Abstract
Little is known about the stability of HIV-1 cross-neutralizing responses. Taking into account the fact that neutralization breadth has been positively associated with plasma viral load, there is no explanation for the presence of broadly neutralizing responses in a group of patients on treatment with undetectable viremia. In addition, the B-cell profile responsible for broadly cross-neutralizing responses is unknown. Here we studied the evolution of neutralizing responses and the B-cell subpopulation distribution in a group of patients with broadly cross-reactive HIV-1-neutralizing activity. We studied neutralization breadth evolution in a group of six previously identified broadly cross-neutralizing patients and six control patients during a 6-year period with a previously described minipanel of recombinant viruses from five different subtypes. B-cell subpopulation distribution during the study was also determined by multiparametric flow cytometry. Broadly cross-neutralizing activity was transient in four broad cross-neutralizers and stable, up to 4.6 years, in the other two. In four out of five broad cross-neutralizers who initiated treatment, a neutralization breadth loss occurred after viremia had been suppressed for as much as 20 months. B-cell subpopulation analyses revealed a significant increase in the frequency of naive B cells in broadly cross-reactive samples, compared with samples with less neutralization breadth (increased from 44% to 62%). We also observed a significant decrease in tissue-like and activated memory B cells (decreased from 19% to 12% and from 17% to 9%, respectively). Our data suggest that HIV-1 broadly cross-neutralizing activity is variable over time and associated with detectable viremia and partial B-cell restoration.
INTRODUCTION
Most successful vaccines induce neutralizing antibodies, and their role in protective immunity is well established (1). Due to the ability of viruses to evade antibody recognition, an antibody-based HIV-1 vaccine will likely require the induction of broadly neutralizing antibodies (bNAbs). Development of an effective HIV-1 vaccine is especially challenging considering that the virus has evolved several mechanisms to evade antibody-mediated neutralization (1–4). Despite these mechanisms, many HIV-infected individuals are able to generate neutralizing antibodies (NAbs). In addition, some chronically infected patients are able to mount a strong cross-reactive neutralizing response with the ability to neutralize several HIV-1 isolates from different clades (5–8). The percentage of patients able to develop bNAbs is low but higher than initially estimated. In some studies, sera from 10 to 25% of the patients displayed broadly neutralizing activity (5–9).
Antibody responses against viral envelope glycoproteins emerge during the first 2 weeks of HIV-1 infection. However, these antibodies are nonneutralizing and fail to inactivate the infecting virus (10, 11). Autologous neutralizing antibodies increase in number during the first months of infection (12), and cross-neutralizing antibody responses have been shown to emerge on average at 2.5 years after infection (13). The subsequent evolution of these responses in HIV-1-infected patients is not well understood. Neutralization breadth has been positively correlated with plasma viral load (5, 9, 13, 14). However, this correlation contrasts with our report in which broad neutralizing responses were detected in patients on combination antiretroviral therapy (cART), despite having undetectable viremia (15). A better understanding of how broadly cross-reactive neutralizing activity (bCrNA) develops and evolves in infected patients may provide important clues for vaccine design. To date, most of the studies analyzing the breadth of neutralizing responses in HIV-1-infected patients have been cross-sectional. Only a few studies have carried out a follow up of these responses, and none of these studies included patients on cART (5, 12, 13, 16, 17).
The frequency and phenotype of different B-cell subpopulations in patients with bCrNA is another aspect that remains poorly understood. Previous reports have shown that HIV-1 infection leads directly or indirectly to several perturbations on most immune system cells, including B lymphocytes. It has been hypothesized that ongoing HIV-1 replication produces B-cell abnormalities, such as increases in the production of IgG (hypergammaglobulinemia) (18, 19), increases in polyclonal activation (20), increases in cell turnover (20, 21), increases in expression of activation markers (22, 23), increases in the differentiation of B cells in plasmablasts (4, 24, 40), augmented B-cell autoreactivity (25), and increases in the frequency of B-cell malignancies and imbalance of different B-cell subpopulations (26, 27). Many of these defects (i.e., imbalance of B-cell subpopulations) appear to be partly reversed after 12 months of antiretroviral therapy (28).
In a previous cross-sectional study (15), we screened 508 serum samples from 364 patients (173 treated and 191 untreated) for broadly cross-reactive neutralizing activity using a strategy based on the use of recombinant viruses. In that study (15), we identified 12 patients that were capable of neutralizing viruses across 5 subtypes (here termed broadly cross-neutralizing [bCrN] patients). We were also able to confirm the presence of broadly IgG-associated cross-reactive neutralizing responses in a group of patients on antiretroviral treatment, despite their having undetectable viremia (15). In the present study, we evaluated the evolution of neutralization breadth in a group of 6 previously identified bCrN and 6 control patients for a 6-year period. Our data suggest that broadly cross-reactive HIV-1-neutralizing activity is variable over time and is associated with detectable viremia and partial restoration of B-cell subpopulations.
MATERIALS AND METHODS
Study participants.
Serum samples were obtained from HIV-1-infected patients treated at Hospital Clinic (Barcelona, Spain). Medical visits were scheduled at approximately 6-month intervals or more often as necessary for appropriate clinical care. bCrN individuals were identified previously (15), and control patients were selected on the basis of having shown no significant neutralization breadth in the previous cross-sectional study. Control patients were also matched for age (25 to 57 years old), CD4+ counts (291 to 759 cells/μl), CD8+ counts (576 to 1,698 cells/μl), and plasma viral loads (1.7 to 4.94 logs) (Table 1). All the individuals gave informed written consent, and the study was reviewed and approved by the Institutional Ethical Committee Board of the Hospital Clinic (Barcelona, Spain).
Table 1.
Clinical characteristics of patientsa
| Characteristic | Broad cross-neutralizer patients |
Control patients |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 181 | 308 | 363 | 488 | 528 | 541 | 108 | 279 | 296 | 326 | 405 | 423 | |
| Risk group | MSM | MSM | MSM | Unknown | MSM | MSM | IDU | MSM | MSM | MSM | HTX | MSM |
| Peak VL (log) | 4.60 | 4.44 | 3.90 | 3.71 | 5.01 | 4.89 | 5.69 | 5.63 | 5.08 | 5.04 | 4.23 | 4.69 |
| Nadir CD4+ level (cells/μl) | 266 | 410 | 537 | 291 | 240 | 286 | 219 | 260 | 305 | 340 | 408 | 525 |
| Nadir CD8+ level (cells/μl) | 285 | 948 | 661 | 630 | 644 | 390 | 394 | 549 | 847 | 458 | 377 | 374 |
| TPS (months) | 185.3 | 127.4 | 97.3 | 65.6 | 62 | 67.4 | 225.6 | 84.6 | 91.3 | 97.7 | 85.9 | 78.4 |
| Beginning of the study (−71 to −37 months)b | ||||||||||||
| Age (yrs) | 57 | 38 | 34 | 36 | 28 | 54 | 42 | 31 | 36 | 36 | 34 | 25 |
| cART | — | — | — | — | — | — | — | — | — | — | — | — |
| VL (log) | 4.04 | 3.31 | 3.48 | 2.6 | 4.9 | 4.51 | 4.75 | 3.94 | 3.53 | 3.86 | 2.81 | 4.04 |
| No. of CD4+ T cells/μl | 567 | 506 | 758 | 646 | 556 | 606 | 595 | 645 | 509 | 425 | 436 | 600 |
| No. of CD8+ T cells/μl | 756 | 1,720 | 924 | 1,115 | 1,290 | ND | 1,388 | 1,290 | 1,136 | 962 | 377 | 726 |
| Time zeroc | ||||||||||||
| cART | ATP | ATP | — | ATP | — | ATP | ATP | — | — | ATP | — | — |
| VL (log)d | <1.7 | <1.7 | 3.87 | <1.7 | 4.43 | <1.7 | <1.7 | 4.94 | 4.61 | <1.7 | 4.24 | 3.51 |
| No. of CD4+ T cells/μl | 437 | 530 | 581 | 291 | 759 | 499 | 759 | 399 | 331 | 703 | 590 | 666 |
| No. of CD8+ T cells/μl | 857 | 1,698 | 664 | 1,029 | 1,029 | 576 | 1,621 | 1,386 | 1,284 | 836 | 859 | 1,030 |
| End of the study (18 to 34 months)e | ||||||||||||
| cART | ATP | ATP | — | ATP | ATP | ATP | ATP | ATP | ATP | ATP | ATP | — |
| VL (log)d | <1.56 | <1.56 | 3.49 | <1.56 | <1.56 | <1.56 | <1.7 | 1.85 | 2.85 | <1.7 | <1.7 | 4.21 |
| No. of CD4+ T cells/μl | 539 | 819 | 664 | 402 | 638 | 484 | 688 | 514 | 593 | 551 | 600 | 525 |
| No. of CD8+ T cells/μl | 1,174 | 1,580 | 979 | 816 | 646 | 390 | 1,383 | 1,601 | 2,093 | 893 | 570 | 1,047 |
All patients were male. MSM, men that have sex with men; IDU, injecting drug user; HTX, heterosexual; VL, viral load; TPS, time elapsed between first HIV-1-positive serology and time zero; ATP: Atripla (efavirenz + emtricitabine + tenofovir). —, untreated.
Time (months) before the last time analyzed in the previous study.
Last time (months) included in the previous study.
The limit of detection was 1.7 log or 1.56 log (corresponding to 50 or 36 copies/ml, respectively), depending on the commercial kit used.
Time (months) after the last time included in the previous study (15).
Immunoglobulin G (IgG) purification.
IgGs were isolated from sera using a protein G HP spin trap (GE Health Care, United Kingdom) and extensively dialyzed with Spectra/Por Float-a-Lyzer G2 50-kDa-cutoff membranes (Spectrum Laboratories Inc.) following the manufacturer's instructions. IgG quantification was done by a mini-Bradford assay on a microplate spectrophotometer (Tecan Trading AG, Switzerland).
Neutralization assays.
Purified IgGs were tested at a 0.2-mg/ml concentration (corresponding to a dilution range of 1/40 to 1/80) against a minipanel of 6 recombinant viruses from different tropisms and 5 different subtypes using TZM-bl cells, as previously described (15). Serial IgG concentrations (0.3 to 0.001 mg/ml) from patients 181, 308, and 528 were also tested against the same recombinant virus panel. The minipanel of recombinant viruses was previously generated by replacing the env sequence of HIV NL4-3 with env sequences from isolates of 5 different subtypes (clades and tier categorizations are given in parentheses): VI191 (A, tier 2), 92BR025 (C, tier 1B), 92UG024 (D, tier 2), CM244 (AE, tier 2), and AC10 (B, tier 2) (15). Strain NL4-3 (clade B, tier 1A) was included in the minipanel as a neutralization-sensitive control, and an amphotropic vesicular stomatitis virus (VSV) Env pseudotyped on an HIV-1 core was included as a specificity control. Virus stocks were produced by transfection of 293T cells using the calcium phosphate method according to the manufacturer's recommendations (ProFection mammalian transfection system; Promega, Madison, WI). VSV-pseudotyped virus stocks were produced by cotransfecting 293T cells with pNL4-3ΔenvFL (29) and pVSV-G plasmids as described above. Neutralization activity for all purified IgGs was measured in triplicate as a reduction in infectivity using a luciferase reporter gene assay after what is considered a single-round infection of TZM-bl cells. A serum sample was considered to be capable of neutralizing a virus when purified IgGs from the corresponding serum reduced viral infectivity by a minimum of 50% at a 0.2-mg/ml concentration. We considered that a serum sample displayed bCrNA when the corresponding purified IgGs were capable of neutralizing viruses across 4 or more subtypes, out of the minipanel described above, with no significant neutralization of the VSV control.
Cell separation and flow-cytometric analysis of peripheral B cells.
Due to limited sample availability, we could analyze only 15 samples from 6 bCrN patients and 18 samples from 6 control patients before treatment (patients 181, 541, and 405, one sample each; patients 308, 488, 528, 108, 296, and 326, three samples each; patients 279, 363, and 423, four samples each; patient 363, five samples). We also analyzed frequencies of various B-cell subpopulations in 15 additional samples after initiating antiretroviral treatment from four bCrN individuals (patients 308 and 108, two samples each; patients 181 and 488, three samples each) and five controls (patients 541, 279, 296, 326, and 405, one sample each). Peripheral blood mononuclear cells (PBMC) were obtained by Ficoll centrifugation (Accuspin system, Histopaque-1077; Sigma Diagnostics). PBMC were then aliquoted at 10 million cells per vial and cryopreserved in liquid nitrogen until needed for flow cytometry. For flow-cytometric analysis, PBMC were thawed, washed twice with phosphate-buffered saline (PBS), and then resuspended in RPMI medium supplemented with 10% fetal bovine serum for 1 h at 37°C. Multiparametric surface staining was performed with the following reactive dye and antibodies: blue fluorescent reactive dye (live/dead stain; Invitrogen), anti-CD19 conjugated to peridinin chlorophyll protein (PerCP) and Cy5.5, anti-CD3–Pacific Blue, anti-CD27–phycoerythrin (PE), anti-CD10–allophycocyanin (APC), anti-CD21–fluorescein isothiocyanate (FITC), and anti-CD20–APC-H7 (all antibodies were purchased from BD, Becton Dickinson). PBMC were incubated with antibodies at 4°C for 1 h. Cells were then washed twice with 2 ml of PBS with bovine serum albumin (BSA; 0.5%) and 0.1% sodium azide. After being washed, cells were resuspended in 400 μl fluorescence-activated cell sorting (FACS) buffer containing 1% paraformaldehyde. Fluorescence-minus-one (FMO) and doublet exclusion controls were also used to delineate the populations of interest. One to two million events were acquired on an LSRFortessa cell analyzer (BD, Becton Dickinson), and data were analyzed using FlowJo software (version 7.2.4; TreeStar Inc.). The B-cell gating strategy is shown in Fig. S1 in the supplemental material.
Statistics.
Analyses were performed using GraphPad Prism 5 (GraphPad Software). Mann-Whitney U tests were used for comparisons of continuous variables between groups. Simple comparisons were made with use of a two-sided alpha level of 0.05.
RESULTS
Neutralization breadth stability in bCrN and control patients.
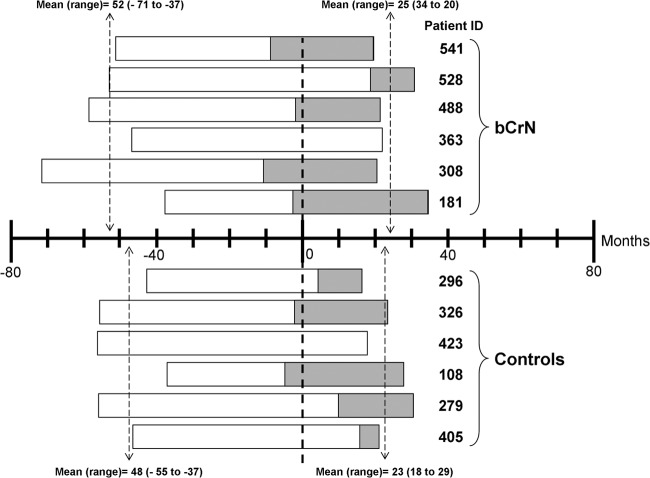
A group of six out of 12 bCrN patients previously identified (15), together with a six-patient control group, were studied for a 6-year period. In the present study, time zero corresponds to the last time point analyzed in the previous study, and a chart indicating the study period for each patient is shown in Fig. 1. The remaining six bCrN patients from the previous study were not included for sample availability reasons. Clinical characteristics of the six bCrN patients and controls at the beginning of the study, at time zero, and at the end of the study are described in Materials and Methods and shown in Table 1.
Fig 1.
Study chart. Time zero corresponds to the last time point analyzed in the previous study (15). The period included in the present study before and after the previous cross-sectional study (mean and range) is indicated. The periods in which some patients were on cART are also indicated.
Detectable broadly cross-neutralizing responses, defined as neutralization of at least one virus included in the minipanel from at least four different subtypes, were observed in our cohort during the time of the study. Several groups have tested the neutralizing activity in a large group of samples on different virus panels and reported that screening for a reduced panel (6 viruses or less) of selected viruses provided similar information on the presence of cross-reactive neutralizing activity as screening for a large virus panel (30–33). The broadly neutralizing activity of the patients selected with our 6-virus panel was also confirmed previously with an extended panel, including 25 additional viruses from different subtypes (15).
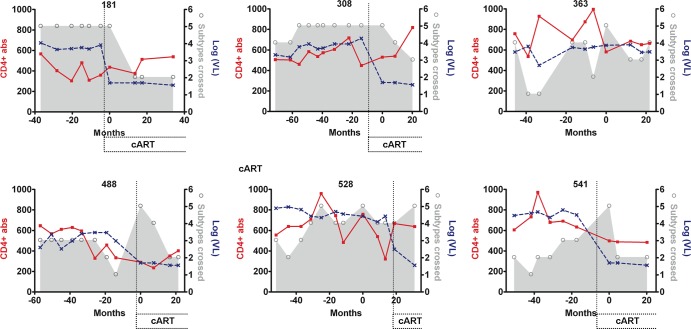
The stability of the bCrNA varied among patients. Patients 363, 488, and 541 showed neutralizing activity across 5 subtypes at only one of the time points analyzed (Fig. 2). Especially significant were the cases of patients 181 and 308, who showed neutralizing activity across 5 subtypes for 3.1- and 4.6-year periods, respectively (Fig. 2; also, see Table S1 in the supplemental material). Patient 528 showed a significant cross-reactive neutralizing response (viruses from four or more subtypes neutralized) for a long period of time (5.2 years). However, the breadth of his antibody-mediated neutralizing response reached 5-subtype neutralization only intermittently (Fig. 2; also, see Table S1 in the supplemental material). In order to check for fluctuations in neutralization titers during the long periods of apparent neutralization stability, we made complete neutralization curves with samples corresponding to periods of more than 3 years of broadly cross-reactive neutralizing responses (patients 181, 308, and 528). The 50% inhibitory concentrations (IC50s) corresponding to these curves are shown in Table 2 and in Fig. S2 in the supplemental material. This new set of experiments showed that the corresponding neutralizing activity had some fluctuations in neutralization titers during the periods with broad cross-reactive neutralizing activity. However, the ability to neutralize viruses across 5 subtypes at an IgG concentration of 0.2 mg/ml was not lost during these periods.
Fig 2.
Changes in neutralization breadth, CD4+ T cells, and viral loads in the bCrN group. Neutralization breadth values (shown by the gray area and open circles) indicate the number of viruses from different subtypes neutralized out of a previously described minipanel (15). The number of CD4+ T cells/mm3 (CD4+ abs) is represented by a solid red line. The viral loads [log(VL), in copies/ml] are represented by a dotted blue line. The periods of time in which some patients were on cART are indicated. Time zero corresponds to the last time point included in the previous study (15). Antibodies capable of neutralizing across 5 subtypes were detected in our cohort of bCrN patients for a period up to 4.6 years. Four out of five bCrN patients that initiated cART showed a loss of neutralization breadth over time.
Table 2.
IgG neutralization data corresponding to periods with broadly cross-reactive neutralizing responses in patients 181, 308 and 528a
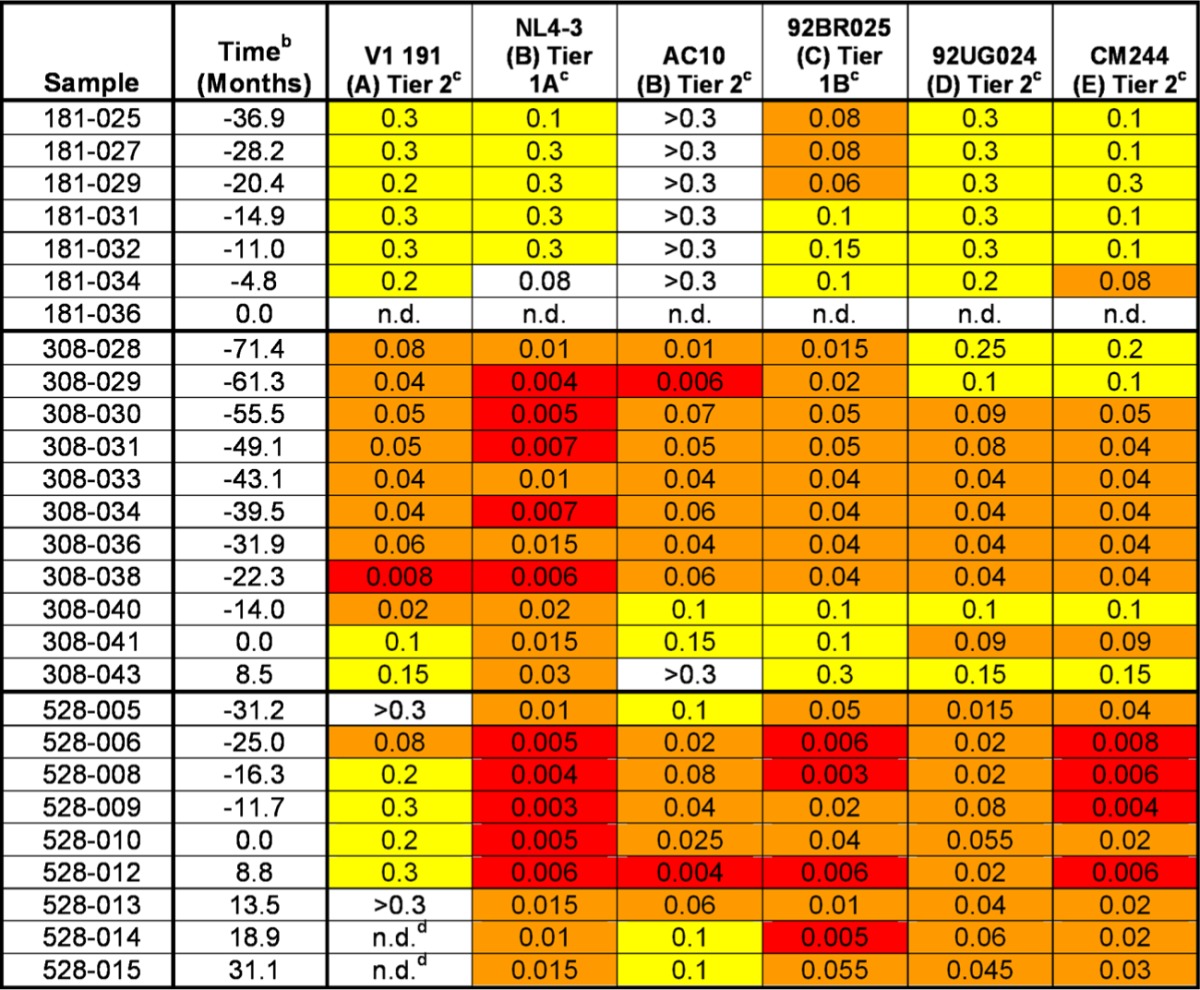
Values are concentrations of purified IgG that reduces the infectivity by 50% (IC50). White indicates that 50% infectivity reduction was reached at the highest concentration tested (0.3 mg/ml); yellow indicates that the IC50 was ≤0.3 but ≥0.1 mg/ml; orange indicates that the IC50 is <0.1 but >0.01 mg/ml; red indicates that the IC50 is <0.01.
Time zero corresponds to the last time point included in the previous study.
Letters in parentheses are clades.
ND, not done for sample availability reasons.
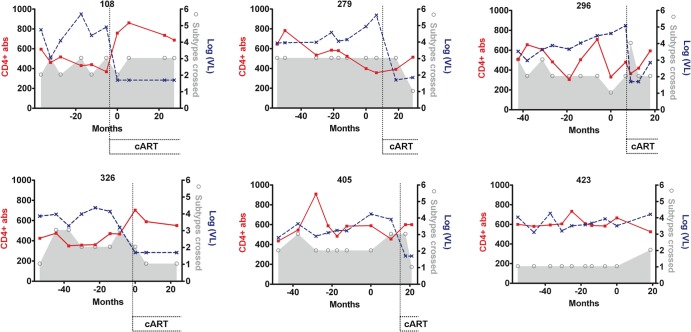
Only one patient in the control group (296) showed a transient bCrNA during the time of the study (neutralization across 4 subtypes at only one of the time points analyzed) (Fig. 3; also, see Table S2 in the supplemental material). We found no significant direct correlation between stability of bCrNA during the study and nadir CD4+ T counts, nadir CD8+ T cell counts, peak viremia, or time since first HIV-positive serology (Fig. 3; also, see Table S2 in the supplemental material).
Fig 3.
Changes in neutralization breadth, CD4+ T cells, and viral loads in the patient control group. Neutralization breadth values (shown by the gray area and open circles) indicate the number of viruses from different subtypes neutralized out of a previously described minipanel (15). The number of CD4+ T cells/mm3 (CD4+ abs) is represented by a solid red line. The viral loads [log(VL), in copies/ml] are represented by a dotted blue line. The periods of time in which some patients were on cART are indicated. Time zero corresponds to the last time point included in the previous study (15).
Impact of viral load suppression on neutralization breadth.
Five out of six patients from both bCrN and control groups initiated combination antiretroviral therapy (cART) during the study, reaching undetectable viremia (<50 copies/ml). Patients 363 and 423 (a bCrN patient and a control patient, respectively) remained untreated (Fig. 1, 2, and 3).
Within the five bCrN patients that received cART and reached undetectable viremia during the follow-up, four showed significant declines in the levels of neutralization breadth (neutralization of viruses from five to three or fewer subtypes), with a delay in loss of breadth after viremia suppression of 4 to 20 months (patients 541 and 308, respectively). The IC50 values corresponding to the periods of broad cross-neutralizing activity in patient 308 (Table 2; also, see Fig. S2 in the supplemental material) indicated that the loss in neutralizing activity initiated at the same time as treatment-associated viral loss, but the ability to neutralize some of the virus from the minipanel was not lost until 20 months after viremia became undetectable. The dynamic link between neutralization breadth loss and decay of viremia in these patients could not be determined, because there were no samples corresponding to intermediate time points available. For patient 528, neutralization breadth loss was not detected after suppression of viremia within the time of the study. This could be explained by the fact that this patient reached undetectable viremia only at the last time point analyzed (12.2 months since cART initiation) (Fig. 2). We did not observe significant differences between neutralization breadth before and after treatment in the control group. Due to their low neutralization breadth, we would probably need an extended panel of viruses to detect neutralization breadth losses in control patients.
Next, we investigated the potential relationship between neutralization breadth and plasma viral load levels in our patients. A univariate model analysis showed that the overall neutralization breadth was similar for plasma samples from patients with detectable and undetectable viremia (Fig. 4A). Considering that it has been previously reported that virus from the secondary lymphoid tissue reservoir is not cleared until after 6 months of potent therapy (34), we compared the neutralization breadths in plasma samples from viremic patients and patients with less than 6 months of undetectable viremia with those in plasma samples from patients with at least 6 months of undetectable viremia and found that the neutralization breadth was significantly higher for the first group (P = 0.0495) (Fig. 3B). This analysis confirmed that plasma viral load had a significant impact on neutralization breadth even when cART patients were included in the study.
Fig 4.
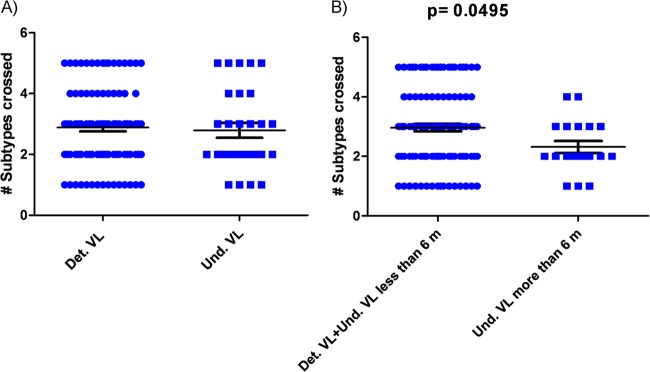
Breadth of the plasma NAb responses and plasma viral load (VL) levels. (A) Analysis of the overall neutralization breadth for plasma samples from patients with detectable (Det.) and undetectable (Und.) viremia (bCrN and control patients). (B) Comparison of neutralization breadth in plasma samples from patients with at least 6 months of undetectable viremia with the group of plasma samples from viremic patients and patients with less than 6 months of undetectable viremia. Horizontal bars within the point plots indicate the median subtype crossed ± the standard error of the mean (SEM). Significance between groups is indicated above the groups. Mann-Whitney U tests were used for comparisons between groups. Simple comparisons were made with use of a two-sided alpha level of 0.05.
B-cell subpopulations before and after initiation of cART.
In order to confirm the previously reported effect of ART on the B-cell phenotypic profile, we evaluated frequencies of various B-cell subpopulations in bCrN and control patients before and after initiation of cART. The average times on treatment for the samples from bCrN and control patients were similar (17.6 ± 12.5 and 16.9 ± 12.7 months, respectively). Based on previous studies (26), CD19+ B cells in peripheral blood of HIV-infected individuals can be divided into the following 6 subpopulations, listed in order of increasing level of differentiation: immature/transitional (CD10+ CD27−), naive (CD10− CD27+ CD21hi), tissue-like memory (CD10− CD27− CD21lo), resting/memory (CD10+ CD27+ CD21hi), and activated/memory (CD10− CD27+ CD21lo) B cells as well as plasmablasts (CD10− CD27++ CD20− CD21lo).
B-cell subpopulation analysis revealed the following changes in mean frequencies as a result of initiation of cART: immature transitional B cells decreased from 6% to 3%; naive B cells increased from 48% to 64%; tissue-like memory B cells decreased from 18% to 11%; activated memory B cells decreased from 15% to 9%, and plasmablasts decreased from 0.07% to 0.04% (Fig. 5). We did not observe any changes in the proportion of resting memory B cells (13%). The decreases in immature, tissue-like memory, and activated memory B cells and the increase in naive B cells were statistically significant. However, the decrease in plasmablast proportion did not reach statistical significance (Fig. 5).
Fig 5.
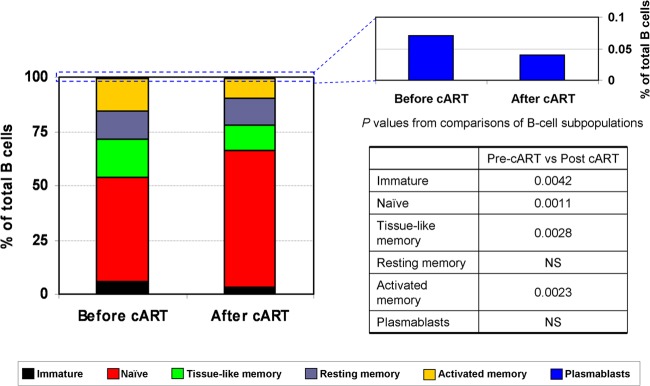
Distribution of B-cell subpopulations before and after cART. The percentages of cells in each of the six B-cell subpopulations for samples from patients before and after cART are shown. The mean frequencies for different B-cell subpopulations are denoted by different colors. P values from comparisons of B-cell subpopulations are also shown. NS, not significant.
Despite our having not found any increase in the percentage of resting memory B cells, these results are in good agreement with previous reports that describe a normalization of B-cell subpopulations after antiretroviral treatment, reflected by an increase in naive and resting memory B cells and a decrease of the 2 apoptosis-prone subpopulations of B cells (immature transitional and mature activated B cells) (28, 35).
B-cell subpopulations in samples with different neutralization breadths.
We analyzed next the frequencies of various B-cell subpopulations in our patients. In this analysis, we included only samples from before initiation of cART, in order to avoid the effect of treatment-associated suppression of viremia on B-cell phenotype and function described above and reported previously (28).
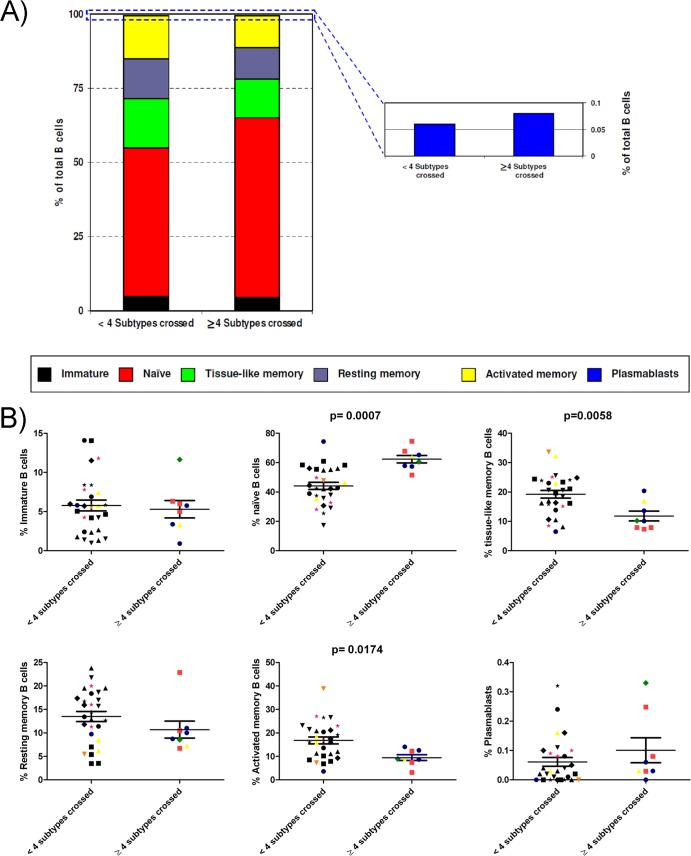
Data on all untreated patients were compiled and analyzed by comparing samples from patients capable of neutralizing viruses across 4 or more subtypes and samples from patients that neutralized viruses from fewer than 4 subtypes. Analysis of the B-cell subpopulations revealed the following mean frequencies in the two groups, respectively: immature transitional B cells, 5% and 6%; naive B cells, 62% and 44%; tissue-like memory B cells, 12% and 19%; resting memory B cells, 11% and 13%; activated memory B cells, 9% and 17%; and plasmablasts, 0.10% and 0.06% (Fig. 6). This analysis revealed a higher percentage of naive B cells and plasmablasts and a lower percentage of tissue-like memory, resting memory, and activated memory B cells in patients that neutralize viruses across 4 or more subtypes. However, as shown in Fig. 6B, only the differences in naive, tissue-like memory, and activated memory B cells were statistically significant (P = 0.0007, P = 0.0058, and P = 0.0174, respectively). No differences in the percentage of immature B cells between the two groups of samples were observed in this analysis. We also compared samples from patients capable of neutralizing across 5 subtypes and samples with lower neutralization breadths. In this analysis, we obtained similar results, but the differences were not statistically significant, probably due to the small number of samples capable of neutralizing across 5 subtypes (data not shown).
Fig 6.
Distribution of B-cell subpopulations in samples with different neutralization breadths. (A) The percentages of cells in each of the six B-cell subpopulations were measured for samples from patients capable of neutralizing viruses across 4 or more subtypes and patients that neutralized viruses from fewer than 4 subtypes. The mean frequencies for different B-cell subpopulations are denoted by different colors. (B) Statistical analysis of each B-cell subpopulation. Horizontal bars within the point plots indicate the median percentage for each group ± the SEM. Significance between groups is indicated above the groups. Each type of symbol corresponds to a different patient. Black symbols, control patients; green diamonds, patient 181; red squares, patient 308; blue circles, patient 363; pink stars, patient 488; yellow triangles, patient 528; and orange inverted triangles, patient 541. Mann-Whitney U tests were used for comparisons between groups. Simple comparisons were made with use of a two-sided alpha level of 0.05.
Our findings indicate that the frequency of naive B cells was significantly higher in patients capable of neutralizing viruses across 4 or more subtypes and close to the average of 65% reported for healthy individuals (36). In contrast, the frequency of tissue-like memory B cells was significantly lower in patients with a broader neutralizing activity than in patients with less neutralization breadth, consistent with the concept that these are exhausted B cells induced by chronic HIV-induced immune activation (37). The frequency of activated memory B cells was also significantly lower in patients capable of neutralizing viruses across 4 subtypes or more than in patients with less neutralization breadth, indicating a reduction of the aberrant increase in B-cell activation associated with chronic HIV infection that was first reported several years ago (35).
HIV infection is associated with a number of perturbations in the B-cell compartment, including the overrepresentation of subpopulations of B cells in the blood that are thought to arise as a result of HIV-induced immune activation and CD4+ T cell lymphopenia. Taken together, these results showed that the balance within B-cell subsets in patients with bCrNA was partially restored compared with the proportions observed in patients with less neutralization breadth, including proportions closer to the ones reported for healthy individuals.
DISCUSSION
The inability to elicit broad and potent cross-reactive anti-HIV neutralizing antibodies by immunization has been a major obstacle for the development of an effective vaccine against HIV. However, we have evidence that the induction of this type of response is feasible, since there are some chronically infected patients with high titers of bNAbs (5). Currently, little is known about the stability of broadly neutralizing responses, the impact that the loss of viremia has on such response, and the B-cell phenotype associated with this response. We carried out the present study in order to characterize the evolution of neutralization breadth and the impact of potent antiretroviral treatment in six bCrN. Our definition of bCrNA is close to the definition used by Simek et al. for elite neutralizing activity, which considers elite activity to be “the ability to neutralize, on average, more than one pseudovirus at an IC50 titer of 300 within a clade group and across at least four clade groups” (32). While we were unable to completely satisfy these criteria, since our 6-virus panel included only single variants of subtypes A, C, D, and AE, the bCrN individuals included in the present study could neutralize viruses across 5 subtypes (A, B, C, D, and AE). In addition, we characterized the frequency of different B-cell subpopulations associated with bCrNA. This study describes the long-term stability of antibody-mediated neutralization breadth in a group of bCrN. Furthermore, this is the first report describing the impact of viremia decay associated with potent antiretroviral treatment on neutralization breadth.
Previous studies hypothesized that the induction of broadly neutralizing antibodies requires prolonged exposure to the antigen and does not develop until 2 to 4 years postinfection (13). At this point, it is likely that is too late for these antibodies to have an effect due to the presence of a widespread infection and an irreversibly damaged immune system. The present study shows that, a normalized B-cell profile is also required to promote the development of broadly neutralizing responses. On the basis of our results, we suggest that the problematic induction of broadly neutralizing antibodies could be explained by the need to match two factors that seem to be incompatible: a prolonged antigen exposure characteristic of a chronic infection and a low damaged-B-cell profile. On the basis of present knowledge, it has been suggested that, in order to be effective, broadly neutralizing antibodies should be in place before infection. The good news in the face of a preventive vaccine would be that the induction of broadly cross-reactive HIV-1 neutralizing antibodies might not be so problematic in noninfected individuals that have a healthy B-cell profile. However, the requirement of long periods of antigen exposure to induce broad neutralizing responses may still be an important obstacle for vaccine development, and its relevance in noninfected individuals still needs to be determined.
According to numerous studies, the breadth of plasma cross-neutralizing antibody activities in HIV-1 infected subjects positively correlates with plasma viral load. However, in a previous study, we confirmed the presence of a broad IgG-associated neutralizing response in patients on antiretroviral treatment, despite having undetectable viremia (15). In the present study, we show new evidence supporting a hypothesis that makes both observations compatible. We studied the evolution of neutralization breadth in a group of bCrN patients and found that a delay in neutralization breadth loss occurs after viremia has been suppressed for as much as 4 to 20 months. As a result of this delay, in a cross-sectional study, aviremic patients with broad neutralizing responses can be found. Similar to the decline of neutralization breadth following cART engagement observed in the present study, a decline of CTL responses has also been observed in chronically infected patients after initiation of treatment (38, 39). This decline has also been associated with reduced viral replication and consequently with reduced CTL antigen stimulation. The identification of the factors associated with the maintenance of bCrNA, both in the presence and in the absence of viremia, may provide information valuable for improving the stability of an effective humoral immune response induced by vaccination. However, these studies are difficult to carry out due to the low percentage of bCrN patients within the group of HIV-1-infected patients (around 2%) (15).
Several studies have demonstrated a significant recovery of B-cell numbers concomitant with a reduction in HIV-1 plasma viremia by cART. This increase has been associated with a normalization of B-cell subpopulations by reduction in the frequency of apoptosis-prone B-cell subpopulations associated with cART. The improved B-cell profile may also explain the benefits of cART in improving B-cell responses to specific immunogens. In contrast, in the present study we observed a decrease in the breadth of the neutralizing response against HIV concomitant with the improvement of the B-cell profile associated with a cART-induced decay in viremia. We hypothesize that, even in the presence of an improved B-cell profile, a minimum level of antigen exposure is required to develop bCrNA.
Overall, our findings indicate that there are both immunological and virological determinants necessary to generate bCrNA. Our results indicate that, in addition to long periods of viremia, the presence of a normalized B-cell repertoire is required for the induction of broadly neutralizing responses.
Supplementary Material
ACKNOWLEDGMENTS
We thank Ana García and María T. García for technical assistance and Richard Diesso for assistance in preparing the manuscript. We also thank Julià Blanco and Jorge Carrillo for help with interpretation of B-cell results. TZM-bl cells were obtained from John C. Kappes, Xiaoyun Wu, and Tranzyme Inc. through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
This work was supported by the Instituto de Salud Carlos III (FIS PS09/01459, FIS PS09/01297, FIS PS09/00283, and FIS PI10/02984), Ayuda para el fomento de la traslación de la aplicación terapeútica de medicamentos de uso humano, huérfanos y terapias avanzadas (TRA-094), Red de Investigación de SIDA (RD06/0006) to V.S.-M., Ministerio de Sanidad (EC10-153), SAF 2012-39075 to F.G., Fundación para la Investigación y la Prevención del Sida en España (FIPSE 36780/08), Ministerio de Ciencia y Tecnología to E.Y. (RYC-2007-00788), and Portuguese Foundation for Science and Technology to C.B.F.
Footnotes
Published ahead of print 4 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.02155-13.
REFERENCES
- 1.Klasse PJ, Sanders RW, Cerutti A, Moore JP. 2012. How can HIV-type-1-Env immunogenicity be improved to facilitate antibody-based vaccine development? AIDS Res. Hum. Retroviruses 28:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reitter JN, Means RE, Desrosiers RC. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4:679–684 [DOI] [PubMed] [Google Scholar]
- 3.Zhu P, Chertova E, Bess J, Jr, Lifson JD, Arthur LO, Liu J, et al. 2003. Electron tomography analysis of envelope glycoprotein trimers on HIV and simian immunodeficiency virus virions. Proc. Natl. Acad. Sci. U. S. A. 100:15812–15817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poignard P, Moulard M, Golez E, Vivona V, Franti M, Venturini S, et al. 2003. Heterogeneity of envelope molecules expressed on primary human immunodeficiency virus type 1 particles as probed by the binding of neutralizing and nonneutralizing antibodies. J. Virol. 77:353–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sather DN, Armann J, Ching LK, Mavrantoni A, Sellhorn G, Caldwell Z, et al. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J. Virol. 83:757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binley JM, Lybarger EA, Crooks ET, Seaman MS, Gray E, Davis KL, et al. 2008. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J. Virol. 82:11651–11668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray ES, Taylor N, Wycuff D, Moore PL, Tomaras GD, Wibmer CK, et al. 2009. Antibody specificities associated with neutralization breadth in plasma from human immunodeficiency virus type 1 subtype C-infected blood donors. J. Virol. 83:8925–8937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Migueles SA, Welcher B, Svehla K, Phogat A, Louder MK, et al. 2007. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat. Med. 13:1032–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doria-Rose NA, Klein RM, Daniels MG, O'Dell S, Nason M, Lapedes A, et al. 2010. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J. Virol. 84:1631–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, et al. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 82:12449–12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rong R, Li B, Lynch RM, Haaland RE, Murphy MK, Mulenga J, et al. 2009. Escape from autologous neutralizing antibodies in acute/early subtype C HIV-1 infection requires multiple pathways. PLoS Pathog. 5:e1000594. 10.1371/journal.ppat.1000594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richman DD, Wrin T, Little SJ, Petropoulos CJ. 2003. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc. Natl. Acad. Sci. U. S. A. 100:4144–4149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikell I, Sather DN, Kalams SA, Altfeld M, Alter G, Stamatatos L. 2011. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog. 7:e1001251. 10.1371/journal.ppat.1001251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sajadi MM, Guan Y, DeVico AL, Seaman MS, Hossain M, Lewis GK, et al. 2011. Correlation between circulating HIV-1 RNA and broad HIV-1 neutralizing antibody activity. J. Acquir. Immune Defic. Syndr. 57:9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medina-Ramirez M, Sanchez-Merino V, Sanchez-Palomino S, Merino-Mansilla A, Ferreira CB, Perez I, et al. 2011. Broadly cross-neutralizing antibodies in HIV-1 patients with undetectable viremia. J. Virol. 85:5804–5813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Euler Z, van den Kerkhof TL, van Gils MJ, Burger JA, Edo-Matas D, Phung P, et al. 2012. Longitudinal analysis of early HIV-1-specific neutralizing activity in an elite neutralizer and in five patients who developed cross-reactive neutralizing activity. J. Virol. 86:2045–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, et al. 2011. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J. Virol. 85:4828–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moir S, Malaspina A, Ogwaro KM, Donoghue ET, Hallahan CW, Ehler LA, et al. 2001. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc. Natl. Acad. Sci. U. S. A. 98:10362–10367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Notermans DW, de Jong JJ, Goudsmit J, Bakker M, Roos MT, Nijholt L, et al. 2001. Potent antiretroviral therapy initiates normalization of hypergammaglobulinemia and a decline in HIV type 1-specific antibody responses. AIDS Res. Hum. Retroviruses 17:1003–1008 [DOI] [PubMed] [Google Scholar]
- 20.Shirai A, Cosentino M, Leitman-Klinman SF, Klinman DM. 1992. Human immunodeficiency virus infection induces both polyclonal and virus-specific B cell activation. J. Clin. Invest. 89:561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovacs JA, Lempicki RA, Sidorov IA, Adelsberger JW, Herpin B, Metcalf JA, et al. 2001. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J. Exp. Med. 194:1731–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Maza O, Crabb E, Mitsuyasu RT, Fahey JL, Giorgi JV. 1987. Infection with the human immunodeficiency virus (HIV) is associated with an in vivo increase in B lymphocyte activation and immaturity. J. Immunol. 138:3720–3724 [PubMed] [Google Scholar]
- 23.De Milito A, Nilsson A, Titanji K, Thorstensson R, Reizenstein E, Narita M, et al. 2004. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 103:2180–2186 [DOI] [PubMed] [Google Scholar]
- 24.Conge AM, Tarte K, Reynes J, Segondy M, Gerfaux J, Zembala M, et al. 1998. Impairment of B-lymphocyte differentiation induced by dual triggering of the B-cell antigen receptor and CD40 in advanced HIV-1-disease. AIDS 12:1437–1449 [DOI] [PubMed] [Google Scholar]
- 25.Scherer EM, Zwick MB, Teyton L, Burton DR. 2007. Difficulties in eliciting broadly neutralizing anti-HIV antibodies are not explained by cardiolipin autoreactivity. AIDS 21:2131–2139 [DOI] [PubMed] [Google Scholar]
- 26.Moir S, Fauci AS. 2009. B cells in HIV infection and disease. Nat. Rev. Immunol. 9:235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Roos AJ, Mirick DK, Edlefsen KL, LaCroix AZ, Kopecky KJ, Madeleine MM, et al. 2012. Markers of B-cell activation in relation to risk of non-Hodgkin lymphoma. Cancer Res. 72:4733–4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moir S, Malaspina A, Ho J, Wang W, Dipoto AC, O'Shea MA, et al. 2008. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. J. Infect. Dis. 197:572–579 [DOI] [PubMed] [Google Scholar]
- 29.Newman RM, Hall L, Connole M, Chen GL, Sato S, Yuste E, et al. 2006. Balancing selection and the evolution of functional polymorphism in Old World monkey TRIM5alpha. Proc. Natl. Acad. Sci. U. S. A. 103:19134–19139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doria-Rose NA, Klein RM, Manion MM, O'Dell S, Phogat A, Chakrabarti B, et al. 2009. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J. Virol. 83:188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Euler Z, van Gils MJ, Bunnik EM, Phung P, Schweighardt B, Wrin T, et al. 2010. Cross-reactive neutralizing humoral immunity does not protect from HIV type 1 disease progression. J. Infect. Dis. 201:1045–1053 [DOI] [PubMed] [Google Scholar]
- 32.Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, et al. 2009. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J. Virol. 83:7337–7348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Euler Z, van Gils MJ, Boeser-Nunnink BD, Schuitemaker H, van Manen D. 2013. Genome-wide association study on the development of cross-reactive neutralizing antibodies in HIV-1 infected individuals. PLoS One 8:e54684. 10.1371/journal.pone.0054684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lafeuillade A, Chollet L, Hittinger G, Profizi N, Costes O, Poggi C. 1998. Residual human immunodeficiency virus type 1 RNA in lymphoid tissue of patients with sustained plasma RNA of <200 copies/mL. J. Infect. Dis. 177:235–238 [DOI] [PubMed] [Google Scholar]
- 35.Moir S, Buckner CM, Ho J, Wang W, Chen J, Waldner AJ, et al. 2010. B cells in early and chronic HIV infection: evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood 116:5571–5579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caraux A, Klein B, Paiva B, Bret C, Schmitz A, Fuhler GM, et al. 2010. Circulating human B and plasma cells. Age-associated changes in counts and detailed characterization of circulating normal C. Haematologica 95:1016–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moir S, Ho J, Malaspina A, Wang W, Dipoto AC, O'Shea MA, et al. 2008. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 205:1797–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogg GS, Jin X, Bonhoeffer S, Moss P, Nowak MA, Monard S, et al. 1999. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J. Virol. 73:797–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalams SA, Goulder PJ, Shea AK, Jones NG, Trocha AK, Ogg GS, et al. 1999. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J. Virol. 73:6721–6728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagase H, Agematsu K, Kitano K, Takamoto M, Okubo Y, Komiyama A, et al. 2001. Mechanism of hypergammaglobulinemia by HIV infection: circulating memory B-cell reduction with plasmacytosis. Clin. Immunol. 100:250–259 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.