Abstract
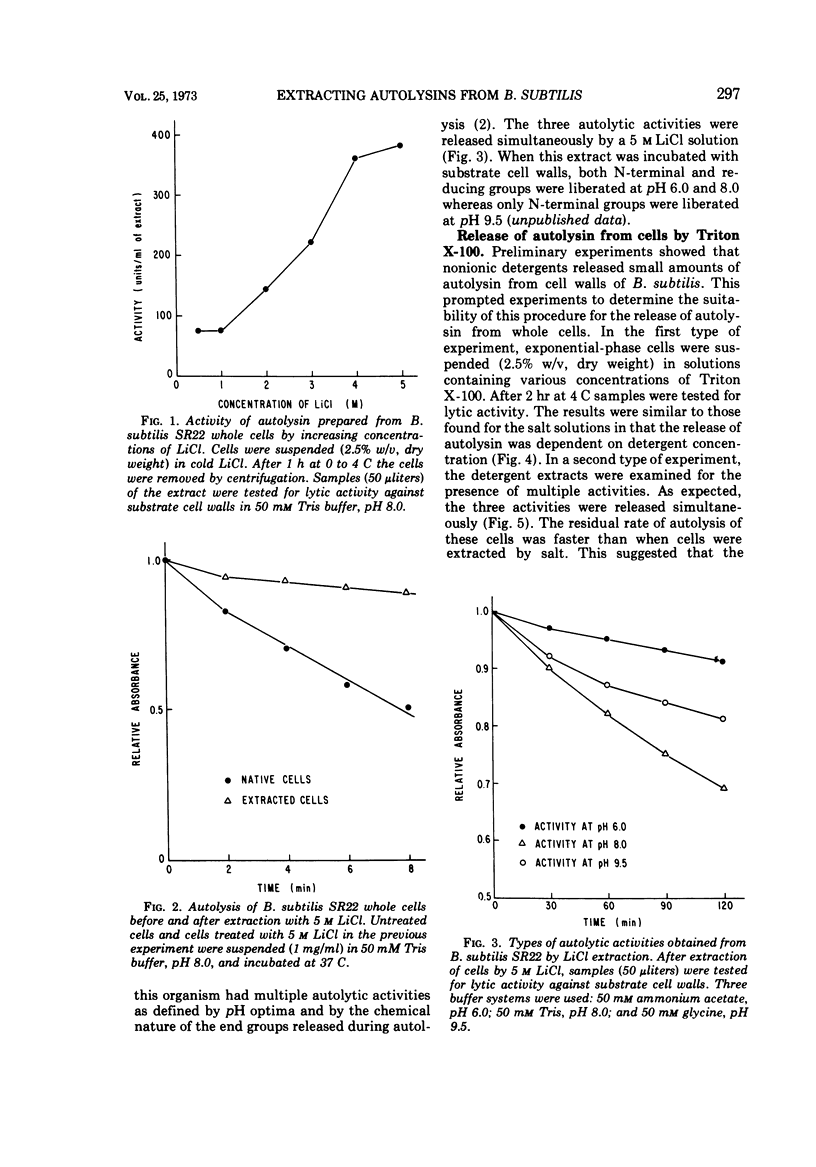
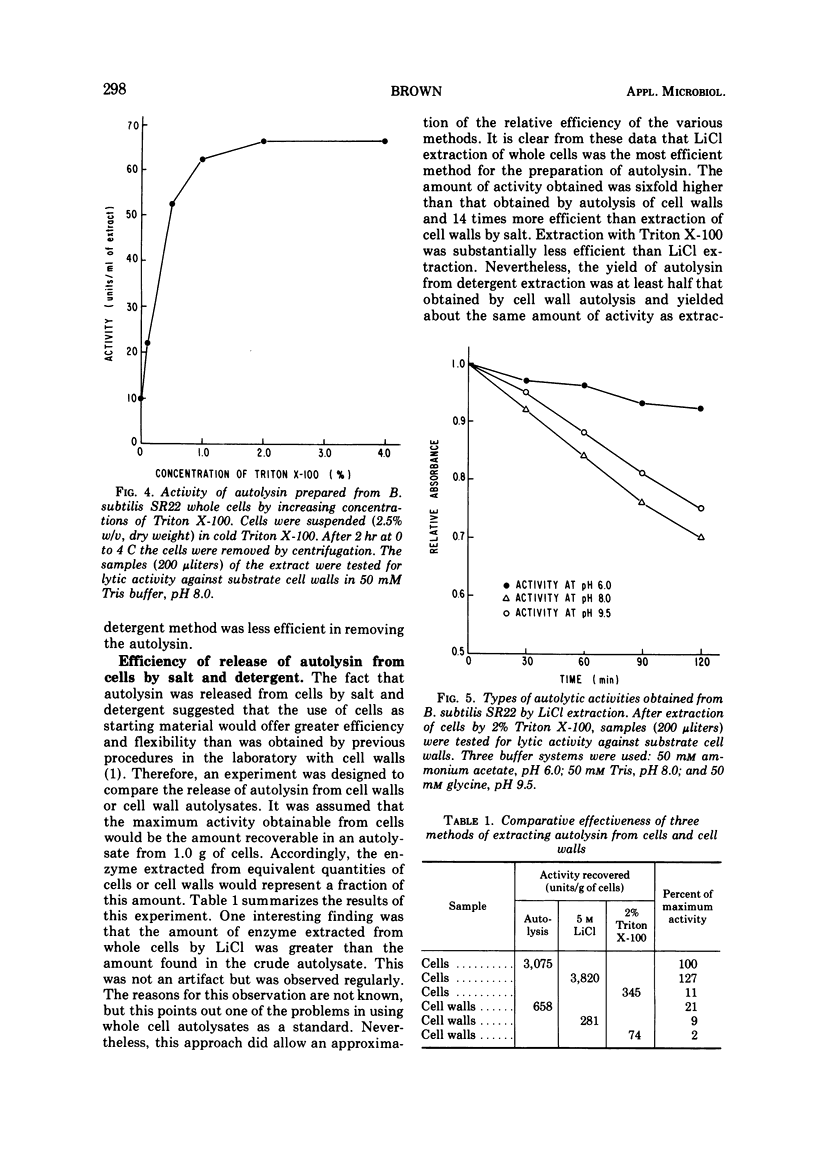
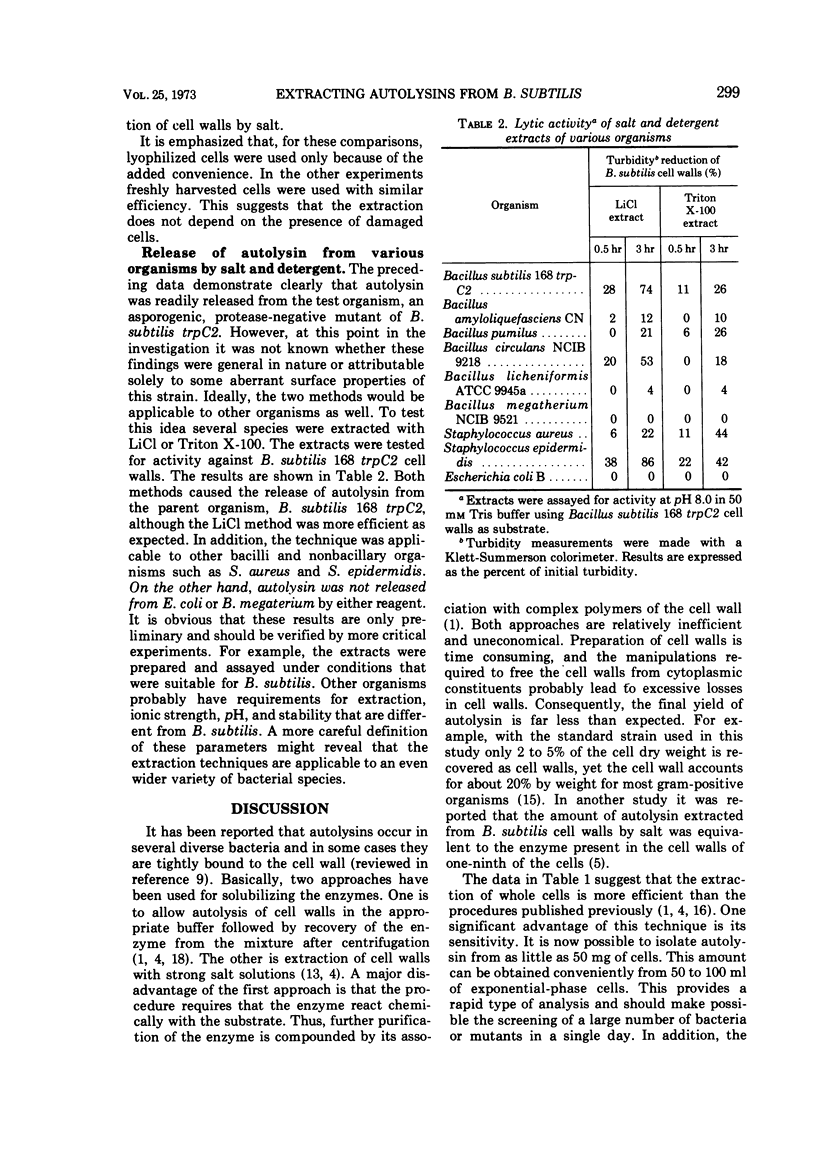
Two procedures are described for the extraction of autolysins from whole cells. One method uses 5 M LiCl at 4 C. The amount of enzyme obtained by this method is six times more than that obtained by autolysis of cell walls and fourteen times more than that obtained by extracting cell walls with LiCl. With the other method, cells are extracted with 2% Triton X-100. This is less efficient than the LiCl method but yields about one-half the amount of enzyme obtained by cell wall autolysis and about the same amount as obtained by extracting cell walls with salt. Both procedures yield autolysin with multiple pH optima. Autolysins can be extracted from several bacterial species by either the LiCl or the detergent method. The data suggest that these techniques have sufficient sensitivity to detect small differences in autolytic activity among mutants and various organisms and are also suitable for large-scale isolation of autolysin for purification and characterization studies.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown W. C., Fraser D. K., Young F. E. Problems in purification of a Bacillus subtilis autolytic enzyme caused by association with teichoic acid. Biochim Biophys Acta. 1970 Feb 11;198(2):308–315. doi: 10.1016/0005-2744(70)90063-x. [DOI] [PubMed] [Google Scholar]
- Brown W. C., Young F. E. Dynamic interactions between cell wall polymers, extracellular proteases and autolytic enzymes. Biochem Biophys Res Commun. 1970 Feb 20;38(4):564–568. doi: 10.1016/0006-291x(70)90618-2. [DOI] [PubMed] [Google Scholar]
- Coles N. W., Gross R. Liberation of surface-located penicillinase from Staphylococcus aureus. Biochem J. 1967 Mar;102(3):742–747. doi: 10.1042/bj1020742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P. Autolysin(s) of Bacillus subtilis as dechaining enzyme. J Bacteriol. 1970 Aug;103(2):494–499. doi: 10.1128/jb.103.2.494-499.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P. Cell wall binding properties of the Bacillus subtilis autolysin(s). J Bacteriol. 1970 Aug;103(2):488–493. doi: 10.1128/jb.103.2.488-493.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Ward J. B. N-acetylmuramyl-L-alanine amidase of Bacillus licheniformis and its L-form. J Bacteriol. 1972 Jun;110(3):878–888. doi: 10.1128/jb.110.3.878-888.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C., Rogers H. J. Autolytic enzymes in growth of bacteria. Nature. 1971 Jan 22;229(5282):272–273. doi: 10.1038/229272a0. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Hoch J. A. Genetic analysis of pleiotropic negative sporulation mutants in Bacillus subtilis. J Bacteriol. 1971 Mar;105(3):896–901. doi: 10.1128/jb.105.3.896-901.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malveaux F. J., San Clemente C. L. Elution of loosely bound acid phosphatase from Staphylococcus aureus. Appl Microbiol. 1967 Jul;15(4):738–743. doi: 10.1128/am.15.4.738-743.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser J. L., Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970 Jan 25;245(2):287–298. [PubMed] [Google Scholar]
- Pooley H. M., Porres-Juan J. M., Shockman G. D. Dissociation of an autolytic enzyme-cell wall complex by treatment with unusually high concentrations of salt. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1134–1140. doi: 10.1016/0006-291x(70)90357-8. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Cheney M. C. Autolytic enzyme system of Streptococcus faecalis. V. Nature of the autolysin-cell wall complex and its relationship to properties of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Jun;98(3):1199–1207. doi: 10.1128/jb.98.3.1199-1207.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Martin J. T. Autolytic enzyme system of Streptococcus faecalis. IV. Electron microscopic observations of autolysin and lysozyme action. J Bacteriol. 1968 Nov;96(5):1803–1810. doi: 10.1128/jb.96.5.1803-1810.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Pooley H. M., Thompson J. S. Autolytic enzyme system of Streptococcus faecalis. 3. Localization of the autolysin at the sites of cell wall synthesis. J Bacteriol. 1967 Nov;94(5):1525–1530. doi: 10.1128/jb.94.5.1525-1530.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]
- Tipper D. J. Mechanism of autolysis of isolated cell walls of Staphylococcus aureus. J Bacteriol. 1969 Feb;97(2):837–847. doi: 10.1128/jb.97.2.837-847.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Weimberg R., Orton W. L. Elution of Acid Phosphatase from the Cell Surface of Saccharomyces mellis by Potassium Chloride. J Bacteriol. 1965 Jul;90(1):82–94. doi: 10.1128/jb.90.1.82-94.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. A., Tristram H. Localization in the Cell and Extraction of Alkaline Phosphatase from Bacillus subtilis. J Bacteriol. 1970 Dec;104(3):1045–1051. doi: 10.1128/jb.104.3.1045-1051.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


