is associated with relapsing disease [9, 25-28], and extremely low ADAMTS13 during the acute illness has been associated with increased mortality [29]. ADAMTS13 inhibitors also have been correlated with relapses [25, 27, 28, 30], delayed response to plasma exchange [26], refractory disease and death [9, 26, 31]. Relapses of TTP are essentially always accompanied by severe ADAMTS13 deficiency, and several recent reports suggest that preemptive treatment with rituximab to normalize ADAMTS13 levels can prevent these relapses [32-35].
None of these studies was randomized and their conclusions must be considered tentative. Nevertheless, they provide a strong rationale for investigating the utility of sensitive ADAMTS13 assays to stratify patients with TTP at diagnosis and monitor ADAMTS13 activity as a biomarker of response to therapy. One study using FRETS-rVWF71 assays is in progress to determine whether early treatment of TTP with low-dose rituximab can restore normal ADAMTS13 levels, improve early outcomes and prevent relapses (NCT01554514).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01 HL72917, R01 HL89746, and U54 HL112303.
Footnotes
Addendum
J. Muia designed and performed research, analyzed and interpreted data, and wrote the manuscript. W. Gao, S. L. Haberichter, L. Dolatshahi, J. Zhu, L. A. Westfield, S. C. Covill and K. D. Friedman, designed and performed research, analyzed and interpreted data, and reviewed the manuscript. J. E. Sadler. designed research, analyzed and interpreted data, and wrote the manuscript.
Disclosure of Conflict of Interest
The authors state that they have no conflict of interest.
References
- 1.Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, Krause M, Scharrer I, Aumann V, Mittler U, Solenthaler M, Lammle B. von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. 1998;339:1578–84. doi: 10.1056/NEJM199811263392202. [DOI] [PubMed] [Google Scholar]
- 2.Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339:1585–94. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, Spasoff RA, Canadian Apheresis Study Group Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med. 1991;325:393–7. doi: 10.1056/NEJM199108083250604. [DOI] [PubMed] [Google Scholar]
- 4.Furlan M, Robles R, Lammle B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87:4223–34. [PubMed] [Google Scholar]
- 5.Tsai HM. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 1996;87:4235–44. [PubMed] [Google Scholar]
- 6.Gerritsen HE, Turecek PL, Schwarz HP, Lammle B, Furlan M. Assay of von Willebrand factor (vWF)-cleaving protease based on decreased collagen binding affinity of degraded vWF: a tool for the diagnosis of thrombotic thrombocytopenic purpura (TTP) Thromb Haemost. 1999;82:1386–9. [PubMed] [Google Scholar]
- 7.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129:93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 8.Tripodi A, Peyvandi F, Chantarangkul V, Palla R, Afrasiabi A, Canciani MT, Chung DW, Ferrari S, Fujimura Y, Karimi M, Kokame K, Kremer Hovinga JA, Lammle B, de Meyer SF, Plaimauer B, Vanhoorelbeke K, Varadi K, Mannucci PM. Second international collaborative study evaluating performance characteristics of methods measuring the von Willebrand factor cleaving protease (ADAMTS-13) J Thromb Haemost. 2008;6:1534–41. doi: 10.1111/j.1538-7836.2008.03099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kremer Hovinga JA, Vesely SK, Terrell DR, Lammle B, George JN. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115:1500–11. doi: 10.1182/blood-2009-09-243790. [DOI] [PubMed] [Google Scholar]
- 10.Palla R, Valsecchi C, Bajetta M, Spreafico M, De Cristofaro R, Peyvandi F. Evaluation of assay methods to measure plasma ADAMTS13 activity in thrombotic microangiopathies. Thromb Haemost. 2011;105:381–5. doi: 10.1160/TH10-06-0417. [DOI] [PubMed] [Google Scholar]
- 11.Di Stasio E, Lancellotti S, Peyvandi F, Palla R, Mannucci PM, De Cristofaro R. Mechanistic studies on ADAMTS13 catalysis. Biophys J. 2008;95:2450–61. doi: 10.1529/biophysj.108.131532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer SC, Sulzer I, Lammle B, Kremer Hovinga JA. Hyperbilirubinemia interferes with ADAMTS-13 activity measurement by FRETS-VWF73 assay: diagnostic relevance in patients suffering from acute thrombotic microangiopathies. J Thromb Haemost. 2007;5:866–7. doi: 10.1111/j.1538-7836.2007.02438.x. [DOI] [PubMed] [Google Scholar]
- 13.Froehlich-Zahnd R, George JN, Vesely SK, Terrell DR, Aboulfatova K, Dong JF, Luken BM, Voorberg J, Budde U, Sulzer I, Lammle B, Kremer Hovinga JA. Evidence for a role of anti-ADAMTS13 autoantibodies despite normal ADAMTS13 activity in recurrent thrombotic thrombocytopenic purpura. Haematologica. 2012;97:297–303. doi: 10.3324/haematol.2011.051433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsushita T, Sadler JE. Identification of amino acid residues essential for von Willebrand factor binding to platelet glycoprotein Ib. Charged-to-alanine scanning mutagenesis of the A1 domain of human von Willebrand factor. J Biol Chem. 1995;270:13406–14. doi: 10.1074/jbc.270.22.13406. [DOI] [PubMed] [Google Scholar]
- 15.Aslanidis C, de Jong PJ. Ligation-independent cloning of PCR products (LIC-PCR) Nucleic Acids Res. 1990;18:6069–74. doi: 10.1093/nar/18.20.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapust RB, Waugh DS. Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci. 1999;8:1668–74. doi: 10.1110/ps.8.8.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feys HB, Liu F, Dong N, Pareyn I, Vauterin S, Vandeputte N, Noppe W, Ruan C, Deckmyn H, Vanhoorelbeke K. ADAMTS-13 plasma level determination uncovers antigen absence in acquired thrombotic thrombocytopenic purpura and ethnic differences. J Thromb Haemost. 2006;4:955–62. doi: 10.1111/j.1538-7836.2006.01833.x. [DOI] [PubMed] [Google Scholar]
- 18.Feys HB, Anderson PJ, Vanhoorelbeke K, Majerus EM, Sadler JE. Multi-step binding of ADAMTS-13 to von Willebrand factor. J Thromb Haemost. 2009;7:2088–95. doi: 10.1111/j.1538-7836.2009.03620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson PJ, Kokame K, Sadler JE. Zinc and calcium ions cooperatively modulate ADAMTS13 activity. J Biol Chem. 2006;281:850–7. doi: 10.1074/jbc.M504540200. [DOI] [PubMed] [Google Scholar]
- 20.Studt JD, Kremer Hovinga JA, Antoine G, Hermann M, Rieger M, Scheiflinger F, Lammle B. Fatal congenital thrombotic thrombocytopenic purpura with apparent ADAMTS13 inhibitor: in vitro inhibition of ADAMTS13 activity by hemoglobin. Blood. 2005;105:542–4. doi: 10.1182/blood-2004-06-2096. [DOI] [PubMed] [Google Scholar]
- 21.NCCLS . NCCLS document EP17-A. NCCLS; USA: 2004. Protocols for determination of limits of detection and limits of quantitation; approved guideline; pp. 1–39. 940 West Valley Road, Suite 1400, Wayne, PA. [Google Scholar]
- 22.Raife TJ, Cao W, Atkinson BS, Bedell B, Montgomery RR, Lentz SR, Johnson GF, Zheng XL. Leukocyte proteases cleave von Willebrand factor at or near the ADAMTS13 cleavage site. Blood. 2009;114:1666–74. doi: 10.1182/blood-2009-01-195461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kremer Hovinga JA, Mottini M, Lammle B. Measurement of ADAMTS-13 activity in plasma by the FRETS-VWF73 assay: comparison with other assay methods. J Thromb Haemost. 2006;4:1146–8. doi: 10.1111/j.1538-7836.2006.01904.x. [DOI] [PubMed] [Google Scholar]
- 24.Lotta LA, Wu HM, Mackie IJ, Noris M, Veyradier A, Scully MA, Remuzzi G, Coppo P, Liesner R, Donadelli R, Loirat C, Gibbs RA, Horne A, Yang S, Garagiola I, Musallam KM, Peyvandi F. Residual plasmatic activity of ADAMTS13 is correlated with phenotype severity in congenital thrombotic thrombocytopenic purpura. Blood. 2012;120:440–8. doi: 10.1182/blood-2012-01-403113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng XL, Kaufman RM, Goodnough LT, Sadler JE. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Blood. 2004;103:4043–9. doi: 10.1182/blood-2003-11-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coppo P, Wolf M, Veyradier A, Bussel A, Malot S, Millot GA, Daubin C, Bordessoule D, Pene F, Mira JP, Heshmati F, Maury E, Guidet B, Boulanger E, Galicier L, Parquet N, Vernant JP, Rondeau E, Azoulay E, Schlemmer B, Microangiopathies RdE. Prognostic value of inhibitory anti-ADAMTS13 antibodies in adult-acquired thrombotic thrombocytopenic purpura. Br J Haematol. 2006;132:66–74. doi: 10.1111/j.1365-2141.2005.05837.x. [DOI] [PubMed] [Google Scholar]
- 27.Peyvandi F, Lavoretano S, Palla R, Feys HB, Vanhoorelbeke K, Battaglioli T, Valsecchi C, Canciani MT, Fabris F, Zver S, Réti M, Mikovic D, Karimi M, Giuffrida G, Laurenti L, Mannucci PM. ADAMTS13 and anti-ADAMTS13 antibodies are markers for recurrence of acquired thrombotic thrombocytopenic purpura during remission. Haematologica. 2008;93:69–76. doi: 10.3324/haematol.11739. [DOI] [PubMed] [Google Scholar]
- 28.Bettoni G, Palla R, Valsecchi C, Consonni D, Lotta LA, Trisolini SM, Mancini I, Musallam KM, Rosendaal FR, Peyvandi F. ADAMTS-13 activity and autoantibodies classes and subclasses as prognostic predictors in acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2012;10:1556–65. doi: 10.1111/j.1538-7836.2012.04808.x. [DOI] [PubMed] [Google Scholar]
- 29.Yang S, Jin M, Lin S, Cataland S, Wu H. ADAMTS13 activity and antigen during therapy and follow-up of patients with idiopathic thrombotic thrombocytopenic purpura: correlation with clinical outcome. Haematologica. 2011;96:1521–7. doi: 10.3324/haematol.2011.042945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrari S, Scheiflinger F, Rieger M, Mudde G, Wolf M, Coppo P, Girma JP, Azoulay E, Brun-Buisson C, Fakhouri F, Mira JP, Oksenhendler E, Poullin P, Rondeau E, Schleinitz N, Schlemmer B, Teboul JL, Vanhille P, Vernant JP, Meyer D, Veyradier A. Prognostic value of anti-ADAMTS 13 antibody features (Ig isotype, titer, and inhibitory effect) in a cohort of 35 adult French patients undergoing a first episode of thrombotic microangiopathy with undetectable ADAMTS 13 activity. Blood. 2007;109:2815–22. doi: 10.1182/blood-2006-02-006064. [DOI] [PubMed] [Google Scholar]
- 31.Tsai HM, Li A, Rock G. Inhibitors of von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura. Clin Lab. 2001;47:387–92. [PubMed] [Google Scholar]
- 32.Scully M, McDonald V, Cavenagh J, Hunt BJ, Longair I, Cohen H, Machin SJ. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood. 2011;118:1746–53. doi: 10.1182/blood-2011-03-341131. [DOI] [PubMed] [Google Scholar]
- 33.Froissart A, Buffet M, Veyradier A, Poullin P, Provot F, Malot S, Schwarzinger M, Galicier L, Vanhille P, Vernant J, Bordessoule D, Guidet B, Azoulay E, Rondeau E, Mira J, Wynckel A, Clabault K, Choukroun G, Presne C, Pourrat J, Hamidou M, Coppo P. Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Experience of the French Thrombotic Microangiopathies Reference Center. Crit Care Med. 2011;40:104–11. doi: 10.1097/CCM.0b013e31822e9d66. [DOI] [PubMed] [Google Scholar]
- 34.Knovich MA, Farland A, Owen J. Long-term management of acquired thrombotic thrombocytopenic purpura using serial plasma ADAMTS13 measurements. Eur J Haematol. 2012;88:518–25. doi: 10.1111/j.1600-0609.2012.01767.x. [DOI] [PubMed] [Google Scholar]
- 35.Westwood JP, Webster H, McGuckin S, McDonald V, Machin SJ, Scully M. Rituximab for thrombotic thrombocytopenic purpura: benefit of early administration during acute episodes and use of prophylaxis to prevent relapse. J Thromb Haemost. 2013;11:481–90. doi: 10.1111/jth.12114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.