Abstract
Acyclic nucleoside phosphonates (ANPs), such as (S)-1-[(3-hydroxy-2-phosphonomethoxy)propyl)]cytosine (HPMPC), are an important group of broad-spectrum antiviral agents with activity against DNA viruses. In this report, we present the in vitro potencies of novel ANPs against gammaherpesviruses, including Kaposi's sarcoma-associated herpesvirus, Epstein-Barr virus (EBV), and three animal gammaherpesviruses. 1-(S)-[3-hydroxy-2-(phosphonomethoxy)propyl]-5-azacytosine (HPMP-5-azaC), (S)-9-[3-hydroxy-2-(phosphonomethoxy)propyl]-3-deazaadenine (3-deaza-HPMPA), and their cyclic derivatives have emerged as highly potent antigammaherpesvirus agents. Interestingly, cyclic prodrugs of ANPs exhibited reduced activities against EBV strain P3HR-1, but not against EBV strain Akata. Cell culture metabolism studies with HPMPC and cyclic HPMPC revealed that these differences were attributable to an altered drug metabolism in P3HR-1 cells after EBV reactivation and, more specifically, to a reduced hydrolysis of cyclic HPMPC by cyclic CMP phosphodiesterase. We did not correlate this effect with phosphodiesterase downregulation, or to functional mutations. Instead, altered cyclic AMP levels in P3HR-1 cells indicated a competitive inhibition of the phosphodiesterase by this cyclic nucleotide. Finally, both HPMPC and HPMP-5-azaC emerged as highly effective inhibitors in vivo through significant inhibition of murine gammaherpesvirus replication and dissemination. With the current need for potent antigammaherpesvirus agents, our findings underline the requirement of appropriate surrogate viruses for antiviral susceptibility testing and highlight HPMP-5-azaC as a promising compound for future clinical development.
INTRODUCTION
Kaposi's sarcoma-associated herpesvirus (KSHV) and Epstein-Barr virus (EBV) are lymphotropic viruses that are characterized by their abilities to induce various tumors and lymphoproliferative diseases, particularly in immunocompromised patients (1, 2). The lack of permissive replication systems and appropriate in vivo animal model systems have hampered the study of both KSHV and EBV (3, 4). Experiments involving the lytic cycle require reactivation of KSHV and EBV in latently infected B cells by using phorbol esters or IgG (5–7). Alternatively, the use of closely related animal gammaherpesviruses, such as murine gammaherpesvirus 68 (MHV-68), herpesvirus saimiri (HVS), and rhesus rhadinovirus (RRV), can overcome these difficulties and are commonly used as surrogate viruses to study EBV and KSHV pathogenesis. These viruses are capable of replicating to high titers and form plaques in different cell types (8–10). Moreover, infection of laboratory mice with MHV-68 has been generally used as a small animal model that offers relevant aspects for KSHV and EBV (9, 11). Major features of gammaherpesvirus pathogenesis are similar in humans and mice, including the initial acute respiratory infection and the establishment of viral latency in B cells (12).
The antiviral drug class of acyclic nucleoside phosphonates (ANPs) encompasses (S)-1-[(3-hydroxy-2-(phosphomethoxy)propyl)]cytosine (HPMPC, or cidofovir), formally licensed for the treatment of cytomegalovirus (CMV) retinitis in AIDS patients, as well as 9-[2-(phosphonomethoxy)ethyl]adenine (PMEA, or adefovir) and (R)-9-[2-(phosphonomethoxy)propyl]adenine (PMPA, or tenofovir), which are both active against hepatitis B virus (HBV) and human immunodeficiency virus (HIV) infections. These nucleotide analogs contain a phosphonate group that is linked to the acyclic nucleoside moiety through a stable P—C bond, which cannot be cleaved by cellular esterases (13). ANPs need to be phosphorylated by cellular kinases to their diphosphate forms to become biologically active, and their selectivity is based on inhibition of reverse transcriptase and/or viral DNA polymerase (14).
New derivatives of ANPs containing a 3-hydroxy-2-(phosphonomethoxy)propyl (HPMP) group or a 2-phosphonomethoxyethyl (PME) group as an acyclic nucleoside moiety have been developed. These analogs are derivatives of diaminopurine (i.e., HPMPDAP and PMEDAP) or diaminopyrimidine (i.e., HPMPO-DAPy and PMEO-DAPy), in which the base moiety is linked via an ether linkage to the aliphatic phosphonate side chain through an oxygen atom at the C-6 position of the pyrimidine ring. A new generation of ANPs encompasses HPMP derivatives with a 5-azacytosine (5-azaC) moiety, with HPMP-5-azaC the leading compound. In addition, cyclic prodrugs of ANPs have been developed, such as cyclic HPMPC, which was reported to exhibit similar antiviral activity as HPMPC but reduced nephrotoxicity (15). These novel ANPs have been shown to exert antiherpesvirus activity and yield great potential for the treatment of various DNA virus and retrovirus infections (13, 16, 17). However, their potencies against gammaherpesviruses have not yet been investigated.
Although no antiviral drugs are currently licensed for treatment of KSHV or EBV infections, several antiherpetic agents have been shown to inhibit these viruses in vitro, particularly those that target the viral DNA polymerase, such as acyclovir, ganciclovir, foscarnet, and HPMPC (18–22). Besides the inhibition of virus lytic replication, HPMPC elicits antitumor activity by induction of apoptosis, and this effect was also demonstrated against nasopharyngeal carcinoma (associated with EBV infection) xenografts in nude mice (23, 24). On the other hand, maribavir (MBV), a benzimidazole riboside, has potent antiviral activity against EBV and is in the late stage of clinical development for human CMV diseases. The mechanism of action remains partially unclear, but it was recently shown to involve the inhibition of the viral protein kinase BGLF4 and thus is independent of the viral DNA polymerase (25). The risk of developing virus-associated diseases is higher in immunocompromised patients with high EBV or KSHV loads, and therefore reducing viral loads by a combination of therapy with antivirals may have positive effects on the onset and disease progression (26, 27). The use of antivirals for the treatment of infectious mononucleosis (IM) in immunocompetent patients is still debatable, because the symptoms of IM are subtle at onset and the disease has a long incubation period (4 to 6 weeks), resulting in a late diagnosis (28). However, in patients with severe EBV-induced infectious mononucleosis, antiviral therapy may be considered as an adjunct to corticosteroid treatment (29). New and promising therapeutic approaches to EBV and KSHV-associated malignancies are under investigation and consist of the induction of virus replication followed by administration of viral DNA polymerase inhibitors, as well as targeting viral latency (30, 31).
In this study, we evaluated the in vitro activities and selectivities of various ANPs, including cyclic HPMP analogs, against gammaherpesvirus replication. Interestingly, the study revealed notable differences in the anti-EBV activities between the noncyclic and cyclic forms of ANPs in P3HR-1 cells, but not in Akata cells. Drug metabolism studies with HPMPC and cyclic HPMPC were performed in these cell lines, and the involvement of cyclic AMP (cAMP) and the cellular 2′-3′-cyclic nucleotide 3′-phosphodiesterase (CNP; EC 3.1.4.37) in the altered drug metabolism in induced P3HR-1 cells was investigated. Finally, the antiviral efficacy of a potent ANP, HPMP-5-azaC, was examined in a mouse model for gammaherpesvirus infection.
MATERIALS AND METHODS
Cells and viruses.
KSHV-infected BCBL-1 cells (NIH AIDS Research and Reference Reagent Program), JSC-1 cells (ATCC CRL-2769), EBV-infected P3HR-1 cells (ATCC HTB-62), and Akata 2000 cells (kindly provided by P. J. Farrell, Imperial College Faculty of Medicine, St. Mary's Campus, London, United Kingdom) were cultured in RPMI 1640 medium (Life Technologies Europe BV, Ghent, Belgium). Murine fibroblasts (NIH 3T3 cells; ATCC CRL-1685), owl monkey kidney cells (OMK; ATCC CRL-1556), and rhesus monkey fibroblasts (RF; kindly provided by S. Wong, Oregon Health and Science University, Beaverton, OR) were grown in Dulbecco's modified eagle's medium (DMEM). All media were supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM l-glutamine, 1% nonessential amino acids, 1% sodium pyruvate, and 1% HEPES. Cultures were incubated at 37°C and 5% CO2. The following viral strains were used: MHV-68 clone G2.4 (provided by A. A. Nash, Edinburgh, United Kingdom); HVS strain C-488 (ATCC VR-1414); RRV strain 17577 (kindly provided by S. Wong, Oregon Health and Science University, Beaverton, OR). These strains were grown in NIH 3T3, OMK, and RF cells, respectively.
Compounds.
The sources of the compounds were as follows: HPMPC, cyclic HPMPC, and PMEA were obtained from Gilead Sciences, Foster City, CA. The following ANPs (and their cyclic analogs) were synthesized at the Institute of Organic Chemistry and Biochemistry, Prague, Czech Republic: HDP-HPMPC (hexadecyloxypropyl-HPMPC), HPMP-5-azaC, HPMPA, 3-deaza-HPMPA, 7-deaza-HPMPA, HPMPDAP, HPMPO-DAPy, 9-[2-(phosphonomethoxy)ethyl]-2,6-diaminopurine (PMEDAP), and 2,4-diamino-6-[2-(phosphonomethoxy)ethoxy]pyrimidine (PMEO-DAPy). The compound structures were previously published (32).
[5-3H]HPMPC (MT-833; specific activity, 26.0 Ci/mmol) and cyclic [5-3H]HPMPC (MT-1713; specific activity, 23 Ci/mmol) were custom synthesized by Moravek Biochemicals (Brea, CA).
KSHV and EBV antiviral assays.
For the antiviral assays, cells were seeded in 48-well plates at a density of 3 × 105 cells/ml (BCBL-1) or 106 cells/ml (JSC-1, Akata, and P3HR-1). Virus replication was induced by addition of 20 ng/ml 12-O-tetradecanoylphorbol 13-acetate (TPA; Sigma) to the cells or 0.1% rabbit anti-human IgG (A0423; Dako) to Akata cells. After 24 h of induction, cells were washed and resuspended in fresh medium in the presence of various concentrations of antiviral drugs. At day 5 postinduction, total DNA was extracted (QIAamp DNA kit; Qiagen, Benelux BV, Venlo, Netherlands), and viral DNA was quantified by real-time quantitative PCR (qPCR) using an ABI Prism 7500 sequence detection system (Life Technologies). The sequences of the PCR primers for the detection of the target genes of KSHV (ORF73) and EBV (BNRF1) have been described elsewhere (18). The 50% and 90% effective concentrations (EC50 and EC90) were calculated by using regression analysis. These EC50 and EC90 values are the concentrations required to reduce KSHV and EBV DNA synthesis in TPA-induced cells by 50% and 90%, respectively.
CPE reduction assays.
NIH 3T3, OMK, and RF cells were grown in 96-well plates and infected with MHV-68, HVS, or RRV, respectively, at a multiplicity of infection of approximately 0.02 PFU/cell. Following 2 h of virus adsorption at 37°C, serial dilutions of test compounds were added in duplicate. Viral infections and dilutions of the drugs were performed in DMEM containing 2% FCS. At day 5 (for MHV-68 and HVS) or 8 (for RRV) postinfection (p.i.), the cytopathic effect (CPE) was evaluated microscopically, and EC50s were determined as the compound concentrations required to reduce the virus-induced CPE by 50%.
Cytotoxicity assays.
The cytotoxicity of the antiviral compounds was determined based on the inhibition of cell growth. In 48-well plates, uninduced BCBL-1 and P3HR-1 cells were grown in culture medium in the presence or absence of serial dilutions of the test compounds. After 3 days of incubation, cells were counted with a Coulter counter (Analis, Namur, Belgium). In 96-well plates, NIH 3T3, OMK, and RF cells were grown at a density of 3 × 103 cells/well. The next day, various concentrations of the compounds were added. The cells were counted after 3 days of incubation. The 50% cytostatic concentrations (CC50s) were defined as the concentration of compound required to reduce cell growth by 50%. Selectivity indices were determined by calculation of the CC50/EC50 ratio.
Intracellular metabolism of HPMPC and cyclic HPMPC.
P3HR-1 and Akata cells were seeded at a density of 1.5 × 106 cells/ml and grown in the presence or absence of 20 ng/ml TPA or 0.1% IgG. The next day, 10 μCi [5-3H]HPMPC] or cyclic [5-3H[HPMPC] was added to the medium. Unradiolabeled compound was added to obtain a final concentration of HPMPC or cyclic HPMPC of 10 μM. After 24, 72, or 120 h, cell extracts were obtained as previously described (33). Briefly, cells were washed three times with ice-cold serum-free medium and centrifuged at 13,000 rpm for 10 min, and cell pellets were extracted with 300 μl ice-cold 67% methanol in water. Extracts were incubated for 10 min on ice and centrifuged at 13,000 rpm for 10 min. Supernatants and cell pellets were stored at −20°C until further use. For high-performance liquid chromatography (HPLC) analysis, 200-μl aliquots of the supernatants were injected onto an anion-exchange Partisphere SAX column (dimensions, 4.6 mm by 125 mm; Whatman, Maidstone, United Kingdom). The buffer composition and gradient system were the same as described previously (33). One-minute fractions of the eluate were collected, mixed with Hisafe 3 cocktail (PerkinElmer, Waltham, MA), and analyzed for radioactivity in a scintillation counter. The different metabolites were identified based on their retention times.
To determine incorporation levels of [5-3H]HPMPC and cyclic [5-3H]HPMPC into total cellular DNA, the methanol-insoluble pellets were digested in 500 ml 5 N sodium hydroxide during 24 h of incubation at 37°C. After neutralization with 500 ml 5 N hydrochloride, digested samples were mixed with Hisafe 3 cocktail and analyzed for total radioactivity.
Western blotting.
Western blot assays were performed by using mouse monoclonal antibody specific to CNP (1:500 dilution; ab6319; Abcam, Cambridge, MA) and goat anti-β-actin (1:500; sc1615; Santa Cruz Biotechnology, Santa Cruz, CA) as the primary antibodies and polyclonal goat anti-mouse antibody (1:10,000; P0447; Dako, Belgium) or polyclonal rabbit anti-goat antibody (1:10,000; P0449; Dako) conjugated to horseradish peroxidase as the secondary antibodies. Films were scanned, and quantification of the bands was obtained by applying ImageJ software. The area under the time-concentration curve for CNP was normalized to that of β-actin.
Sequencing.
After extracting total RNA from P3HR-1 and Akata cells with TRIzol reagent (Life Technologies) and an RNeasy minikit (Qiagen), cDNA was obtained using a first-strand cDNA synthesis kit (GE Healthcare). The entire CNP mRNA was amplified by PCR by using FastStart high-fidelity DNA polymerase (Roche Applied Science, Mannheim, Germany) following the manufacturer's instructions. PCR products were purified with a QIAquick purification kit (Qiagen), and the amplicons were sequenced using a cycle sequencing BigDye Terminator kit version 3.1 on an ABI 3730 sequencing system (Applied Biosystems) and a set of primers spanning the entire coding region of the CNP gene. The sequencing results were computer assembled and compared with the sequence of the reference sequence (NM_033133.4) by using the software SeqScape version 2.7 (Applied Biosystems).
Quantification of cAMP.
P3HR-1, Akata, JSC-1, and BCBL-1 cells were induced with 20 ng/ml TPA or 0.1% IgG. After 24, 72, or 120 h, the concentration of cAMP was quantified in the cell lysates by using a cAMP (direct) enzyme immunoassay kit according to the manufacturer's instructions (Enzo Life Sciences, Antwerp, Belgium).
Animal studies.
All animal work was approved by the KU Leuven Ethics Committee for Animal Care and Use (permit number P097-2010). All animal experiments were conducted in accordance with the Belgian and European guidelines for the protection of vertebrate animals used for experimental and other scientific purposes.
BALB/c mice (age, 4 weeks) were inoculated intranasally with 10,000 PFU MHV-68 under anesthesia with ketamine-xylazine in saline. HPMPC and HPMP-5-azaC were administered intraperitoneally (i.p.) at a dose of 25 mg/kg of body weight/day for 5 consecutive days (starting 2 h p.i.). At days 6 and 12 p.i., five mice per group were sacrificed by injection of pentobarbital, and lungs, mediastinal lymph nodes (MLNs), and spleens were harvested. Tissue sections were stored (i) at −20°C in phosphate-buffered saline for DNA extraction, (ii) at −80°C in RNAlater (Ambion) for RNA extraction, and (iii) fixed in 10% buffered formalin. Tissues were embedded in paraffin, and 5-μm sections were stained with hematoxylin-eosin for histopathological examination.
Quantification of viral DNA load and determination of relative ORF73 and gB expression levels in the tissues were performed as previously described (34). Briefly, tissues were homogenized and DNA was extracted by using a QIAamp DNA minikit (Qiagen), and DNA was quantified by qPCR. RNA was extracted by using a combination of TRIzol reagent (Invitrogen) and an RNeasy minikit (Qiagen). One-step reverse transcription-qPCR was performed using a TaqMan RNA-to-CT 1-step kit (Applied Biosystems) for relative quantitation of RNA.
RESULTS
In vitro antiviral activities of ANPs against gammaherpesviruses.
The activities and selectivities of ANPs against KSHV, EBV, MHV-68, HVS, and RRV are summarized in Table 1 and Table 2. Overall, HPMP derivatives were potent inhibitors of KSHV replication in BCBL-1 cells. With the exception of cyclic HPMPO-DAPy, EC50s ranged from 0.3 to 5.1 μM. The highest anti-KSHV activity was found with 3-deaza-HPMPA. HPMP-5-azaC displayed the highest selectivity, with a selectivity index (SI) of >714 and an EC90 of 9.6 μM. In contrast, PMEA, PMEDAP, and PMEO-DAPy demonstrated relatively weak antiviral activities and no selectivity against KSHV in BCBL-1 cells.
Table 1.
Inhibitory effects of ANPs on the replication of human gammaherpesviruses
| Compound | KSHV |
EBV |
||||||
|---|---|---|---|---|---|---|---|---|
| Antiviral activity (μM)a |
CC50 (μM)b | SIc | Antiviral activity (μM)a |
CC50 (μM)b | SIc | |||
| EC50 | EC90 | EC50 | EC90 | |||||
| HPMPC | 1.3 ± 0.29 | 2.2 ± 1.3 | 609 ± 92 | 468 | 1.9 ± 1.6 | 20 ± 1.6 | >500 | >263 |
| Cyclic HPMPC | 2.4 ± 1.0 | 3.7 ± 3.7 | 589 ± 121 | 245 | 41 ± 24 | 256 ± 286 | >500 | >15 |
| HDP-HPMPC | 0.7 ± 0.1 | 59 ± 18 | >125 | >179 | 0.3 ± 0.2 | 5.2 ± 5.9 | >125 | >417 |
| HPMP-5-azaC | 0.7 ± 0.4 | 9.6 ± 7.1 | >500 | >714 | 3.2 ± 1.8 | 50 ± 40 | >500 | >156 |
| Cyclic HPMP-5-azaC | 3.1 ± 0.8 | 4.6 ± 11 | 462 ± 31 | 149 | 1.1 ± 0.2 | 7.6 ± 9.2 | >500 | >455 |
| HPMPA | 0.7 ± 0.7 | ≥96 | 406 ± 3 | 580 | 3.6 ± 5.3 | 36 ± 36 | >500 | >139 |
| Cyclic HPMPA | 0.7 ± 0.4 | ≥112 | 428 ± 189 | 611 | 39 ± 25 | 312 ± 217 | >500 | >13 |
| 3-Deaza-HPMPA | 0.3 ± 0.2 | 3.0 ± 1.7 | 97 ± 46 | 323 | 0.7 ± 0.7 | 28 ± 22 | >500 | >714 |
| Cyclic 3-deaza-HPMPA | 1.8 ± 0.4 | 3.9 ± 3.2 | 84 ± 70 | 47 | 39 ± 9 | 496 ± 127 | >500 | >139 |
| 7-Deaza-HPMPA | 5.0 ± 0.3 | 12 ± 6.6 | 30 ± 10 | 6 | 5.3 ± 9.3 | 66 ± 0 | ≥470 | 89 |
| Cyclic 7-deaza-HPMPA | 1.4 ± 5.6 | 2.5 ± 0.4 | 116 ± 25 | 83 | ≥239 | >500 | >500 | 2 |
| HPMPDAP | 0.9 ± 0.3 | 31 ± 22 | 459 ± 157 | 510 | 38 ± 28 | ≥336 | >500 | >13 |
| Cyclic HPMPDAP | 2.0 ± 1.3 | 7.3 ± 6.7 | 107 ± 47 | 54 | >500 | >500 | >500 | 1.0 |
| HPMPO-DAPy | 5.1 ± 1.4 | 17 ± 0 | 139 ± 129 | 27 | 59 ± 24 | >500 | >500 | >8 |
| Cyclic HPMPO-DAPy | 111 ± 20 | > 500 | 627 ± 211 | 6 | >500 | >500 | >500 | 1.0 |
| PMEA | 44 ± 15 | ≥172 | 44 ± 26 | 1 | 13 ± 3.3 | 55 ± 29 | >500 | >38 |
| PMEDAP | 16 ± 7.6 | >174 | 14 ± 7 | 1 | 7.6 ± 4.2 | 90 ± 73 | >500 | >66 |
| PMEO-DAPy | 12 ± 5.0 | ≥159 | 27 ± 11 | 2 | 5.0 ± 2.3 | 23 ± 3.8 | >500 | >100 |
The concentrations required to reduce KSHV or EBV DNA synthesis in TPA-stimulated BCBL-1 or P3HR-1 cells by 50 or 90%. Means ± standard deviations are shown where applicable.
The concentration required to reduce the growth of uninduced BCBL-1 or P3HR-1 cells by 50%. Means ± standard deviations are shown where applicable.
The selectivity index (ratio of the CC50 to the EC50).
Table 2.
Inhibitory effects of ANPs on the replication of animal gammaherpesviruses
| Compound | MHV-68 |
HVS |
RRV |
||||||
|---|---|---|---|---|---|---|---|---|---|
| EC50a (μM) | CC50b (μM) | SIc | EC50a (μM) | CC50b (μM) | SIc | EC50a (μM) | CC50b (μM) | SIc | |
| HPMPC | 1.6 ± 0.2 | 98 ± 25 | 61 | 3.5 ± 2.2 | >500 | >143 | 0.2 ± 0.1 | 578 ± 295 | 2,627 |
| Cyclic HPMPC | 2.5 ± 1.2 | 680 ± 199 | 272 | 1.4 ± 0.7 | >500 | >357 | 0.2 ± 0 | >500 | >2,941 |
| HPMP-5-azaC | 1.8 ± 1.4 | 93 ± 36 | 52 | 2.1 ± 1.8 | 732 ± 130 | 348 | 0.2 ± 0.01 | 539 ± 125 | 2,695 |
| Cyclic HPMP-5-azaC | 4.6 ± 2.9 | 195 ± 114 | 42 | 1.5 ± 0.4 | 275 ± 53 | 183 | 1.1 ± 0.8 | 313 ± 164 | 285 |
| HPMPA | 3.0 ± 3.3 | 30 ± 13 | 10 | 4 ± 2.3 | 396 ± 241 | 99 | 0.3 ± 0.3 | 353 ± 3 | 1,177 |
| Cyclic HPMPA | 1.4 ± 0.4 | 63 ± 25 | 45 | 8.4 ± 4.2 | >500 | >60 | 0.3 ± 0 | 182 ± 109 | 607 |
| 3-Deaza-HPMPA | 0.2 ± 0.1 | 17 ± 13 | 85 | 1.0 ± 0.3 | 119 ± 63 | 119 | 0.3 ± 0.03 | 116 ± 76 | 387 |
| Cyclic 3-deaza-HPMPA | 0.4 ± 0.3 | 49 ± 7 | 123 | 2.8 ± 1.4 | 538 ± 289 | 192 | 1.4 ± 2.1 | 190 ± 32 | 136 |
| 7-Deaza-HPMPA | 0.7 ± 0.7 | 4.6 ± 1.3 | 7 | 56 ± 33 | >500 | 9 | 0.7 ± 0.3 | 46 ± 13 | 66 |
| Cyclic 7-deaza-HPMPA | 13 ± 6 | 137 ± 46 | 11 | 88 ± 74 | >500 | 6 | 1.8 ± 0 | 637 ± 320 | 354 |
| HPMPDAP | 0.9 ± 0.3 | 25 ± 14 | 28 | 28 ± 6 | >500 | >18 | 2.8 ± 3.1 | >500 | >179 |
| Cyclic HPMPDAP | 17 ± 0 | 7.7 ± 6.3 | <1 | 67 ± 13 | >500 | >3 | 3.3 ± 1.7 | >500 | >152 |
| HPMPO-DAPy | 0.1 ± 0.07 | 1.0 ± 1.0 | 10 | 68 ± 17 | >500 | >7 | 5.2 ± 0.64 | >500 | > 96 |
| Cyclic HPMPO-DAPy | 6.7 ± 0 | 211 ± 57 | 31 | 215 ± 10 | >500 | >2 | 15 ± 3.7 | 476 ± 238 | 32 |
| PMEA | 0.7 ± 0 | 12 ± 5 | 17 | 172 ± 81 | >500 | >3 | 238 ± 55 | 586 ± 187 | 2 |
| PMEDAP | 0.1 ± 0.03 | 2.8 ± 1.4 | 28 | 59 ± 7.1 | >500 | >8 | 73 ± 7.0 | >500 | >7 |
| PMEO-DAPy | 0.1 ± 0.08 | 2.3 ± 1.5 | 23 | 413 ± 208 | 583 ± 102 | 1 | 242 ± 121 | 242 ± 87 | 1 |
Concentration required for 50% inhibition of CPE. Values are means ± standard deviations where applicable.
Concentration required to reduce the growth of NIH 3T3, OMK, and RF cells by 50%. Values are means ± standard deviations where applicable.
Ratio of the CC50 to the EC50.
Compared to HPMPC (EC50, 1.9 μM), HDP-HPMPC and 3-deaza-HPMPA were more active against EBV strain P3HR-1, with EC50s of 0.3 μM and 0.7 μM, respectively. HPMPC, HPMP-5-azaC, cyclic HPMP-5-azaC, HPMPA, and 7-deaza-HPMPA showed similar anti-EBV activities, with EC50s ranging from 1.9 to 5.3 μM. Weak or no inhibition of EBV replication was observed for HPMPDAP, HPMPO-DAPy, or cyclic HPMP derivatives, with the exception of cyclic HPMP-5-azaC. PMEA, PMEDAP, and PMEO-DAPy showed moderate EC50s, in the range of 5 to 13 μM.
Regarding the inhibition of MHV-68 replication, HPMPC, HPMP-5-azaC, HPMPA, and their cyclic forms exhibited EC50s in the range of 1.4 to 4.6 μM and SIs between 10 and 272. The other drugs (except for cyclic 7-deaza HPMPA, cyclic HPMPDAP, and cyclic HPMPO-DAPy) were even more inhibitory against MHV-68 replication than HPMPC and HPMPA, with EC50s of <1 μM.
HVS was selectively inhibited by HPMPC (SI, >143), its 5-azaC derivative (SI, 348), HPMPA (SI, 99), and its 3-deaza derivative (SI, 119), as well as by their respective cyclic forms. Poor activities and selectivities were obtained for the other ANP derivatives.
The replication of RRV was markedly inhibited by HPMPC, HPMP-5-azaC, and 3-deaza- and 7-deaza-HPMPA, as well as their cyclic derivatives, with EC50s ranging from 0.2 to 1.8 μM. Moderate activities but high selectivities were found for HPMPDAP, HPMPO-DAPy, and their cyclic forms. Poor activities and no selectivity were determined for PMEA, PMEDAP, and PMEO-DAPy against this virus.
Among the ANPs tested against the five gammaherpesviruses, the highest selectivity indices were found for HPMPC, HPMP-5-azaC, 3-deaza-HPMPA, and their cyclic derivatives. Compared to BCBL-1, P3HR-1, OMK, and RF cells, NIH 3T3 cells appeared to be more susceptible to the cytostatic effects of ANPs, and therefore relatively lower SIs were determined against MHV-68 for NIH 3T3 cells.
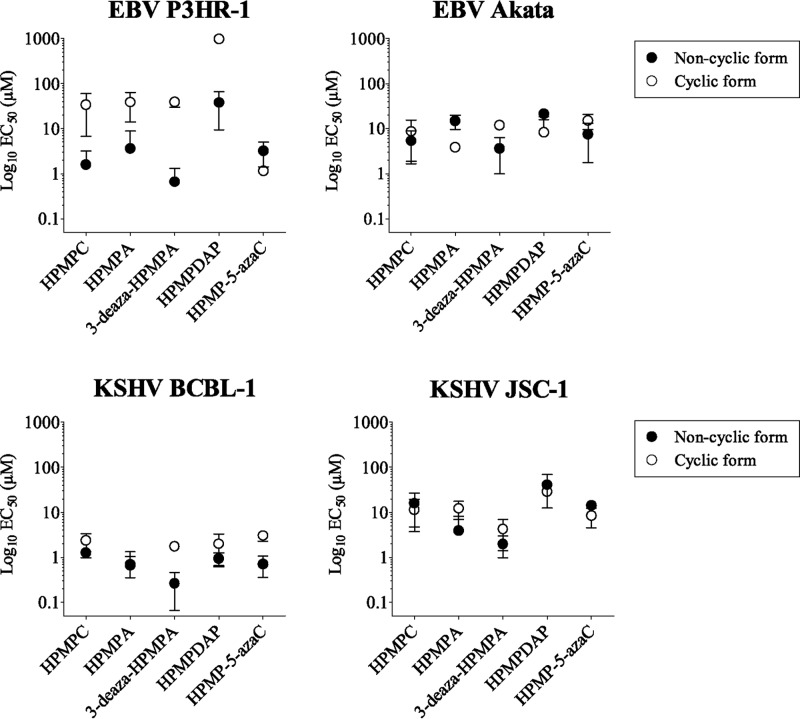
Reduced activities of cyclic HPMP derivatives against EBV in P3HR-1 cells. (i) Activities of cyclic and noncyclic forms of ANPs in different EBV- and KSHV-positive B-cell lines.
Unlike what was seen with KSHV, the cyclic prodrugs of HPMPC, HPMPA, 3-deaza-HPMPA, 7-deaza-HPMPA, HPMPDAP, and HPMPO-DAPy were 10- to 50-fold less active against EBV than their noncyclic forms, e.g., the EC50s of HPMPC and cyclic HPMPC were 2 and 41 μM in P3HR-1 cells, respectively. Among the cyclic HPMP derivatives, cyclic HPMP-5-azaC was found to be an exception, showing a similar EC50 as HPMP-5-azaC against the EBV P3HR-1 strain (1 and 3 μM, respectively).
To determine whether these differences in antiviral activity reflected a particular feature of EBV and/or the P3HR-1 cell line, we evaluated the inhibitory activities of several ANPs and their cyclic forms in the EBV-positive Akata cell line, as well as in the KSHV-positive JSC-1 cell line (Fig. 1). No discrepancies in antiviral activities between noncyclic versus cyclic forms of ANPs were noticed in Akata cells, e.g., the EC50s determined for HPMPC and cyclic HPMPC were 5.4 and 8.7 μM, respectively. In addition, we found similar activities for the cyclic and noncyclic compounds against KSHV in JSC-1 cells. Thus, the reduced antiviral activities of cyclic HPMP derivatives were only observed against the EBV P3HR-1 strain. We hypothesized that an impaired conversion of the cyclic forms into their parent counterparts, e.g., cyclic HPMPC into HPMPC, was responsible for the reduced activities of the cyclic prodrugs against EBV strain P3HR-1.
Fig 1.
Inhibitory effects of HPMP derivatives against KSHV and EBV replication in different B-cell lines. EC50s of ANPs against KSHV in BCBL-1 and JSC-1 cells, and against EBV in P3HR-1 and Akata cells were plotted as the mean of at least three independent experiments ± the standard deviation. The EC50 was defined as the concentration required for 50% inhibition of viral DNA load.
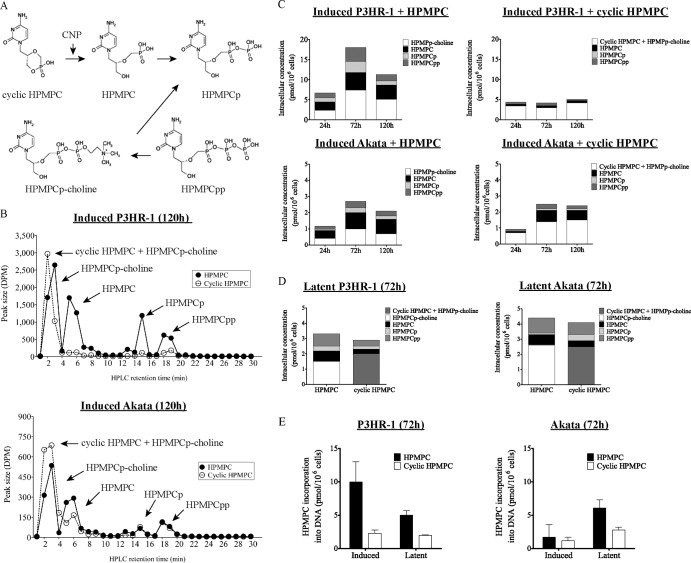
(ii) Metabolism of HPMPC and cyclic HPMPC in P3HR-1 and Akata cells.
After cellular uptake, cyclic HPMPC is efficiently converted into its parent form HPMPC by the phosphodiesterase CNP (35). Further metabolic conversion of HPMPC leads to the production of three metabolites: HPMPC monophosphate (HPMPCp), HPMPC diphosphate (HPMPCpp), and the choline adduct (HPMPCp-choline), which is the intracellular reservoir form of HPMPC (Fig. 2A). We studied the metabolism of HPMPC and cyclic HPMPC in the two EBV-infected cell lines. A kinetic study was performed to determine the concentrations of drug metabolites after 24, 72, and 120 h of incubation with 10 μM [5-3H]HPMPC or cyclic [5-3H]HPMPC in P3HR-1 and Akata cells that were induced into the EBV lytic cycle by either TPA or IgG (Fig. 2C). A representative chromatogram of HPMPC and cyclic HPMPC metabolism at 120 h is shown in Fig. 2B. Under the assay conditions used, cyclic HPMPC coeluted with HPMPCp-choline (retention time, 1 to 3 min). As a consequence, the radioactivity results in the first 3 min of the chromatograms obtained from cells incubated with cyclic HPMPC showed a mixture of cyclic HPMPC and HPMPCp-choline.
Fig 2.
Metabolism of [5-3H]HPMPC and cyclic [5-3H]HPMPC in P3HR-1 and Akata cells after 120 h. (A) Activation pathway for HPMPC and cyclic HPMPC. CNP, cellular 2′,3′-cyclic nucleotide 3′-phosphodiesterase. (B) Representative HPLC result showing the metabolism of 10 μM [5-3H]HPMPC and cyclic [5-3H]HPMPC in induced P3HR-1 and Akata cells after 120 h of exposure to the indicated compound. The arrows point to the different metabolites of HPMPC and cyclic HPMPC. Note the differences between values on the y axis. (C) At the designated time points, intracellular concentrations of HPMPC, HPMPCp, HPMPCpp, HPMPp-choline, and a mixture of cyclic HPMPC and HPMPCp-choline were determined in P3HR-1 and Akata cells after EBV reactivation. The means of two independent experiments were plotted. (D) Intracellular concentrations of HPMPC metabolites in latently infected cells at 72 h after addition of HPMPC or cyclic HPMPC were plotted as the means of two independent experiments. (E) Incorporation of HPMPC into DNA of P3HR-1 and Akata cells in the latent and lytic stages of the virus life cycle after 72 h of incubation.
The metabolic profiles of HPMPC versus cyclic HPMPC showed interesting differences in induced P3HR-1 cells, as well as between P3HR-1 and Akata cells (Fig. 2C). The total amount of radioactivity of all metabolites after incubation with HPMPC was markedly increased in P3HR-1 cells compared to Akata cells (7 to 18 pmol/106 cells versus 1 to 3 pmol/106 cells). Also, after addition of HPMPC or cyclic HPMPC to Akata cells, similar levels of total radioactivity were seen at 24, 72, and 120 h. In contrast, following incubation of P3HR-1 cells with cyclic HPMPC, the total radioactivity was 2- to 4-fold reduced from that for the cells incubated with HPMPC (e.g., concentrations at 72 h after addition of cyclic HPMPC or HPMPC were 4 pmol/106 cells or 18 pmol/106 cells, respectively).
We observed another striking dissimilarity in the metabolism of cyclic HPMPC in induced P3HR-1 at the three designated time points. As shown in Fig. 2C, the concentrations of metabolites that were formed in these cells were low when cyclic HPMPC was added, compared to HPMPC addition. After 72 and 120 h of incubation with cyclic HPMPC, the relative proportion of drug metabolites did not increase. Unlike P3HR-1 cells, Akata cells were found to produce relatively similar concentrations of drug metabolites when cells were incubated with HPMPC or with cyclic HPMPC. Taken together, these findings pointed toward an impaired metabolism of cyclic HPMPC in P3HR-1 cells, in particular, the conversion of cyclic HPMPC into HPMPC.
A similar experiment was conducted in latently infected P3HR-1 and Akata cells, which were incubated with [5-3H]HPMPC or cyclic [5-3H]HPMPC during 72 h, since the altered drug metabolism was the most pronounced at this time point in activated cells. Both latently EBV-infected cell lines had comparable amounts of total radioactivity after incubation with either HPMPC or cyclic HPMPC, i.e., 3 to 4 pmol/106 cells (Fig. 2D). Although, the metabolite concentrations in P3HR-1 cells were slightly lower after addition of cyclic HPMPC than with HPMPC, we did not consider that the metabolism of cyclic HPMPC was altered, such as in induced P3HR-1 cells. The drug metabolites produced in Akata cells incubated with both compounds accounted for similar relative concentrations. The amount of the active metabolite HPMPCpp was shown to be slightly lower in both cell lines when incubated with cyclic HPMPC than with HPMPC.
We further examined the incorporation of radioactivity into total cellular DNA after incubating the cells with cyclic HPMPC or HPMPC. As shown in Fig. 2E, the incorporated radioactivity after 72 h of incubation of P3HR-1 cells with cyclic HPMPC was 4-fold above the cultures receiving HPMPC. In contrast, in Akata cells, DNA incorporation levels were comparable after incubation with cyclic HPMPC or HPMPC. Both latently infected cell lines showed somewhat reduced incorporation of HPMPC into DNA after addition of cyclic HPMPC, but no differences in drug incorporation were observed in P3HR-1 versus Akata cells. Hence, the altered drug metabolism was exclusively observed in induced P3HR-1 cells.
(iii) Characterization of CNP expression in EBV- and KSHV-infected cells.
The cellular CMP phosphodiesterase CNP has been reported to convert cyclic HPMPC into HPMPC (35). To examine whether reduced expression of CNP may be responsible for the altered cyclic HPMPC metabolism in induced P3HR-1 cells, we performed Western blot analysis to determine the protein levels of this enzyme in different B-cell lines, including P3HR-1 and Akata, as well as two KSHV-positive cell lines, BCBL-1 and JSC-1. Relative quantification of the band intensities showed no significant differences in the amount of CNP between the different cell lines, neither in latently infected cells nor in cells induced into the virus lytic cycle (Fig. 3). Even though there was a trend toward an elevated CNP protein level in JSC-1 cells, this difference was not significant.
Fig 3.

Expression of CNP in EBV- and/or KSHV-positive cells. The Western blot shows CNP in induced and latently infected B cells at 120 h (upper panel) and band intensities relative to actin (lower panel).
(iv) Sequence analysis of CNP cDNA derived from different B-cell lines.
We performed genotypic analysis of the cDNA for CNP obtained from P3HR-1, Akata, BCBL-1, and JSC-1 cells to identify any mutations that could potentially affect the enzymatic activity. In one allele of the coding sequence of CNP derived from P3HR-1 cells, a missense mutation was identified at nucleotide position 620, resulting in a Q207R amino acid change. This mutation was mapped to the CNPase domain of the protein but was previously reported as a single-nucleotide polymorphism (reference SNP rs34353668). Therefore, this amino acid change is not expected to affect the enzymatic activity.
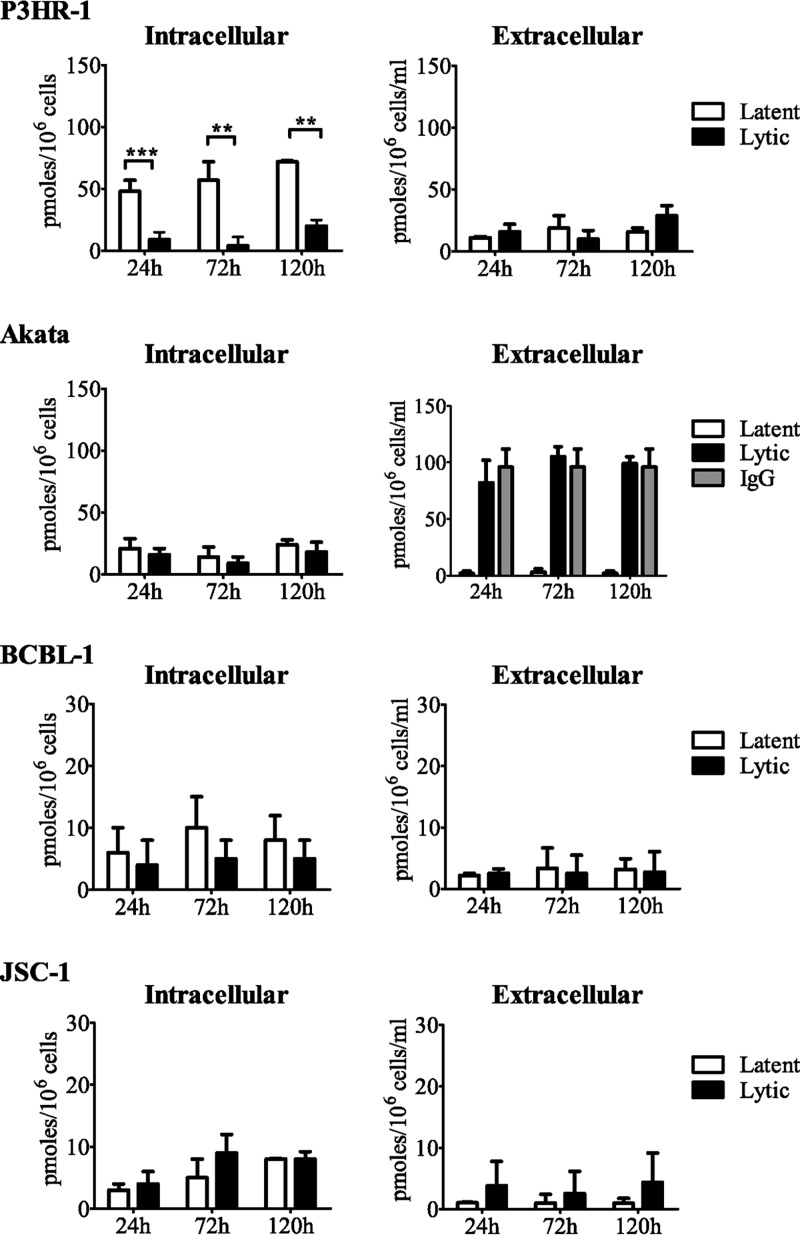
(v) cAMP levels in latently infected cells and in cells induced into the virus lytic cycle.
Mendel et al. previously demonstrated that cyclic HPMPC is an efficient substrate for CNP and that it competes with the natural substrates cAMP and cCMP (35). Therefore, we hypothesized that enhanced conversion of cAMP might compete with cyclic HPMPC for hydrolysis by CNP in P3HR-1 cells, but not in Akata cells. Therefore, we performed an enzyme-linked immunosorbent assay (ELISA) to quantify the intracellular cAMP levels in latently infected cells and in induced P3HR-1, Akata, BCBL-1, and JSC-1 cells at 24, 72, and 120 h (Fig. 4). The levels of cAMP were comparable in latently infected Akata cells, BCBL-1 cells, and JSC-1 cells, and similar levels were also found after induction of the virus lytic cycle, with mean levels ranging from 5 to 20 pmol/106 cells. In contrast, cAMP levels in latently infected P3HR-1 cells were as high as 70 pmol/106 cells, but after EBV reactivation, the cAMP levels decreased to approximately 10 pmol/106 cells.
Fig 4.
Intracellular and extracellular cAMP levels in P3HR-1, Akata, BCBL-1, and JSC-1 cells. Cells were cultured for 24, 72, or 120 h in the presence or absence of the inducing agent (TPA or IgG), and cAMP levels were measured by ELISA. The intracellular cAMP levels represent the amount of cAMP in 106 cells, and the extracellular levels are expressed as the amount of cAMP in 1 ml of medium containing 106 cells. Each bar represents the mean ± standard error of the mean of at least two independent experiments. **, P < 0.01; ***, P < 0.001.
The reduction of intracellular cAMP concentration, as observed after reactivation of EBV in P3HR-1 cells, may be the result of increased cAMP efflux from the cells and/or its increased hydrolysis. We quantified the extracellular cAMP levels in P3HR-1 cells, yet observed no differences in cAMP levels between the latent and lytic states (approximately 10 pmol/106 cells/ml) (Fig. 4). Thus, the decrease in the intracellular cAMP level after EBV reactivation in P3HR-1 cells appears to result from enhanced degradation of cAMP to AMP by phosphodiesterases. Supernatants of BCBL-1, JSC-1, and latently infected Akata cells showed comparable cAMP levels, ranging from 1 to 4 pmol/106 cells/ml. The high cAMP levels obtained in IgG-induced Akata cells were due to interference of the IgG with the ELISA, as reported by the manufacturer. To confirm this, we included an IgG control sample consisting of medium and IgG, and it showed cAMP levels similar to those found in the supernatants of Akata cells in the lytic state.
Efficacy of HPMPC and HPMP-5-azaC treatment in MHV-68-infected mice.
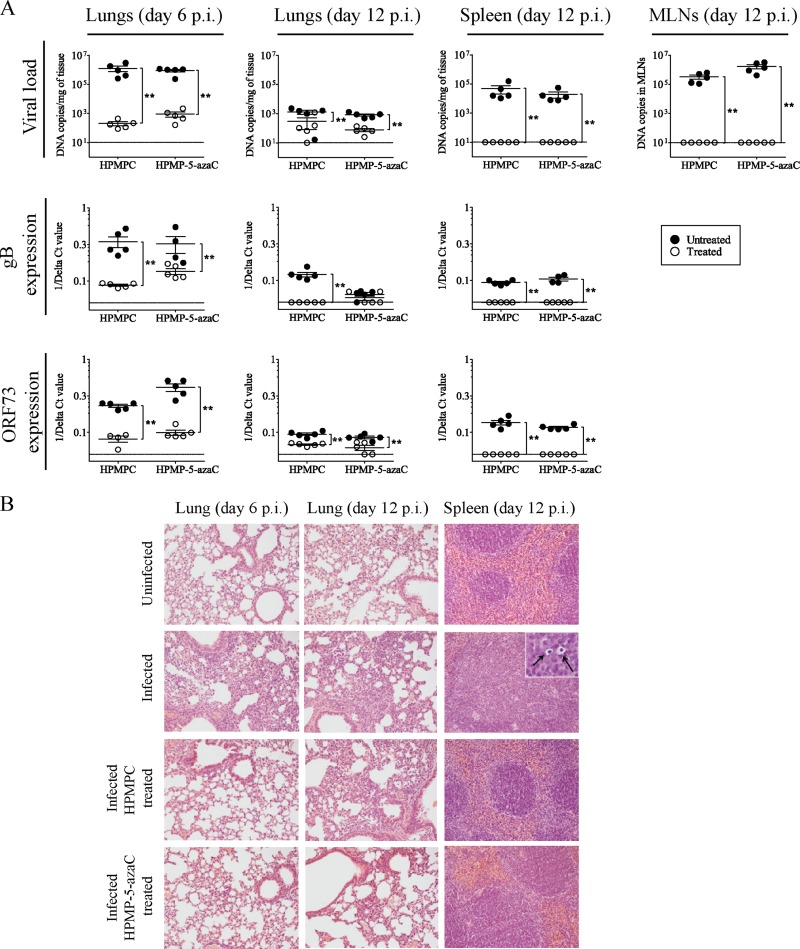
In this study, we showed that HPMP-5-azaC possesses potent in vitro activity and selectivity against all five gammaherpesviruses tested and, moreover, its cyclic derivative was highly active against EBV in P3HR-1 cells. Therefore, we evaluated the in vivo efficacies of HPMPC and HPMP-5-azaC in BALB/c mice intranasally infected with MHV-68. Drug efficacy was evaluated at two time points to determine the inhibition of acute MHV-68 replication in the lungs (at day 6 p.i.), as well as the prevention of establishment of viral latency in the spleen at an early time point (at day 12 p.i.). Both time points were selected based on previous kinetic experiments in which latent (ORF73) and lytic (gB) MHV-68 gene expression was determined in this mouse model (34). Infected mice were treated i.p. with 25 mg per kg per day of HPMPC or HPMP-5-azaC during 5 consecutive days. Animals were sacrificed at day 6 or day 12 p.i., and viral DNA loads were recorded for lungs, spleens, and MLNs. A significant decrease in MHV-68 DNA copies was observed in the lungs of HPMPC- and HPMP-5-azaC-treated mice at day 6 p.i. and day 12 p.i. (Fig. 5A). At day 12 p.i., infected untreated animals showed high numbers of viral DNA copies in MLNs (ranging from 105 to 106 copies) and spleens (104 to 105 copies/mg). In contrast, no viral DNA was detected in these tissues in mice treated with HPMPC or HPMP-5-azaC.
Fig 5.
Analysis of MHV-68 infection in different organs of untreated and treated mice at acute and latent stages of infection. (A) DNA copy numbers were detected in the lungs, MLNs, and spleens of mice infected by the intranasal route with 104 PFU MHV-68 and treated with HPMPC or HPMP-5-azaC (i.p.) for 5 consecutive days. Each group contained five mice. Results are presented as the mean log viral copy number per mg of tissue ± the standard deviation. The levels of MHV-68 gB and ORF73 expression, normalized to the endogenous control, glyceraldehyde-3-phosphate dehydrogenase, were measured relative to the infected control by using the comparative threshold cycle (CT) method (ΔΔCT method). Statistical significance was calculated using the Mann-Whitney U test. *, P < 0.05; **, P < 0.01. (B) Hematoxylin and eosin staining of lung tissues of uninfected mice, MHV-68-infected mice, HPMPC-treated mice, and HPMP-5-azaC-treated mice at days 6 and 12 p.i. Histopathology of the spleen at day 12 p.i. is shown in the panels on the right, and arrows indicate the presence of prominent macrophages in the spleens of infected mice. Magnification, ×20 (inset, ×100).
We further evaluated the impact of antiviral treatment on the lytic and latent stages of MHV-68 infection at the end of treatment (day 6 p.i.) and after treatment (day 12 p.i.) by determining the ORF73 (latent) and gB (lytic) gene expression levels in lungs and spleen tissues. Compared to the untreated infected control, drug treatment resulted in a 300-fold (HPMPC) and 20-fold (HPMP-5-azaC) decrease in gB expression at day 6 p.i. in lung tissue (Fig. 5A). Both drugs reduced the levels of the ORF73 transcript by 300-fold at this time point. No gB expression was found in the lungs of HPMPC-treated mice at day 12 p.i., whereas two out of five HPMP-5-azaC-treated mice showed low gB expression levels. ORF73 expression was reduced by approximately 10-fold in the lungs of treated mice at day 12 p.i. compared to the untreated controls. In the spleen tissue, the gB and ORF73 transcripts were not detected in drug-treated mice.
Histological examination revealed the presence of an inflammatory response in the lungs of infected mice at day 6 p.i. that was characterized by an increased interstitial cellularity in perivascular and peribronchial locations (Fig. 5B). At day 12 p.i., the inflammation in the lungs was dominated by mononuclear inflammatory cells. Following HPMPC or HPMP-5-azaC treatment, lungs of infected mice showed few inflammatory cells at day 6 p.i., but numbers of these cells were increased by day 12 p.i. Enlargement of the MLNs and splenomegaly were observed in infected mice but not in drug-treated mice. Large, cell-rich, and poorly delineated follicles, as well as tingible body macrophages (Fig. 5B, black arrows) were observed in the spleens of infected mice but were not seen in the spleens of HPMPC- or HPMP-5-azaC-treated mice.
DISCUSSION
In this report, we evaluated the effects of ANPs, including some novel derivatives, on the lytic state of gammaherpesvirus replication. Based on the antiproliferative activity of HPMPC, further investigations are required to determine whether these compounds also have an effect on KSHV-infected tumor cells or EBV-transformed cells (24). These studies are of interest since there is a need for new and potent compounds to clear latently infected cells to prevent reoccurrence of viral replication. The broad-spectrum antiviral activities of ANPs depend on the nature of the acyclic side chain and the base moiety (14). We showed that modification of the acyclic side chains from a PME group to an HPMP group resulted in enhancement of antiviral activity against gammaherpesviruses, with the exception of MHV-68. In contrast, substitution of an adenine, as in HPMPA, to a cytosine, as in HPMPC, had no effect on antigammaherpesvirus activity. In addition, the 5-azacytosine analog showed similar in vitro antiviral potency as the parent compound, HPMPC. Modification of the adenine base in HPMPA and PMEA resulted in different profiles of antiviral activity against gammaherpesviruses. Substitution of the purine base to a diaminopurine, such as in HPMPDAP, caused a significant decrease in anti-EBV, anti-HVS, and anti-RRV activities but did not modulate the activities against KSHV or MHV-68. In contrast to HPMPDAP, PMEDAP showed slightly higher activity against gammaherpesviruses than PMEA. The diaminopyrimidine counterpart HPMPO-DAPy possessed a slightly decreased inhibitory activity against gammaherpesviruses (except for MHV-68) compared to HPMPDAP. The inhibitory effects of PMEO-DAPy on EBV, KSHV, and MHV-68 replication were comparable to those of PMEDAP; however, PMEO-DAPy had lower potency against HVS and RRV (Table 1 and Table 2). The divergent antigammaherpesvirus activities of particular ANPs, mostly adenine derivatives, could not be linked to the genetic relationship among the gammaherpesviruses tested. Hence, this study highlights the requirement of different animal gammaherpesviruses, such as MHV-68, HVS, and RRV, for antiviral susceptibility testing, in addition to KSHV and EBV, in order to consider the viruses appropriate surrogate viruses for drug-related studies.
Cyclic prodrugs of ANPs, including cyclic HPMPC and cyclic HPMPA, have been described to possess similar activities as their noncyclic forms when evaluated against alpha- and betaherpesviruses, i.e., herpes simplex virus type 1 (HSV-1), HSV-2, varicella-zoster virus, and human cytomegalovirus (36). Previously, Lin and colleagues investigated the inhibitory effects of HPMPA and cyclic HPMPA on the replication of EBV in P3HR-1 cells (21). They reported that cyclic HPMPA showed a 19-fold decrease in anti-EBV activity compared to HPMPA. Remarkably, in our study not only cyclic HPMPA but also all cyclic HPMP derivatives, with the exception of cyclic HPMP-5-azaC, showed consistently diminished inhibitory effects on EBV replication in P3HR-1 cells compared to their parent compounds (ranging from 10- to 50-fold increases in EC50s). On the contrary, we did not observe differences in antiviral activities of these compounds against the EBV Akata strain. These antiviral data agree with our finding that the induced P3HR-1 cells, but not the induced Akata cells, showed reduced HPMPC incorporation into cellular DNA when incubated with cyclic HPMPC compared to HPMPC. Of note, diminished anti-MHV-68 activities were also observed for the cyclic prodrugs of 7-deaza-HPMPA, HPMPDAP, and HPMPO-DAPy, in comparison with their parent drugs (Table 2), and these findings must be further investigated to explain the differences in anti-MHV-68 activities.
Diminished incorporation of HPMPC in induced P3HR-1 cells treated with cyclic HPMPC was the direct consequence of an altered metabolism of cyclic HPMPC compared to HPMPC in this cell line. The activation of HPMPC and cyclic HPMPC is the net result of different processes that involve drug uptake, intracellular hydrolysis of cyclic HPMPC, intracellular phosphorylation of HPMPC, and efflux of cyclic HPMPC and/or HPMPC from the cells (15). Interestingly, we observed that in induced P3HR-1 cells, cyclic HPMPC was less efficient (than HPMPC) in delivering HPMPC metabolites, including HPMPCpp, the active form that leads to DNA incorporation. In induced Akata cells, HPMPC and cyclic HPMPC had comparable metabolism and, consequently, similar levels of drug incorporation into cellular DNA. The different behaviors between the P3HR-1 and Akata cells were only observed after virus induction, since the latently infected cells showed a similar metabolic profile when exposed to the compounds. Thus, we demonstrated an enhanced ability of P3HR-1 cells to metabolize HPMPC after induction of the EBV lytic cycle, but a decreased ability to metabolize cyclic HPMPC compared to Akata cells.
The reduced concentrations of HPMPC and its phosphorylated metabolites in induced P3HR-1 cells incubated with cyclic HPMPC suggested that these cells display impaired conversion of cyclic HPMPC into HPMPC. It was previously reported that this conversion is mediated by the intracellular cyclic CMP phosphodiesterase CNP (37). In the current study, we demonstrated that induction of EBV replication in P3HR-1 cells did not lead to an absence or diminished expression of CNP. The enzyme was equally expressed in different B-cell lines and, in addition, its expression was independent from the virus that reactivated it (EBV or KSHV), as well as from the inducing agent (TPA or IgG). The enzymatic activity of CNP was not considered to be affected by the Q207R substitution that we identified in the CNP gene of P3HR-1 cells, since this mutation was previously described as a genetic polymorphism. Hence, the reduced capacity for CNP-mediated hydrolysis of cyclic HPMPC appears not be an intrinsic feature of the P3HR-1 cells. Thus, other factors, such as altered competition with natural CNP substrates (i.e., cAMP and cCMP), may be involved.
Infections with viruses such as HSV are known to alter the intracellular levels of cyclic nucleotides, such as decreases in cAMP levels (38). We observed that latently infected P3HR-1 cells contain notably higher levels of cAMP than other gammaherpesvirus-infected B cells. Previously, high cAMP levels were associated with maintenance of viral latency in Burkitt's lymphoma cells (39). The contribution of the cAMP/protein kinase A signaling pathway in EBV latency was attributed to inhibition of the transactivating immediate-early gene BZLF-1, as well as to regulation of the activity and transcription of the latency C promoter (Cp) (39–41). Cp drives the transcription of six EBNA genes and is active in the EBV type III latency program, such as in the EBV P3HR-1 strain, but inactive in the EBV type I latency program (expressing only EBNA1), such as in the EBV Akata strain (42). Thus, activation and stimulation of Cp are important to control the expression of the different latency programs in EBV-infected cells, and this promoter is regulated by viral and cellular factors, such as cAMP.
Because of the inhibitory effect of cAMP on EBV replication in P3HR-1 cells, cAMP levels might need to be reduced in order for the virus to replicate more efficiently. Indeed, rapid cAMP degradation was triggered upon EBV reactivation. Reduction of intracellular cAMP levels is accomplished by its export across the plasma membrane, as well as by phosphodiesterase-mediated degradation (43). Since we were not able to correlate the decrease in intracellular cAMP to an increase of cAMP efflux in P3HR-1 cells, degradation by phosphodiesterases is most likely responsible for the rapid elimination of cAMP in these cells. Also, we do not exclude an indirect effect of TPA treatment on cAMP levels, since phorbol esters have been shown to influence cAMP phosphodiesterase activities in certain cell types through activation of protein kinase C (44).
Taken together, these data strongly indicate that the increased conversion of cAMP in induced P3HR-1 cells could competitively counteract cyclic HPMPC hydrolysis by CNP (Fig. 6). Mendel et al. showed that cAMP, cCMP, and cyclic HPMPC compete with each other for the active site of this enzyme and that the catalytic efficiency is 10- to 20-fold higher for cAMP and cCMP than for cyclic HPMPC (37). Additionally, we may assume that the cyclic forms of other ANPs, cAMP and cCMP analogs, are also hydrolyzed by cAMP- and/or cCMP-converting phosphodiesterases besides CNP. Moreover, P3HR-1 cells are derived from the Jijoye cell line and, in contrast to Jijoye cells, P3HR-1 cells carry a deletion in the EBV genome. We can speculate that the presence of this mutation might also influence the metabolism of cyclic prodrugs. While it was not investigated here, additional studies with Jijoye cells might help to clear this point.
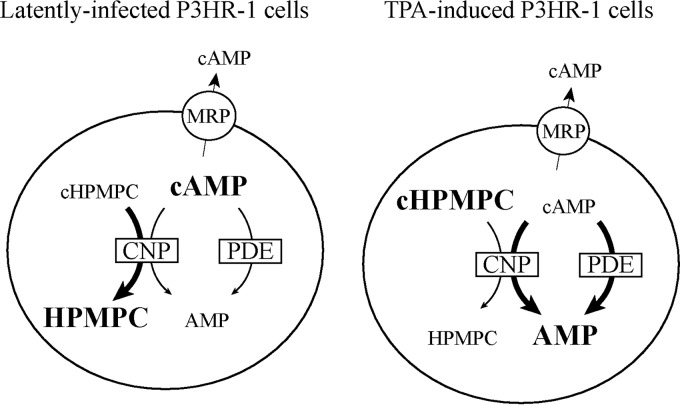
Fig 6.
Proposed mechanism for reduced conversion of cyclic HPMPC in P3HR-1 cells after EBV reactivation. Cyclic HPMPC (cHPMPC) and cAMP are hydrolyzed by the phosphodiesterase CNP and act competitively for the active site of the enzyme (37). In addition, cAMP is known to be hydrolyzed by other phosphodiesterases (PDE) (47). Cyclic nucleotides are also subject to cellular efflux by membrane proteins, such as the multidrug resistance proteins MRP4, MRP5, and MRP8 (48). In latently infected P3HR-1 cells, high intracellular cAMP levels suggested low hydrolysis of this cyclic nucleotide by CNP and other PDE. Upon EBV reactivation, hydrolysis of cAMP by CNP and PDE is considerably increased. The cAMP that is bound to CNP competitively inhibits conversion of cyclic HPMPC by CNP.
Cyclic HPMP-5-azaC was the only cyclic HPMP derivative for which the antiviral activity against EBV in P3HR-1 cells was comparable to that of the noncyclic form. Interestingly, HPMP-5-azaC is known to possess a different pharmacologic profile than HPMPC, since HPMP-5-azaC displays markedly higher phosphorylation to its active metabolite and higher consecutive incorporation into DNA (33). Although diminished conversion of cyclic HPMP-5-azaC in P3HR-1 cells might also occur, this might be compensated by the efficient activation of HPMP-5-azaC, leading to higher incorporation of HPMP-5-azaC into DNA at levels comparable to those of cyclic HPMPC and other HPMP derivatives.
We also demonstrated that, in vivo, HPMP-5-azaC has comparable anti-MHV-68 efficacy as HPMPC. MHV-68 replication was greatly reduced in the lungs of infected mice treated i.p. with HPMPC or HPMP-5-azaC. Although drug treatment was only effective against the lytic cycle of the virus, ongoing productive replication is essential for maintaining high levels of latently infected cells. In line with this, levels of the ORF73 transcript were markedly reduced in drug-treated mice. Until 12 days p.i., MHV-68 did not spread from the lungs to other organs, such as MLNs and spleens, in the mice treated with HPMPC or HPMP-5-azaC. This prolonged effect may be attributed to the long intracellular half-life of some ANP metabolites, such as the choline adducts of the cytosine derivatives (33, 45). In this report, we focused on the efficacy of HPMP-5-azaC to inhibit (i) virus replication in the lungs and (ii) establishment of latent infection in the spleen, rather than on the progression of infection during long-term latency. As virus still resides in the lungs of drug-treated mice, we expect MHV-68 to reactivate at later time points. Similar observations were made in previous studies that examined the efficacy of HPMPC or nucleoside analogs (45, 46).
In conclusion, we have performed a comprehensive investigation of the antigammaherpesvirus activity of several novel ANPs. HPMPC, HPMP-5-azaC, 3-deaza-HPMPA, and their cyclic derivatives emerged as the most potent antigammaherpesvirus agents. Additionally, we observed that cyclic HPMP derivatives have reduced anti-EBV activities in induced P3HR-1 cells, and this effect appeared to be specific for this EBV-infected cell line. Our findings further suggested that the regulation of the different virus latency patterns and reactivation among two EBV-infected cell lines might be indirectly involved in the altered metabolism of cyclic HPMPC and likely of other cyclic prodrugs. Finally, the in vitro and in vivo data presented here demonstrate that HPMP-5-azaC may be an attractive candidate for the development of antigammaherpesvirus drugs and of DNA viruses in general.
ACKNOWLEDGMENTS
We thank Wim van Dam for his excellent technical assistance.
This work was supported by grants from the Geconcentererde Onderzoeksacties (krediet number 10/014), the Fonds voor Wetenschappelijk Onderzoek (krediet number G-0608-08 of the KU Leuven), the Subvention for Development of Research Organization (RVO 61388963), and the Ministry of Industry and Trade of the Czech Republic (FR-TI4/625).
Footnotes
Published ahead of print 11 September 2013
REFERENCES
- 1.Cesarman E. 2011. Gammaherpesvirus and lymphoproliferative disorders in immunocompromised patients. Cancer Lett. 305:163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green M, Michaels MG. 2013. Epstein-Barr virus infection and posttransplant lymphoproliferative disorder. Am. J. Transplant. 13(Suppl 3):41–54 [DOI] [PubMed] [Google Scholar]
- 3.Estep RD, Powers MF, Yen BK, Li H, Wong SW. 2007. Construction of an infectious rhesus rhadinovirus bacterial artificial chromosome for the analysis of Kaposi's sarcoma-associated herpesvirus-related disease development. J. Virol. 81:2957–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin JC. 2000. Strategies for evaluation of antiviral agents against Epstein-Barr virus in culture. Methods Mol. Med. 24:139–150 [DOI] [PubMed] [Google Scholar]
- 5.DeWire SM, McVoy MA, Damania B. 2002. Kinetics of expression of rhesus monkey rhadinovirus (RRV) and identification and characterization of a polycistronic transcript encoding the RRV Orf50/Rta, RRV R8, and R8.1 genes. J. Virol. 76:9819–9831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller G, Heston L, Grogan E, Gradoville L, Rigsby M, Sun R, Shedd D, Kushnaryov VM, Grossberg S, Chang Y. 1997. Selective switch between latency and lytic replication of Kaposi's sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J. Virol. 71:314–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renne R, Lagunoff M, Zhong W, Ganem D. 1996. The size and conformation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) DNA in infected cells and virions. J. Virol. 70:8151–8154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blossom D. 2007. EBV and KSHV, p 1093–1114 In Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K. (ed), Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge, England: [PubMed] [Google Scholar]
- 9.Chang H, Wachtman LM, Pearson CB, Lee JS, Lee HR, Lee SH, Vieira J, Mansfield KG, Jung JU. 2009. Non-human primate model of Kaposi's sarcoma-associated herpesvirus infection. PLoS Pathog. 5(10):e1000606. 10.1371/journal.ppat.1000606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damania B. 2004. Oncogenic gamma-herpesviruses: comparison of viral proteins involved in tumorigenesis. Nat. Rev. Microbiol. 2:656–668 [DOI] [PubMed] [Google Scholar]
- 11.Forrest J, Krug L, Speck SH. 2009. Murine gammaherpesvirus 68 infection of mice: a small animal model for characterizing basic aspects of gamma-herpesvirus pathogenesis, p 735–762 In Dammania B, Pipas J. (ed), DNA tumor viruses. Springer, New York, NY [Google Scholar]
- 12.Cipkova-Jarcuskova J, Chalupkova A, Hrabovska Z, Wagnerova M, Mistrikova J. 2013. Biological and pathogenetic characterization of different isolates of murine gammaherpesvirus 68 (MHV-68) in the context of study of human oncogenic gammaherpesviruses. Acta Virol. 57:105–112 [DOI] [PubMed] [Google Scholar]
- 13.De Clercq E. 2011. The clinical potential of the acyclic (and cyclic) nucleoside phosphonates. The magic of the phosphonate bond. Biochem. Pharmacol. 2:99–109 [DOI] [PubMed] [Google Scholar]
- 14.De Clercq E, Holy A. 2005. Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nat. Rev. Drug Discov. 4:928–940 [DOI] [PubMed] [Google Scholar]
- 15.Cundy KC, Barditch-Crovo P, Petty BG, Ruby A, Redpath M, Jaffe HS, Lietman PS. 1999. Clinical pharmacokinetics of 1-[((S)-2-hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl]cytosine in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 43:271–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Clercq E, Andrei G, Balzarini J, Leyssen P, Naesens L, Neyts J, Pannecouque C, Snoeck R, Ying C, Hockova D, Holy A. 2005. Antiviral potential of a new generation of acyclic nucleoside phosphonates, the 6-[2-(phosphonomethoxy)alkoxy]-2,4-diaminopyrimidines. Nucleosides Nucleotides Nucleic Acids 24:331–341 [DOI] [PubMed] [Google Scholar]
- 17.Krecmerova M, Holy A, Piskala A, Masojidkova M, Andrei G, Naesens L, Neyts J, Balzarini J, De CE, Snoeck R. 2007. Antiviral activity of triazine analogues of 1-(S)-[3-hydroxy-2-(phosphonomethoxy)propyl]cytosine (cidofovir) and related compounds. J. Med. Chem. 50:1069–1077 [DOI] [PubMed] [Google Scholar]
- 18.Friedrichs C, Neyts J, Gaspar G, De Clercq E, Wutzler P. 2004. Evaluation of antiviral activity against human herpesvirus 8 (HHV-8) and Epstein-Barr virus (EBV) by a quantitative real-time PCR assay. Antiviral Res. 62:121–123 [DOI] [PubMed] [Google Scholar]
- 19.Lin JC, De Clercq E, Pagano JS. 1987. Novel acyclic adenosine analogs inhibit Epstein-Barr virus replication. Antimicrob. Agents Chemother. 31:1431–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin JC, De Clercq E, Pagano JS. 1991. Inhibitory effects of acyclic nucleoside phosphonate analogs, including (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, on Epstein-Barr virus replication. Antimicrob. Agents Chemother. 35:2440–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meerbach A, Holy A, Wutzler P, De Clercq E, Neyts J. 1998. Inhibitory effects of novel nucleoside and nucleotide analogues on Epstein-Barr virus replication. Antivir. Chem. Chemother. 9:275–282 [DOI] [PubMed] [Google Scholar]
- 22.Neyts J, De Clercq E. 1997. Antiviral drug susceptibility of human herpesvirus 8. Antimicrob. Agents Chemother. 41:2754–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murono S, Raab-Traub N, Pagano JS. 2001. Prevention and inhibition of nasopharyngeal carcinoma growth by antiviral phosphonated nucleoside analogs. Cancer Res. 61:7875–7877 [PubMed] [Google Scholar]
- 24.Neyts J, Sadler R, De Clercq E, Raab-Traub N, Pagano JS. 1998. The antiviral agent cidofovir [(S)-1-(3-hydroxy-2-phosphonyl-methoxypropyl)cytosine] has pronounced activity against nasopharyngeal carcinoma grown in nude mice. Cancer Res. 58:384–388 [PubMed] [Google Scholar]
- 25.Whitehurst CB, Sanders MK, Law M, Wang FZ, Xiong J, Dittmer DP, Pagano JS. 2013. Maribavir inhibits Epstein-Barr virus (EBV) transcription through the EBV protein kinase. J. Virol. 87:5311–5315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gulley ML, Tang W. 2010. Using Epstein-Barr viral load assays to diagnose, monitor, and prevent posttransplant lymphoproliferative disorder. Clin. Microbiol. Rev. 23:350–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcelin AG, Motol J, Guihot A, Caumes E, Viard JP, Dussaix E, Cadranel G, Frances C, Carcelain G, Calvez V, Dupin N. 2007. Relationship between the quantity of Kaposi sarcoma-associated herpesvirus (KSHV) in peripheral blood and effusion fluid samples and KSHV-associated disease. J. Infect. Dis. 196:1163–1166 [DOI] [PubMed] [Google Scholar]
- 28.Rafailidis PI, Mavros MN, Kapaskelis A, Falagas ME. 2010. Antiviral treatment for severe EBV infections in apparently immunocompetent patients. J. Clin. Virol. 49:151–157 [DOI] [PubMed] [Google Scholar]
- 29.Vouloumanou EK, Rafailidis PI, Falagas ME. 2012. Current diagnosis and management of infectious mononucleosis. Curr. Opin. Hematol. 19:14–20 [DOI] [PubMed] [Google Scholar]
- 30.Feng WH, Hong G, Delecluse HJ, Kenney SC. 2004. Lytic induction therapy for Epstein-Barr virus-positive B-cell lymphomas. J. Virol. 78:1893–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gershburg E, Pagano JS. 2005. Epstein-Barr virus infections: prospects for treatment. J. Antimicrob. Chemother. 56:277–281 [DOI] [PubMed] [Google Scholar]
- 32.Kreckmerová M. 2012. Nucleoside and nucleotide analogues for the treatment of herpesvirus infections: current stage and new prospects in the field of acyclic nucleoside phosphonates, p 245–270 In Magel GD, Tyring S. (ed), Herpesviridae: a look into this unique family of viruses. http://www.intechopen.com. 10.5772/28490 [DOI]
- 33.Naesens L, Andrei G, Votruba I, Krecmerova M, Holy A, Neyts J, De Clercq E, Snoeck R. 2008. Intracellular metabolism of the new antiviral compound 1-(S)-[3-hydroxy-2-(phosphonomethoxy)propyl]-5-azacytosine. Biochem. Pharmacol. 76:997–1005 [DOI] [PubMed] [Google Scholar]
- 34.Coen N, Singh U, Vuyyuru V, Van den Oord JJ, Balzarini J, Duraffour S, Snoeck R, Cheng YC, Chu CK, Andrei G. 2013. Activity and mechanism of action of HDVD, a novel pyrimidine nucleoside derivative with high levels of selectivity and potency against gammaherpesviruses. J. Virol. 87:3839–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cihlar T, Chen MS. 1996. Identification of enzymes catalyzing two-step phosphorylation of cidofovir and the effect of cytomegalovirus infection on their activities in host cells. Mol. Pharmacol. 50:1502–1510 [PubMed] [Google Scholar]
- 36.De Clercq E, Sakuma T, Baba M, Pauwels R, Balzarini J, Rosenberg I, Holy A. 1987. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Res. 8:261–272 [DOI] [PubMed] [Google Scholar]
- 37.Mendel DB, Cihlar T, Moon K, Chen MS. 1997. Conversion of 1-[((S)-2-hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl]cytosine to cidofovir by an intracellular cyclic CMP phosphodiesterase. Antimicrob. Agents Chemother. 41:641–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanwick TL, Anderson RW, Nahmias AJ. 1977. Interaction between cyclic nucleotides and herpes simplex viruses: productive infection. Infect. Immun. 18:342–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daibata M, Humphreys RE, Takada K, Sairenji T. 1990. Activation of latent EBV via anti-IgG-triggered, second messenger pathways in the Burkitt's lymphoma cell line Akata. J. Immunol. 144:4788–4793 [PubMed] [Google Scholar]
- 40.Daibata M, Humphreys RE, Sairenji T. 1992. Phosphorylation of the Epstein-Barr virus BZLF1 immediate-early gene product ZEBRA. Virology 188:916–920 [DOI] [PubMed] [Google Scholar]
- 41.Fuentes-Panana EM, Peng R, Brewer G, Tan J, Ling PD. 2000. Regulation of the Epstein-Barr virus C promoter by AUF1 and the cyclic AMP/protein kinase A signaling pathway. J. Virol. 74:8166–8175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernasconi M, Berger C, Sigrist JA, Bonanomi A, Sobek J, Niggli FK, Nadal D. 2006. Quantitative profiling of housekeeping and Epstein-Barr virus gene transcription in Burkitt lymphoma cell lines using an oligonucleotide microarray. Virol. J. 3:43. 10.1186/1743-422X-3-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jedlitschky G, Burchell B, Keppler D. 2000. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J. Biol. Chem. 275:30069–30074 [DOI] [PubMed] [Google Scholar]
- 44.Houslay MD. 1991. ‘Crosstalk': a pivotal role for protein kinase C in modulating relationships between signal transduction pathways. Eur. J. Biochem. 195:9–27 [DOI] [PubMed] [Google Scholar]
- 45.Neyts J, De Clercq E. 1998. In vitro and in vivo inhibition of murine gammaherpesvirus 68 replication by selected antiviral agents. Antimicrob. Agents Chemother. 42:170–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnes A, Dyson H, Sunil-Chandra NP, Collins P, Nash AA. 1999. 2′-Deoxy-5-ethyl-beta-4′-thiouridine inhibits replication of murine gammaherpesvirus and delays the onset of virus latency. Antivir. Chem. Chemother. 10:321–326 [DOI] [PubMed] [Google Scholar]
- 47.Gantner F, Gotz C, Gekeler V, Schudt C, Wendel A, Hatzelmann A. 1998. Phosphodiesterase profile of human B lymphocytes from normal and atopic donors and the effects of PDE inhibition on B cell proliferation. Br. J. Pharmacol. 123:1031–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wielinga PR, van der Heijden I, Reid G, Beijnen JH, Wijnholds J, Borst P. 2003. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J. Biol. Chem. 278:17664–17671 [DOI] [PubMed] [Google Scholar]