Abstract
Bats are considered important animal reservoirs for many viruses pathogenic to humans. An approach based on viral metagenomics was used to study gut specimens from 78 insectivorous bats in Yunnan Province, China. Seventy-four reads were found to be related to group A rotavirus (RVA). Further reverse transcription-PCR screening and viral isolation on cell cultures confirmed the presence of a novel RVA strain, named RVA/Bat-tc/MSLH14/2012/G3P[3], in 1 (6%) of 16 lesser horseshoe bats. Full genomic sequencing analyses showed that MSLH14 possessed the genotype constellation G3-P[3]-I8-R3-C3-M3-A9-N3-T3-E3-H6, which is akin to human and animal rotaviruses believed to be of feline/canine origin. Phylogenetic analysis indicated that VP7 was most closely related to bovine RVA strains from India, whereas VP4 was most closely related to an unusual human RVA strain, CMH222, with animal characteristics isolated in Thailand. The remaining gene segments were only distantly related to a range of animal RVA strains, most of which are believed to be related to feline/canine RVAs. Experimental infection showed that bat RVA strain MSLH14 was highly pathogenic to suckling mice, causing 100% mortality when they were inoculated orally with a titer as low as 5 × 102 50% tissue culture infective doses. As this virus is not closely related to any known RVA strain, it is tempting to speculate that it is a true bat RVA strain rather than a virus transmitted between species. However, further screening of bat populations, preferably juvenile animals, will be crucial in determining whether or not this virus is widely distributed in the bat population.
INTRODUCTION
Group A rotaviruses (RVAs) are the most important pathogens causing severe, acute diarrhea and gastroenteritis in infants and young children less than 5 years old, resulting in an estimated 453,000 deaths each year worldwide, especially in industrializing countries (1, 2). RVAs, of the genus Rotavirus within the family Reoviridae (3), have a wide range of hosts, including humans and the young of many animal species, including horses, cats, dogs, monkeys, rats, cows, pigs, and birds (1). The RVA genome contains 11 segments of double-stranded RNA, with most of them (except for gene segment 11) encoding a single polypeptide, allowing the virus to express six nonstructural proteins (NSPs) and six structural proteins (VPs) (1). Mature RVA particles resemble a wheel with spikes and possess a triple-layer protein capsid (1). The outer capsid is composed of VP7 and VP4, which define the G and P genotypes, respectively, which are often used in a dual classification system (4). Because of the segmented nature of the RVA genome, reassortment can occur after the coinfection of a single cell, resulting in progeny virus with gene segments from both parental strains and, hence, novel characteristics (1). Consequently, the use of only one or two gene segments for classification provides an incomplete description of RVA. Matthijnssens and colleagues therefore developed a sequence-based classification and nomenclature system involving all 11 gene segments, defining genotypes (G, P, I, R, C, M, A, N, T, E, and H genotypes) for each gene segment (VP7, VP4, VP6, VP1, VP2, VP3, NSP1, NSP2, NSP3, NSP4, and NSP5/6, respectively) based on calculated nucleotide sequence identity cutoff values (5–7). Sequence comparison with an increasing number of whole genome sequences has shown that most species possess RVA strains of particular genotype constellations. The vast majority of human RVA strains belong to the major genotype constellations I1-R1-C1-M1-A1-N1-T1-E1-H1 and I2-R2-C2-M2-A2-N2-T2-E2-H2, referred to as Wa-like and DS-1-like, respectively, or to the minor Au-1-like genotype constellation I3-R3-C3-M3-A3/A12-N3-T3-E3-H3/H6, which is believed to be of feline/canine RVA origin (4). Characterization of RVA strains from several other animal species have identified more or less conserved genotype constellations, such as I2-R2-C2-M2-A3/A13-N2-T6-E2-H3 for cattle (8), I1/I5-R1-M1-A1/A8-N1-T1/T7-E1-H1 for pigs (9), I2/I6-R2-C2-M3-A10-N2-T3-E2-H7 for horses (10), and I3-R3-C2-M3-A3/A9-N2-T3-E3-H3/H6 for cats and dogs (11).
Bats are important virus reservoirs and harbor more than 130 viruses, many of which are highly pathogenic to humans, including Ebola virus, Nipah virus, Hendra virus, and Lyssaviruses (12). With high-throughput next-generation sequencing technology, viral metagenomics have been successfully used to explore the bat virome, leading to the discovery of many novel viruses in the last 4 years, such as bat circovirus, bat papillomavirus, bat bocavirus, bat astrovirus, and bat hepatitis virus (13–18). Esona and colleagues first reported a partial genomic sequence of an RVA strain from straw-colored fruit bats in Africa in 2010 (19). Unfortunately, the virus could not be isolated for further characterization. In this study, we report the first isolation and characterization of an RVA strain from a lesser horseshoe bat (Rhinolophus hipposideros) in Yunnan Province, China.
MATERIALS AND METHODS
Sample collection and preparation.
Collection of bats and experimental infection of mice in this study were reviewed and approved by the Administrative Committee on Animal Welfare of the Institute of Military Veterinary, Academy of Military Medical Sciences, China. All animals were treated strictly in accordance to the Principles and Guidelines for Laboratory Animal Medicine (2006) of the Ministry of Science and Technology, China.
Sixteen adult lesser horseshoe bats (Rhinolophus hipposideros) and 62 adult greater horseshoe bats (Rhinolophus ferrumequinum) were captured alive with nets in three neighboring caves in Dehong Prefecture of Yunnan Province, China. All bats were euthanized by intravenous injection of potassium chloride at the local Center for Diseases Control and Prevention, and the guts, along with their contents, and lungs of each bat were collected and immediately frozen separately in a liquid nitrogen tank prior to transportation to the laboratory, where they were stored at −80°C.
Metagenomic analysis, RT-PCR, and sequencing of the complete genome.
Viral metagenomic studies of the guts and lungs were conducted as previously described (20). Briefly, all guts and lungs were pooled and viral nucleic acids were extracted manually from purified virions with the RNeasy Mini kit (Qiagen, Hilden, Germany), reverse transcribed, and randomly amplified. Purified PCR products were ultrasonicated to fragments of about 180 bp prior to ligation to Solexa adaptor and then Solexa sequenced on one lane at the Beijing Genome Institute (Shenzhen, China). Sequence information was obtained by Illumina Sequencing by Synthesis. After removal of the adaptor sequences, reads of over 100 bp were defined as significant data and subjected to BLASTn searches (http://blast.ncbi.nlm.nih.gov/Blast.cgi) against the nonredundant database in GenBank. The BLAST hits were considered significant if the E value was ≤10e-5 (20). Sequences of bacteria and eukaryotes were removed and virus-like sequences were subjected to further analysis. On the basis of the RVA reads obtained, seminested PCR primers (see Table S1 in the supplemental material) targeting an 836-bp fragment of the VP3 gene were designed with Genefisher (http://bibiserv.techfak.uni-bielefeld.de/genefisher2/) for rescreening. Viral RNA was extracted automatically from each gut and lung sample with the RNeasy Mini kit (Qiagen) on a QIAcube (Qiagen). Reverse transcription (RT) was done with the 1st cDNA synthesis kit (TaKaRa, Dalian, China) according to the manufacturer's protocol. The cDNA was amplified with the PCR Master Mix (Tiangen, Beijing, China) with a PCR program consisting of 30 (outer PCR) or 35 (inner PCR) cycles of denaturation at 94°C for 30 s, annealing at 54°C for 30 s, and extension at 72°C for 50 s; double-distilled H2O was used as a negative control. Positive PCR products were commercially sequenced with an ABI 3730 sequencer (Invitrogen, Beijing, China).
To obtain the full genome, 16 degenerate PCR primer pairs (see Table S1 in the supplemental material) targeting the full lengths of all 11 segments were designed with Genefisher on the basis of RVA sequences available in GenBank. Viral cDNA of each segment was synthesized directly from RNA of the positive gut sample prepared as described above and amplified with the Fast HiFidelity PCR kit (Tiangen). The amplicons were ligated into the pMD18-T vector (TaKaRa), used to transfect TOP10 chemically competent Escherichia coli (Tiangen), and then sequenced by the Sanger method in an ABI 3730 sequencer (Invitrogen). Five clones of each amplicon were sequenced.
Cell culture and virus isolation.
RVA-positive samples were ground with serum-free minimum essential medium (MEM; Sigma-Aldrich, St. Louis, MO) and centrifuged at 12,000 × g for 10 min at 4°C. Supernatants were passed through 0.22-μm syringe filters (Sartorius, Göttingen, Germany), and the filtrates were added to rhesus monkey kidney Marc145 cells after trypsin treatment. The cell cultures were observed daily during incubation with 5% CO2 at 37°C and harvested once cytopathic effects (CPE) developed. To develop a serological detection method, hyperimmune serum against the virus was prepared by intramuscular inoculation of adult mice with the isolated virus. The titer of the seventh passage of the virus was determined by assay of 10-fold dilutions in an indirect immunofluorescence assay (IFA) with fluorescein isothiocyanate-conjugated goat anti-mouse antibody (Sigma-Aldrich). For morphological observation, the virus culture at passage 3 was centrifuged at 12,000 × g for 30 min and the sedimented cell debris containing virus was resuspended in SM buffer (50 mM Tris, 10 mM MgSO4, 0.1 M NaCl, pH 7.4) and negatively stained with phosphotungstic acid for observation in a JEM-1200 EXII transmission electron microscope (JEOL, Tokyo, Japan). The virus culture in Marc145 cells (passage 2) was further used to infect immortalized embryonic Myotis petax bat kidney cells (BFK cell line) established by our laboratory (unpublished data). To characterize the electropherotype of the virus, RNA was extracted from the isolated virus, subjected to polyacrylamide gel electrophoresis (PAGE), and visualized by the silver staining method (21).
Experimental infection of mice.
Specific-pathogen-free pregnant Kunming mice (n = 3) and adult mice (n = 4) were obtained from the Breeding Laboratory of Jilin University and housed separately in isolators (Tecniplast, Buguggiate, Italy). Four-day-old suckling mice were divided into three groups of four animals. Two groups were orally inoculated with 5 × 102 and 5 × 105 50% tissue culture infective doses (TCID50) of virus culture, respectively, and the third group was mock infected with uninfected Marc145 cells. All suckling mice were inspected and weighed daily. Small intestine, lung, liver, and brain tissues were immediately collected from dead mice and subjected to RT-PCR analysis and viral isolation. The surviving mice were euthanized at 14 days postinoculation (p.i.). Three adult mice were orally inoculated with 1 × 104 TCID50 of virus, and one was mock infected with Marc145 cells. The animals were inspected daily for diarrhea, and their fresh feces were collected every 12 h p.i. for RT-PCR detection of the virus. The data were analyzed with Statistical Analysis System v9.2 (SAS, Inc., Cary, NC).
Nucleotide sequence analysis and Bayesian evolution estimation.
The nucleotide and amino acid sequences of our bat RVA and other representatives (strain designations are shown in trees [see Fig. 1 to 3]) were aligned with ClustalW version 2.0. Phylogenetic trees were inferred by the neighbor-joining method of MEGA 5.1 with 1,000 bootstrap replicates (22). To identify the genotypes of the different segments of this virus, all segments were genotyped with the online tool RotaC (v2.0; http://rotac.regatools.be/) (23) or after consultation with the Rotavirus Classification Working Group (5–7).
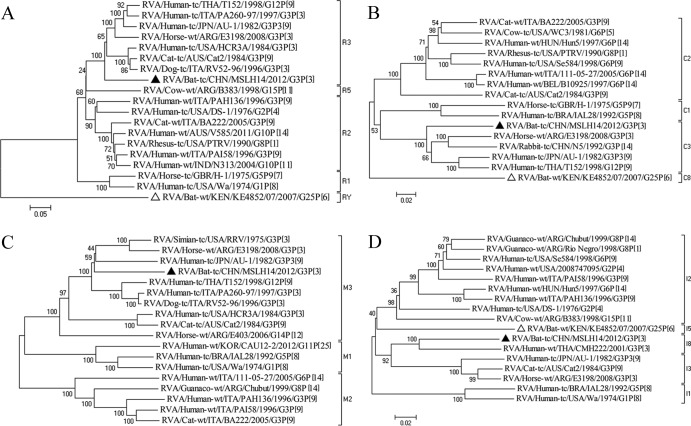
Fig 1.
(A) Bayesian analysis of 818-bp segments of VP7 from G3 RVA strains suggests that MSLH14 (filled triangle) diverged in 1982 from an ancestor most closely related to bovine RVA strains in India. (B) Phylogenetic analysis of a 2,254-bp VP4 segment of MSLH14 (filled triangle) and other representative RVA strains suggests that MSLH14 is most closely related to CMH222.
Fig 3.
Phylogenetic analyses of NSP1 (A), NSP2 (B), NSP3 (C), NSP4 (D), and NSP5 (E) of MSLH14 (filled triangles) and other representative RVA strains. Fruit bat RVA strain KE4852 is indicated by open triangles.
To estimate the evolutionary rate (substitutions per site per year) and the time of the most recent common ancestor (TMRCA) of the G3 genotype, the entire open reading frame (ORF) sequences of 79 VP7 genes together with that of MSLH14 with their isolation times provided by GenBank were used in a Bayesian phylogenetic reconstruction based on the Markov chain Monte Carlo analysis available in the BEAST package v1.6.1 (http://beast.bio.ed.ac.uk/) (24). Sequences were analyzed with the HKY nucleotide substitution model with gamma-distributed rate variation, an uncorrelated lognormal relaxed-clock model, and a Bayesian Skyline coalescent prior (25). The files generated by BEAUTi were run independently four times for 100 million generations and sampled every 10,000 states. The posterior densities were calculated with 10% burn-in and checked for convergence with Tracer v1.5. The maximum clade credibility tree was generated by TreeAnnotator v1.6.1 (http://beast.bio.ed.ac.uk/) and visualized with FigTree v1.3.1 (http://tree.bio.ed.ac.uk/software/figtree/).
Nucleotide sequence accession numbers.
The complete sequences of the 11 genomic segments (excluding primer sequences) obtained in this study have been deposited in GenBank under accession numbers KC960619 to KC960629.
RESULTS
Solexa sequencing and RT-PCR screening.
Solexa sequencing generated 478,416 reads (average length of 142.43 nucleotides [nt]), and 4.63% (22,150) of them were identified as virus-related sequences. Of these sequences, 74 were annotated as belonging to the VP3 gene of RVA and fell into two groups. One group had 53 sequences (length range, 140 to 147 nt) all mapping against nt 14 to 160 of the RVA VP3 gene and showing 95% nucleotide sequence identity to equine rotavirus A strain RVA/Horse-wt/ARG/E3198/2008/G3P[3]. The second group contained 21 sequences (length range, 141 to 145 nt) all mapping to nt 1373 to 1497 of the RVA VP3 gene and showing 87% nucleotide sequence identity to lapine rotavirus strain RVA/Rabbit-tc/CHN/N5/1992/G3P[14]. Subsequently, we screened all bat specimens with an RVA VP3-specific seminested RT-PCR. The gut sample of only 1 of 16 lesser horseshoe bats was RVA positive, and none of the greater horseshoe bat samples was positive for RVA. The virus was named RVA/Bat-tc/CHN/MSLH14/2012G3P[3].
Genotype constellation.
The complete genotype constellation of RVA strain MSLH14 was found to be G3-P[3]-I8-R3-C3-M3-A9-N3-T3-E3-H6. Comparison of this genotype constellation with those of other known RVA strains revealed that the genotypes of eight gene segments of MSLH14 were shared with RVA strains from cats (RVA/Cat-tc/AUS/Cat97/1984/G3P[3]), dogs (RVA/Dog-tc/ITA/RV198-95/1995/G3P[3], RVA/Dog-tc/ITA/RV52-96/1996/G3P[3]), humans (RVA/Human-tc/JPN/AU-1/1982/G3P3[9], RVA/Human-wt/CHN/L621/2006/G3P[9], RVA/Human-wt/CHN/E2451/2011/G3P[9], RVA/Human-tc/THA/T152/1998/G12P[9], RVA/Dog-tc/USA/CU-1/1982/G3P[3], RVA/Dog-tc/AUS/K9/1981/G3P[3], RVA/Dog-tc/USA/A79-10/1979/G3P[3]), and simians (RVA/Rhesus-tc/USA/TUCH/2002/G3P[24], RVA/Simian-tc/USA/RRV/1975/G3P[3]) (Table 1). Most striking was the observation that MSLH14 shared 10 out of 11 gene segments with the unusual equine RVA strain RVA/Horse-wt/ARG/E3198/2008/G3P[3] (Table 1).
Table 1.
Genotype constellation of RVA/Bat-tc/MSLH14/2012/G3P[3] and its nucleotide sequence identities with other representative RVA strains

Gray shading indicates a genotype shared with RVA/Bat-tc/CHN/MSLH14/2012/G3P[3]. The highest identities are in bold italics.
Phylogenetic analyses.
Both VP7 glycoprotein (G genotypes) and VP4 spike protein (P genotypes) elicit neutralizing antibodies, and to date, 27 G and 37 P genotypes from various hosts have been identified (6, 26). The nearly full-length VP7 sequence (1,008 nt) of MSLH14, encoding a 326-amino-acid (aa) ORF, was determined. This segment was most closely related to a number of bovine G3 RVA strains, including the bovine RVA strain RVA/Cow-wt/IND/J63/1996/G3P[X] (94% nucleotide sequence identity) (Fig. 1A). The partial VP4 gene segment (2,342 nt) of MSLH14 encoded 776 aa and showed the highest nucleotide sequence identity (88%) with the P[3] RVA strain RVA/Human-wt/THA/CMH222/2001/G3P[3] isolated from a child with gastroenteritis from Chiang Mai, Thailand, in 2001 (27). In the phylogenetic analyses, MSLH14 also clustered with CMH222 and more distantly with equine, simian, feline, canine, and human P[3] RVA strains (Table 1; Fig. 1B).
The middle layer of an RVA particle is composed entirely of VP6, which is classified into 16 I genotypes. The nearly full length of VP6 of MSLH14 (1,277 nt) encodes 397 aa. As found for VP4, the VP6 gene segment of MSLH14 was most closely related to CMH079 (87% nucleotide sequence identity), followed by CMH222 (86%), the prototype and currently the only two members of the I8 genotype (Table 1; Fig. 2D).
Fig 2.
Phylogenetic analyses of VP1 (A), VP2 (B), VP3 (C), and VP6 (D) of RVA/Bat-tc/MSLH14/2012/G3P[3] (filled triangles) and other representative RVA strains. Sequences from fruit bat strain RVA/Bat-wt/KEN/KE4852/07/2007/G25P[6] are indicated by open triangles. Only the partial VP1 sequence of strain KE4852 (open triangles) was determined, thereby preventing the classification of this gene segment (32).
The RVA core particle is composed of VP2 and a few copies of VP1 and VP3 forming the viral replication complex. These three genes are classified into nine R genotypes (VP1), nine C genotypes (VP2), and eight M genotypes (VP3) (6). The nearly complete VP1 (3,290 nt and 1,088 aa), VP2 (2, 683 nt and 886 aa), and VP3 (2,557 nt and 835 aa) gene segments of RVA strain MSLH14 clustered only distantly (84 to 85%, for VP1, 86 to 88%, for VP2, and 83 to 88%, for VP3) with other feline, canine, human, simian, and equine RVA strains of the R3, C3, and M3 genotypes, respectively (Table 1; Fig. 2A to C).
Five of the RVA gene segments encode five or six nonstructural proteins responsible for immune evasion, viral replication, and morphogenesis (1). RVA NSP1 to NSP5 are classified into 16 A, 10 N, 12 T, 15 E, and 11 H genotypes, respectively (2, 6, 28). Although all of the gene segments encoding the NSPs clearly fall into established genotypes, MSLH14 does not cluster very closely with any of the known RVA strains. The NSP1 segment of MSLH14 (1,545 nt encoding 508 aa) showed the highest nucleotide sequence identity (88%) to RVA/Rhesus-tc/USA/TUCH/2002/G3P[24] in genotype A9, also containing canine RVA strain A79-10 and human RVA strain RVA/human-wt/USA/6212/2003/G3P[9] (Table 1; Fig. 3A). The NSP2 gene segment of MSLH14 (1,011 nt encoding 317 aa) was most closely related (93% nucleotide sequence identity) to RVA/Horse-wt/ARG/E3198/2008/G3P[3], followed by 91% nucleotide sequence identity to two human RVAs detected in Wuhan, China, RVA/Human-tc/CHN/L621/2006/G3P[9] and RVA/Human-wt/CHN/E2451/2011/G3P[9], of the N3 genotype (Table 1; Fig. 3B). The NSP3 (1,023 nt, 311 aa), NSP4 (727 nt, 178 aa), and NSP5 (666 nt, 198 aa) gene segments of MSLH14 clustered close to the root of their respective genotypes, T3, E3, and H6, showing the highest nucleotide sequence identities to RVA/Human-tc/CHN/L621/2006/G3P[9] (88%, NSP3), RVA/Human-wt/CHN/E2451/2011/G3P[9] (92%, NSP4), and RVA/Human-tc/ITA/PA260-97/1997/G3P[3] (94%, NSP5), respectively (Table 1; Fig. 3C to E).
Bayesian analysis of G3 genes.
For Bayesian analysis of the G3 genotypes, VP7 segments of 30 human, 23 equine, 10 bovine, 6 canine, 4 lapine, 3 feline, 1 simian, and 1 caprine RVAs and bat RVA strain MSLH14 were used to estimate the molecular evolutionary rate and TMRCA. Results indicate that the mutation rate of G3 was 1.47 × 10−3 mutations/site/year (95% highest posterior density [HPD] confidence interval, 0.75 to 2.33 × 10−3), which is in the same range as the mutation rates of G9 (1.87 × 10−3) and G12 (1.66 × 10−3) estimated previously (25). Phylogenetic reconstruction suggested that RVA strain MSLH14 and Indian RVA strains from cattle, humans, and horses shared an ancestor in common around 1982 (95% HPD confidence interval, 1945 to 1999) (Fig. 1A).
Virus isolation and RNA PAGE.
The RVA-positive gut sample was inoculated onto Marc145 cells after trypsin treatment and homogenization, and a CPE was observed upon primary passage at 48 h p.i. The infected cells formed foci with a rounded shape and soon detached. The detached cells were connected by cytoplasmic filaments. The CPE spread to the entire cell monolayer within 72 to 96 h p.i. (Fig. 4B). IFA revealed bright green fluorescence filling the cytoplasm of infected cells (Fig. 4B), and the TCID50 of passage 7 for Marc145 cells was 1 × 106/100 μl. A similar CPE was also observed in BFK cells (Fig. 4C and D). By transmission electron microscopy, the RVA isolate from Marc145 cells at passage 2 showed typical rotavirus-like particles (about 70 nm in diameter) lacking VP4 spikes. Double-layer particles (DLPs) lacking VP4 and VP7 and single-layer particles (SLPs or cores also lacking VP6) were additionally observed (Fig. 5B).
Fig 4.
Cells inoculated with RVA-positive samples. (A) Mock-infected Marc145 cells show a normal appearance, and no bright fluorescence was noted by IFA. (B) CPE generated by RVA/Bat-tc/MSLH14/2012/G3P[3] in Marc145 cells, showing many detached infected cells connected by cytoplasmic filaments and colored by bright fluorescence in the cytoplasm. (C) Mock-infected BFK cells. (D) CPE generated by MSLH14 in BFK cells.
Fig 5.

PAGE analysis (A) and morphological observation (B) of strain RVA/Bat-tc/MSLH14/2012/G3P[3]. (A) Typical 4-2-3-2 gene segregation pattern of RVA strains. From left to right, the bonds are VP1, VP2, VP3, VP4, NSP1, VP6, NSP3, VP7, NSP2, NSP4, and NSP5. (B) Many particles lacking VP4 spikes are visible, along with some DLPs lacking VP4 and VP7 and SLPs or cores.
To determine the electropherotype of the bat RVA genome, viral RNA was extracted and subjected to PAGE, followed by silver staining. The result was the typical RVA 4-2-3-2 gene migration pattern (Fig. 5A) (21).
Mouse inoculation test.
All suckling mice orally inoculated with RVA strain MSLH14 developed clinical symptoms, including chilling, sluggish activity, and diminished suckling at 3 or 4 days p.i., while all mock-infected mice remained healthy. Feces were not observed, as they were possibly eaten by the mothers. Four suckling mice inoculated with 5 × 105 TCID50 of virus (group Y) died on days 3, 4, 5, and 7 p.i. with their weights increasing until day 3 p.i., followed by a decrease in weight until death (Fig. 6A). Four suckling mice inoculated with 5 × 102 TCID50 of virus (group D) died on days 6, 7, 8, and 9 p.i. with body weight dynamics resembling those of group Y (Fig. 6B). In contrast, the mock-infected group continually gained weight without the presence of disease (Fig. 6C). The most remarkable gross pathological change in the infected groups was that the intestinal walls of all suckling mice in both groups Y and D became thinner and the intestines swelled with much more yellow fluid content. Notably, some bubbles were observed in the small intestines of groups Y and D. Except for lesions in the gut, no gross pathological changes were observed in the other organs, indicating that strain MSLH14 did not have any major secondary sites of replication. The dates of death showed a normal distribution (W [group Y] = 0.97; P [group Y] = 0.85 [>0.05]; W [group D] = 0.99, P [group D] = 0.97 [>0.05]) and equality of variance (F = 1.75; P = 0.66 [>0.05]) and was analyzed with a t test, showing that the survival time of group Y (M = 4.75 ± 1.71) was significantly shorter than that of group D (M = 7.50 ± 1.29) (t = −2.57, P = 0.04 [<0.05]). To confirm that the death of mice was caused by RVAs, the lungs, livers, guts, and brains of each mouse were subjected to RT-PCR screening. Results showed that all guts were positive for RVAs. Furthermore, two lung samples from mice in group Y were also positive, suggesting that MSLH14 is able to cause (limited) viremia. All positive tissues were ground and added to Marc145 cells, and the same CPE as described above was observed.
Fig 6.
Weight changes and survival curves of mice after oral administration of RVA/Bat-tc/MSLH14/2012/G3P[3]. (A) Group Y inoculated with 5 × 105 TCID50 of virus. (B) Group D inoculated with 5 × 102 TCID50 of virus. (C) Mock-infected group inoculated with MEM.
The adult mice did not develop any clinical symptoms during the 2 weeks of observation following infection with MSLH14, but virus was detected by RT-PCR in their normal-looking feces at 12 to 24 h p.i.
DISCUSSION
G3P[3] and G3P[9] are genotype combinations usually found in RVA strains from cats (Cat2, Cat97, BA222) and dogs (RV198, RV52, A79-10, K9, CU-1) (11, 29–32). A number of scattered reports have identified such feline/canine-like RVA strains (Ro1845, HCR3A, AU-1, E2451, L621, T152, CMH222, PA260, PAH136, PAI58, 6212, CMH120) infecting humans (11, 27, 29, 32–38). More recently, three distinct genotype constellations have been differentiated among feline/canine RVA strains on the basis of analyses of a limited number of full RVA genome sequences (11). In particular, the Au-1 like genotype constellation (I3-R3-C3-M3-A3/12-N3-T3-E3-H3/6) has been reported to circulate to a limited extent in humans in Japan, Israel, Thailand, and Argentina (4). In addition, a number of unusual animal RVA strains have been isolated that are believed to have a distant ancestor in common with feline/canine RVA strains in the majority of their gene segments (11, 27, 29, 32–38). Two uniquely distinct simian RVA strains, RRV and TUCH, and an atypical equine RVA strain, E3198, share multiple genotypes with feline/canine-like RVA strains, suggesting that at least parts of their genomes have an ancestor in common with feline/canine RVA strains (39, 40). Here, we report the detection and isolation of the first RVA strain (RVA/Bat-tc/MSLH14/2012/G3P[3]) from insectivorous bats. MSLH14 possesses the genotype constellation G3-P[3]-I8-R3-C3-M3-A9-N3-T3-E3-H6, which is reminiscent of many of the above-mentioned feline/canine-like RVA strains, suggesting a distant common ancestry (Table 1). Strain MSLH14 is only the third known RVA strain with the I8 (VP6) genotype. Only the unusual human G3P[3] RVA strain CMH222 and G3P[10] RVA strain CMH079 from Thailand also possess this genotype (27, 41) and, interestingly, the VP4 (P[3]) gene of MSLH14 was also found to cluster near CMH222. Although the genetic relationship between MSLH14 and CMH222 is relatively distant, they may have an ancestor in common. Bayesian analysis of MSLH14 suggests that its VP7 gene segment is related to a number of unusual bovine and single isolates of human and equine G3 RVA strains isolated in different regions of India (Fig. 1A). Some of these bovine G3 strains (such as RVA/Cow-wt/IND/RUBV3/2005/G3P[3]) have been further investigated and found to possess the P[3] genotype and bovine-like VP6, NSP4, and NSP5 gene segments, which led the authors to speculate about the potential feline/canine/bovine reassortant nature of these unusual G3 RVA strains (42). Although our study identified only a single lesser horseshoe bat positive for RVA (6%), this positivity rate might be an underestimation for several reasons. (i) The sample number was small and may not represent the real situation in a broader area; (ii) no neonates were involved in this study, whereas RVAs are known to infect mainly infants and young animals; and (iii) adult bats, having robust immune competence, can clear viruses rapidly following infection (43), thereby decreasing the chance of their identification by surveillance. Unfortunately, bat sera were not collected in the present study, not allowing us to perform further rotavirus seroprevalence studies. Therefore, the isolation of only a single RVA strain cannot yet tell us whether or not this G3P[3] bat RVA strain is a genuine bat RVA strain or an unusual one. Esona et al. detected nucleic acids of an RVA (RVA/Bat-wt/KEN/KE4852/07/2007/G25P[6]) in fruit bats in Kenya, which was the first report of an RVA in bats (19). Although this virus was not isolated and its virulence for other animals was unclear, the VP7, VP6, VP1, VP2, NSP2, NSP3, and NSP5 gene segments were (partially) determined. Our analyses indicate that the sequences obtained are only distantly related to the sequences of MSLH14 and do not have a single genotype in common with any of the sequences investigated (Fig. 1 to 3). Taken together, these two bat rotaviruses are not closely related to any known RVA strain, and therefore it can be speculated that these two viruses are true bat RVA strains rather than viruses transmitted between species. To further confirm and better understand the prevalence of RVAs in bats, further molecular and serological investigations of larger numbers and species of adult and neonatal bats in different geographical regions must be performed.
Mice and rabbits have been used to study the pathogenesis and host immune response of RVAs or their vaccine potency (44–49). Ijaz et al. used suckling mice to compare the virulence of bovine RVA (BRV) and murine RVA (MRV) and found that an inoculation dose as low as 100 PFU/mouse of BRV and 10 PFU/mouse of MRV could induce disease in mice (47). In consideration of animal welfare, the 50% lethal dose of MSLH14 was not determined but a preliminary animal test showed that a dose as low as 5 × 102 TCID50 of virus could induce rapid infection of suckling mice with 100% mortality. Inoculation of adult mice with 1 × 104 TCID50 of virus did not induce overt diarrhea, with only a short period of virus shedding in feces between 12 and 24 h p.i. These results indicate that MSLH14 is much more virulent for suckling mice than for adult mice.
RVA infection of children is a common disease in China and is a huge health and financial burden for their families. Duan et al. estimated that about 48% of diarrhea in children is caused by RVAs (50). Surveillance of human RVA infection has been conducted by sentinel hospitals in cooperation with the WHO since 1998, and these surveillance studies have shown the circulation of mainly G1P[8], G3P[4], and G3P[8] in the Chinese human population (38, 50). Furthermore, a number of human RVAs are believed to have originated from animals such as cattle, cats, pigs, and dogs through interspecies transmission and animal-human RVA reassortment (51–53), and therefore, to better control and prevent human RVA infections, investigation and surveillance of RVAs in animals, including wildlife, would be useful. However, RVA surveillance in animals has not been conducted systematically in China, making the background of RVA infection in animals unclear. The present study highlights the potential role of bats as reservoirs of RVAs and additionally highlights the importance of a fundamental knowledge of viruses in wildlife in order to organize better control and prevention.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the NSFC-Yunnan Province Joint Fund (U1036601), the National 973 Program (2012CB722501), and the National 863 Program (2012AA022006) to C. Tu.
Footnotes
Published ahead of print 11 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.02013-13.
REFERENCES
- 1.Estes MK, Kapikian AZ. 2007. Rotaviruses, p. 1917–1974 In Knipe DM, Howley PM. (ed), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 2.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, WHO-Coordinated Global Rotavirus Surveillance Network 2012. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect. Dis. 12:136–141 [DOI] [PubMed] [Google Scholar]
- 3.King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. (ed). 2012. Rotavirus, p 603–613 In Virus taxonomy: eighth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, San Diego, CA [Google Scholar]
- 4.Matthijnssens J, Van Ranst M. 2012. Genotype constellation and evolution of group A rotaviruses infecting humans. Curr. Opin. Virol. 2:426–433 [DOI] [PubMed] [Google Scholar]
- 5.Matthijnssens J, Ciarlet M, Heiman E, Arijs I, Delbeke T, McDonald SM, Palombo EA, Iturriza-Gomara M, Maes P, Patton JT, Rahman M, Van Ranst M. 2008. Full genome-based classification of rotaviruses reveals a common origin between human Wa-like and porcine rotavirus strains and human DS-1-like and bovine rotavirus strains. J. Virol. 82:3204–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthijnssens J, Ciarlet M, McDonald SM, Attoui H, Banyai K, Brister JR, Buesa J, Esona MD, Estes MK, Gentsch JR, Iturriza-Gomara M, Johne R, Kirkwood CD, Martella V, Mertens PP, Nakagomi O, Parreno V, Rahman M, Ruggeri FM, Saif LJ, Santos N, Steyer A, Taniguchi K, Patton JT, Desselberger U, Van Ranst M. 2011. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG). Arch. Virol. 156:1397–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthijnssens J, Ciarlet M, Rahman M, Attoui H, Banyai K, Estes MK, Gentsch JR, Iturriza-Gomara M, Kirkwood CD, Martella V, Mertens PP, Nakagomi O, Patton JT, Ruggeri FM, Saif LJ, Santos N, Steyer A, Taniguchi K, Desselberger U, Van Ranst M. 2008. Recommendations for the classification of group A rotaviruses using all 11 genomic RNA segments. Arch. Virol. 153:1621–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthijnssens J, Potgieter CA, Ciarlet M, Parreno V, Martella V, Banyai K, Garaicoechea L, Palombo EA, Novo L, Zeller M, Arista S, Gerna G, Rahman M, Van Ranst M. 2009. Are human P[14] rotavirus strains the result of interspecies transmissions from sheep or other ungulates that belong to the mammalian order Artiodactyla? J. Virol. 83:2917–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HH, Matthijnssens J, Kim HJ, Kwon HJ, Park JG, Son KY, Ryu EH, Kim DS, Lee WS, Kang MI, Yang DK, Hyun BH, Park SI, Park SJ, Cho KO. 2012. Full-length genomic analysis of porcine G9P[23] and G9P[7] rotavirus strains isolated from pigs with diarrhea in South Korea. Infect. Genet. Evol. 12:1427–1435 [DOI] [PubMed] [Google Scholar]
- 10.Matthijnssens J, Mino S, Papp H, Potgieter C, Novo L, Heylen E, Zeller M, Garaicoechea L, Badaracco A, Lengyel G, Kisfali P, Cullinane A, Collins PJ, Ciarlet M, O'Shea H, Parreno V, Banyai K, Barrandeguy M, Van Ranst M. 2012. Complete molecular genome analyses of equine rotavirus A strains from different continents reveal several novel genotypes and a largely conserved genotype constellation. J. Gen. Virol. 93:866–875 [DOI] [PubMed] [Google Scholar]
- 11.Matthijnssens J, De Grazia S, Piessens J, Heylen E, Zeller M, Giammanco GM, Banyai K, Buonavoglia C, Ciarlet M, Martella V, Van Ranst M. 2011. Multiple reassortment and interspecies transmission events contribute to the diversity of feline, canine and feline/canine-like human group A rotavirus strains. Infect. Genet. Evol. 11:1396–1406 [DOI] [PubMed] [Google Scholar]
- 12.Luis AD, Hayman DT, O'Shea TJ, Cryan PM, Gilbert AT, Pulliam JR, Mills JN, Timonin ME, Willis CK, Cunningham AA, Fooks AR, Rupprecht CE, Wood JL, Webb CT. 2013. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. Biol. Sci. 280:20122753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donaldson EF, Haskew AN, Gates JE, Huynh J, Moore CJ, Frieman MB. 2010. Metagenomic analysis of the viromes of three North American bat species: viral diversity among different bat species that share a common habitat. J. Virol. 84:13004–13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Victoria JG, Wang C, Jones M, Fellers GM, Kunz TH, Delwart E. 2010. Bat guano virome: predominance of dietary viruses from insects and plants plus novel mammalian viruses. J. Virol. 84:6955–6965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge X, Li Y, Yang X, Zhang H, Zhou P, Zhang Y, Shi Z. 2012. Metagenomic analysis of viruses from bat fecal samples reveals many novel viruses in insectivorous bats in China. J. Virol. 86:4620–4630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tse H, Tsang AK, Tsoi HW, Leung AS, Ho CC, Lau SK, Woo PC, Yuen KY. 2012. Identification of a novel bat papillomavirus by metagenomics. PLoS One 7:e43986. 10.1371/journal.pone.0043986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z, Ren X, Yang L, Hu Y, Yang J, He G, Zhang J, Dong J, Sun L, Du J, Liu L, Xue Y, Wang J, Yang F, Zhang S, Jin Q. 2012. Virome analysis for identification of novel mammalian viruses in bat species from Chinese provinces. J. Virol. 86:10999–11012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He B, Fan Q, Yang F, Hu T, Qiu W, Feng Y, Li Z, Li Y, Zhang F, Guo H, Zou X, Tu C. 2013. Hepatitis virus in long-fingered bats, Myanmar. Emerg. Infect. Dis. 19:638–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esona MD, Mijatovic-Rustempasic S, Conrardy C, Tong S, Kuzmin IV, Agwanda B, Breiman RF, Banyai K, Niezgoda M, Rupprecht CE, Gentsch JR, Bowen MD. 2010. Reassortant group A rotavirus from straw-colored fruit bat (Eidolon helvum). Emerg. Infect. Dis. 16:1844–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He B, Li Z, Yang F, Zheng J, Feng Y, Guo H, Li Y, Wang Y, Su N, Zhang F. 2013. Virome profiling of bats from Myanmar by metagenomic analysis of tissue samples reveals more novel mammalian viruses. PLoS One 8:e61950. 10.1371/journal.pone.0061950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herring AJ, Inglis NF, Ojeh CK, Snodgrass DR, Menzies JD. 1982. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J. Clin. Microbiol. 16:473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maes P, Matthijnssens J, Rahman M, Van Ranst M. 2009. RotaC: a web-based tool for the complete genome classification of group A rotaviruses. BMC Microbiol. 9:238. 10.1186/1471-2180-9-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7:214. 10.1186/1471-2148-7-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthijnssens J, Heylen E, Zeller M, Rahman M, Lemey P, Van Ranst M. 2010. Phylodynamic analyses of rotavirus genotypes G9 and G12 underscore their potential for swift global spread. Mol. Biol. Evol. 27:2431–2436 [DOI] [PubMed] [Google Scholar]
- 26.Trojnar E, Sachsenroder J, Twardziok S, Reetz J, Otto PH, Johne R. 2013. Identification of an avian group A rotavirus containing a novel VP4 gene with a close relationship to those of mammalian rotaviruses. J. Gen. Virol. 94:136–142 [DOI] [PubMed] [Google Scholar]
- 27.Khamrin P, Maneekarn N, Peerakome S, Yagyu F, Okitsu S, Ushijima H. 2006. Molecular characterization of a rare G3P[3] human rotavirus reassortant strain reveals evidence for multiple human-animal interspecies transmissions. J. Med. Virol. 78:986–994 [DOI] [PubMed] [Google Scholar]
- 28.Papp H, Al-Mutairi LZ, Chehadeh W, Farkas SL, Lengyel G, Jakab F, Martella V, Szucs G, Banyai K. 2012. Novel NSP4 genotype in a camel G10P[15] rotavirus strain. Acta Microbiol. Immunol. Hung. 59:411–421 [DOI] [PubMed] [Google Scholar]
- 29.Mochizuki M, Nakagomi T, Nakagomi O. 1997. Isolation from diarrheal and asymptomatic kittens of three rotavirus strains that belong to the AU-1 genogroup of human rotaviruses. J. Clin. Microbiol. 35:1272–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martella V, Banyai K, Matthijnssens J, Buonavoglia C, Ciarlet M. 2010. Zoonotic aspects of rotaviruses. Vet. Microbiol. 140:246–255 [DOI] [PubMed] [Google Scholar]
- 31.Martella V, Potgieter AC, Lorusso E, De Grazia S, Giammanco GM, Matthijnssens J, Banyai K, Ciarlet M, Lavazza A, Decaro N, Buonavoglia C. 2011. A feline rotavirus G3P[9] carries traces of multiple reassortment events and resembles rare human G3P[9] rotaviruses. J. Gen. Virol. 92:1214–1221 [DOI] [PubMed] [Google Scholar]
- 32.Tsugawa T, Hoshino Y. 2008. Whole genome sequence and phylogenetic analyses reveal human rotavirus G3P[3] strains Ro1845 and HCR3A are examples of direct virion transmission of canine/feline rotaviruses to humans. Virology 380:344–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagomi O, Ohshima A, Aboudy Y, Shif I, Mochizuki M, Nakagomi T, Gotlieb-Stematsky T. 1990. Molecular identification by RNA-RNA hybridization of a human rotavirus that is closely related to rotaviruses of feline and canine origin. J. Clin. Microbiol. 28:1198–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman M, Matthijnssens J, Yang X, Delbeke T, Arijs I, Taniguchi K, Iturriza-Gomara M, Iftekharuddin N, Azim T, Van Ranst M. 2007. Evolutionary history and global spread of the emerging g12 human rotaviruses. J. Virol. 81:2382–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Grazia S, Giammanco GM, Potgieter CA, Matthijnssens J, Banyai K, Platia MA, Colomba C, Martella V. 2010. Unusual assortment of segments in 2 rare human rotavirus genomes. Emerg. Infect. Dis. 16:859–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant L, Esona M, Gentsch J, Watt J, Reid R, Weatherholtz R, Santosham M, Parashar U, O'Brien K. 2011. Detection of G3P[3] and G3P[9] rotavirus strains in American Indian children with evidence of gene reassortment between human and animal rotaviruses. J. Med. Virol. 83:1288–1299 [DOI] [PubMed] [Google Scholar]
- 37.Khamrin P, Maneekarn N, Peerakome S, Tonusin S, Phan TG, Okitsu S, Ushijima H. 2007. Molecular characterization of rare G3P[9] rotavirus strains isolated from children hospitalized with acute gastroenteritis. J. Med. Virol. 79:843–851 [DOI] [PubMed] [Google Scholar]
- 38.Wang YH, Pang BB, Zhou X, Ghosh S, Tang WF, Peng JS, Hu Q, Zhou DJ, Kobayashi N. 2013. Complex evolutionary patterns of two rare human G3P[9] rotavirus strains possessing a feline/canine-like H6 genotype on an AU-1-like genotype constellation. Infect. Genet. Evol. 16:103–112 [DOI] [PubMed] [Google Scholar]
- 39.Matthijnssens J, Taraporewala ZF, Yang H, Rao S, Yuan L, Cao D, Hoshino Y, Mertens PP, Carner GR, McNeal M, Sestak K, Van Ranst M, Patton JT. 2010. Simian rotaviruses possess divergent gene constellations that originated from interspecies transmission and reassortment. J. Virol. 84:2013–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miño S, Matthijnssens J, Badaracco A, Garaicoechea L, Zeller M, Heylen E, Van Ranst M, Barrandeguy M, Parreno V. 2013. Equine G3P[3] rotavirus strain E3198 related to simian RRV and feline/canine-like rotaviruses based on complete genome analyses. Vet. Microbiol. 161:239–246 [DOI] [PubMed] [Google Scholar]
- 41.Khamrin P, Maneekarn N, Peerakome S, Malasao R, Thongprachum A, Chan-It W, Mizuguchi M, Okitsu S, Ushijima H. 2009. Molecular characterization of VP4, VP6, VP7, NSP4, and NSP5/6 genes identifies an unusual G3P[10] human rotavirus strain. J. Med. Virol. 81:176–182 [DOI] [PubMed] [Google Scholar]
- 42.Ghosh S, Varghese V, Samajdar S, Sinha M, Kobayashi N, Naik TN. 2007. Molecular characterization of bovine group A rotavirus G3P[3] strains. Arch. Virol. 152:1935–1940 [DOI] [PubMed] [Google Scholar]
- 43.Calisher CH, Childs JE, Field HE, Holmes KV, Schountz T. 2006. Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 19:531–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bell LM, Clark HF, O'Brien EA, Kornstein MJ, Plotkin SA, Offit PA. 1987. Gastroenteritis caused by human rotaviruses (serotype three) in a suckling mouse model. Proc. Soc. Exp. Biol. Med. 184:127–132 [DOI] [PubMed] [Google Scholar]
- 45.Conner ME, Estes MK, Graham DY. 1988. Rabbit model of rotavirus infection. J. Virol. 62:1625–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conner ME, Gilger MA, Estes MK, Graham DY. 1991. Serologic and mucosal immune response to rotavirus infection in the rabbit model. J. Virol. 65:2562–2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ijaz MK, Dent Haines DD, Babiuk LA. 1989. Development of a murine model to study the pathogenesis of rotavirus infection. Exp. Mol. Pathol. 51:186–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Offit PA, Clark HF, Kornstein MJ, Plotkin SA. 1984. A murine model for oral infection with a primate rotavirus (simian SA11). J. Virol. 51:233–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ward RL, McNeal MM, Sheridan JF. 1990. Development of an adult mouse model for studies on protection against rotavirus. J. Virol. 64:5070–5075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duan ZJ, Liu N, Yang SH, Zhang J, Sun LW, Tang JY, Jin Y, Du ZQ, Xu J, Wu QB, Tong ZL, Gong ST, Qian Y, Ma JM, Liao XC, Widdowson MA, Jiang B, Fang ZY. 2009. Hospital-based surveillance of rotavirus diarrhea in the People's Republic of China, August 2003-July 2007. J. Infect. Dis. 200(Suppl 1):S167–S173 [DOI] [PubMed] [Google Scholar]
- 51.Gentsch JR, Laird AR, Bielfelt B, Griffin DD, Banyai K, Ramachandran M, Jain V, Cunliffe NA, Nakagomi O, Kirkwood CD, Fischer TK, Parashar UD, Bresee JS, Jiang B, Glass RI. 2005. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. J. Infect. Dis. 192(Suppl 1):S146–S159 [DOI] [PubMed] [Google Scholar]
- 52.Iizuka M, Kaga E, Chiba M, Masamune O, Gerna G, Nakagomi O. 1994. Serotype G6 human rotavirus sharing a conserved genetic constellation with natural reassortants between members of the bovine and AU-1 genogroups. Arch. Virol. 135:427–432 [DOI] [PubMed] [Google Scholar]
- 53.Santos N, Lima RC, Nozawa CM, Linhares RE, Gouvea V. 1999. Detection of porcine rotavirus type G9 and of a mixture of types G1 and G5 associated with Wa-like VP4 specificity: evidence for natural human-porcine genetic reassortment. J. Clin. Microbiol. 37:2734–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.