Abstract
We have reported previously that ocular infection of different strains of mice with recombinant herpes simplex virus 1 (HSV-1) constitutively expressing interleukin-2 (IL-2) provokes central nervous system (CNS) demyelination and optic neuropathy, as determined by changes in visual evoked cortical potentials and pathological changes in the optic nerve and CNS, whereas recombinant viruses expressing IL-4, gamma interferon, IL-12p35, IL-12p40, or IL-12p70 do not induce this neuropathy. The goal of this study was to dissect the mechanism underlying the interplay between the immune system (elevation of IL-2) and an environmental factor (infection with HSV-1) that elicits this pathology. Similar results were obtained upon delivery of IL-2 into the mouse brain using osmotic minipumps or injection of mice with recombinant IL-2 protein, IL-2 DNA, or IL-2 synthetic peptides prior to infection with wild-type (wt) HSV-1 strains McKrae and KOS. The critical role of IL-2 is further supported by our data, indicating that a single mutation at position T27A in IL-2 completely blocks the HSV-1-induced pathology. This study shows a novel model of autoimmunity in which viral infection and enhanced IL-2 cause CNS demyelination.
INTRODUCTION
Several lines of evidence implicate interleukin-2 (IL-2) in the pathology of multiple sclerosis (MS) (1–4). Patients with MS have elevated levels of IL-2 in their sera and cerebrospinal fluid (CSF) (1–4). The soluble IL-2 receptor (sIL-2R) is also elevated in the sera and CSF of patients with MS (3, 5–10). These clinical findings of elevated IL-2 levels (and lower than normal IL-4 levels) in the sera of MS patients suggest a possible link between IL-2 and the onset of MS. Furthermore, supernatants harvested from T lymphocytes of MS patients cause damage to myelin and glial cells in vitro (11, 12), suggesting that the MS T lymphocytes produce demyelination factors and are activated in vivo.
In order to investigate the role of cytokines in demyelination in a viral model of MS, we had constructed a panel of recombinant herpes simplex virus 1 (HSV-1) isolates that constitutively express murine cytokines, including IL-2 (13), IL-4, gamma interferon (IFN-γ), IL-12p35, or IL-12p40 (14–16). Using these viruses, we have shown that infection of different strains of mice with a recombinant HSV-1 strain constitutively expressing IL-2 (HSV–IL-2), but not HSV–IL-4, HSV–IFN-γ, HSV–IL-12p35, or HSV–IL-12p40, results in demyelination of the optic nerves (ONs), the spinal cords (SCs), and the brains of the infected mice, as determined by histologic examination of tissues obtained at necropsy (17, 18). In addition, the HSV–IL-2-infected mice developed optic neuropathy, as determined by changes in the visual evoked cortical potentials (VECPs) (18). Using knockout mice, depletion, and transfer studies, we found that both CD8+ and CD4+ T cells contributed to HSV–IL-2-induced central nervous system (CNS) demyelination, with CD8+ T cells being the primary inducers (19). We have also found that infection of mice with a recombinant HSV-1 isolate expressing IL-12p70 or injection of mice with IL-12p70 DNA blocks the CNS demyelination induced by HSV–IL-2 (19, 20).
Since the recombinant HSV–IL-2 is not a naturally occurring virus, we used an Alzet osmotic minipump to transfer IL-2 into the brains of recipient mice prior to ocular infection with wild-type (wt) HSV-1 (rather than HSV–IL-2). We also tested the effect of administration of IL-2 DNA, IL-2 site-specific mutants, and synthetic peptide fragments prior to infection with wt HSV-1. We demonstrate that a single mutation in amino acid (aa) 27 of IL-2 abolishes CNS demyelination in mice ocularly infected with wt HSV-1. Thus, analysis of the mechanisms by which the enhanced levels of IL-2 in combination with viral infection elicit CNS demyelination suggests that full-length IL-2 is not required to induce CNS demyelination and further suggests that the region of IL-2 that is involved in CNS demyelination involves the region of IL-2 at aa 27. Collectively, these results provide evidence that this HSV-1 model represents a valid alternative to other commonly used models of MS.
MATERIALS AND METHODS
Ethics statement.
All animal procedures were performed in strict accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research (http://www.arvo.org/About_ARVO/Policies/Statement_for_the_Use_of_Animals_in_Ophthalmic_and_Visual_Research/) and the guidelines in the National Research Council's Guide for the Care and Use of Laboratory Animals (21). The animal research protocol was approved by the Institutional Animal Care and Use Committee of Cedars-Sinai Medical Center (protocol 2841).
Mice and viruses.
Female BALB/c and C57BL/6 mice (age, 6 weeks) were purchased from The Jackson Laboratory. Plaque-purified HSV-1 strain McKrae (virulent wild type) or KOS (avirulent wild type) and a recombinant HSV-1 strain expressing IL-2 (13) were grown in rabbit skin (RS) cell monolayers in minimal essential medium (MEM) containing 5% fetal calf serum (FCS), as described previously (13, 14, 16). The McKrae virus strain is virulent at an infectious dose of 2 × 105 PFU/eye, whereas the KOS virus strain and HSV–IL-2 are attenuated. We have shown previously that the recombinant HSV–IL-2 expresses IL-2 at high levels in different tissues (13).
Murine recombinant IL-2 (rIL-2) infusion.
An Alzet miniosmotic pump (Alzet brain infusion kit 1; Cupertino, CA) was intracranially (i.c.) implanted into the back of the skull of female C57BL/6 mice through a small skin incision. Wound edges were repaired with surgical clips. Most animals began to regain weight 3 to 4 days after surgery. The osmotic pumps were loaded under sterile conditions with 30 μg of purified murine IL-2 (mIL-2; Peprotech, Rocky Hill, NJ) in 100 μl of phosphate-buffered saline (PBS), and 1 μg IL-2 was continuously infused for 24 h. Two weeks after IL-2 infusion, some mice were ocularly infected with 2 × 105 PFU/eye of HSV-1 strain McKrae or KOS. Control mice were infused with a similar volume of IL-2 without HSV-1 infection. Mice were sacrificed at 14 days postinfection (p.i.), and CNS tissues were stained with Luxol fast blue (LFB). In addition, in some mice the same osmotic pump was implanted subcutaneously at the side and the mice were ocularly infected with HSV-1 strain McKrae.
DNA injection.
The complete open reading frame (ORF) of murine IL-2 or the IL-2A, IL-2B, IL-2A1, or IL-2A2 fragment, as well as that of full-length IL-2 with one mutation or three mutations, was cloned into the BamHI site of the VR-1055 expression vector as we described previously (22). Full-length natural murine IL-2 and its various forms used in this study were synthesized by GenScript (Piscataway, NJ). Plasmid DNA encoding each full-length sequence or each fragment of IL-2 was purified on a cesium chloride gradient. In each experiment, 10 mice per group were inoculated intramuscularly (i.m.) with 25 μg DNA into each quadriceps on days 0, 7, and 14 before ocular infection. As a negative control, mock-vaccinated mice were similarly injected with vector DNA alone. Injected mice were ocularly infected 4 h after the third injection.
IL-2 protein injection.
Purified rIL-2 was purchased from Peprotech. The rIL-2 was emulsified 1:1 in complete Freund's adjuvant (CFA) or incomplete Freund's adjuvant (IFA). On day 0, 10 mice per group were injected subcutaneously in the shoulder with 10 μg of rIL-2 in CFA; on days 7 and 14, the mice were injected with the rIL-2 in IFA. As negative controls, mice were similarly injected with adjuvants alone. Injected mice were ocularly infected 4 h after the third injection.
Peptide synthesis.
A panel of 32 overlapping murine IL-2 peptides spanning the entire IL-2 protein sequence (15-mers with 10-aa overlaps) was synthesized by Mimotopes (San Diego, CA), and the peptide sequences are as follows: peptide 1, MYSMQLASCVTLTLV; peptide 2, LASCVTLTLVLLVNS; peptide 3, TLTLVLLVNSAPTSS; peptide 4, LLVNSAPTSSSTSSS; peptide 5, APTSSSTSSSTAEAQ; peptide 6, STSSSTAEAQQQQQQ; peptide 7, TAEAQQQQQQQQQQQ; peptide 8, QQQQQQQQQQQHLEQ; peptide 9, QQQQQQHLEQLLMDL; peptide 10, QHLEQLLMDLQELLS; peptide 11, LLMDLQELLSRMENY; peptide 12, QELLSRMENYRNLKL; peptide 13, RMENYRNLKLPRMLT; peptide 14, RNLKLPRMLTFKFYL; peptide 15, PRMLTFKFYLPKQAT; peptide 16, FKFYLPKQATELKDL; peptide 17, PKQATELKDLQCLED; peptide 18, ELKDLQCLEDELGPL; peptide 19, QCLEDELGPLRHVLD; peptide 20, ELGPLRHVLDLTQSK; peptide 21, RHVLDLTQSKSFQLE; peptide 22, LTQSKSFQLEDAENF; peptide 23, SFQLEDAENFISNIR; peptide 24, DAENFISNIRVTVVK; peptide 25, ISNIRVTVVKLKGSD; peptide 26, VTVVKLKGSDNTFEC; peptide 27, LKGSDNTFECQFDDE; peptide 28, NTFECQFDDESATVV; peptide 29, QFDDESATVVDFLRR; peptide 30, SATVVDFLRRWIAFC; peptide 31, DFLRRWIAFCQSIIS; and peptide 32, WIAFCQSIISTSPQ. The purity of the original peptides synthesized was at least 90%. All peptides were dissolved in dimethyl sulfoxide at a concentration of 1 μg/μl and stored at −20°C.
IL-2 peptide injection.
Mice were injected 3 times with 1 μg of each peptide individually or with combined peptides in adjuvant. On day 0, five mice per group were injected subcutaneously in the shoulder with each individual peptide or combinations of peptides in CFA; on days 7 and 14, the mice were injected with each individual peptide or combinations of peptides in IFA. As negative controls, mice were similarly injected with adjuvant alone. Injected mice were ocularly infected 4 h after the third injection.
Ocular infection.
Mice were ocularly infected with 2 × 105 PFU of strain McKrae, strain KOS, or recombinant HSV–IL-2 per eye. Each virus was suspended in 2 μl of tissue culture medium and administered as an eye drop. In contrast to mice with the C57BL/6 background, which are refractory to McKrae infection, mice with the BALB/c background are highly susceptible to McKrae infection. Thus, mice with the BALB/c background were infected with the KOS virus rather than the McKrae virus. Corneal scarification was not used in this study for either strain.
Preparation of ON, SC, and brain for pathological analysis.
The ONs, SCs, and brains of infected mice were removed at necropsy on day 14 p.i. The ONs, SCs, and brains were collected from experimental and control mice and then placed in Tissue-Tek OCT embedding medium (SaKura Fintek, Torrance, CA) and stored at −80°C. Transverse sections of each tissue (thickness, 8 to 10 μm) were cut, air dried overnight, and fixed in acetone for 3 min at 25°C (23). Demyelination in each section was confirmed by monitoring adjacent sections.
Analysis of demyelination using LFB staining.
The presence or absence of demyelination in the ONs, SCs, and brains of infected mice was evaluated using LFB staining of formalin-fixed sections of ON, SC, and brain as we described previously (17). Every 4th section of ON, SC, and brain was stained with LFB.
Statistical analysis.
Fisher's exact tests were performed using the computer program Instat (GraphPad, San Diego, CA) to compare demyelination in infected mice with the absence of demyelination in control groups. Results were considered statistically significant when the P value was <0.05.
RESULTS
Murine IL-2 infusion following ocular HSV-1 infection induces demyelination in mice infected with wt HSV-1.
Our published data establish that ocular infection of mice with HSV–IL-2 elicits demyelination of the optic nerve, spinal cord, and brain (18–20, 24). In these studies, we looked at the time course of appearance of demyelination in mice infected with HSV–IL-2 (18–20, 24). Demyelination was not detected in any of the HSV–IL-2-infected mice on day 3 or 7 p.i.; however, demyelination was initially detected on day 10 p.i. Demyelination was also detected on days 30 and 60 p.i. Overall, the patterns of demyelination plaques observed on days 10, 30, 60, and 75 p.i. were similar to those observed on day 14. Thus, in this study, we looked at demyelination on day 14 p.i. As the recombinant HSV–IL-2 is not a naturally occurring virus, we generated an alternative approach for delivery of the IL-2 using an Alzet microosmotic pump loaded with murine IL-2 protein, as described in Materials and Methods. Mice were ocularly infected with virulent HSV-1 strain McKrae or mock infected and sacrificed at 14 days p.i. Representative photomicrographs of Luxol blue-stained sections of the brain, SC, and ON from mice infected with McKrae and mIL-2, mice not infected but treated with mIL-2, or mice infected and not treated with mIL-2 are shown in Fig. 1. Consistent with the results obtained when mice were infected with HSV–IL-2 (18–20, 24), administration of murine IL-2 using an implanted miniature osmotic pump and ocular infection with wt HSV-1 induced demyelination in the ON, SC, and brain of all mice tested (Fig. 1A, rIL-2 protein + HSV-1) but was not observed in mice treated with murine IL-2 in the absence of ocular infection (Fig. 1A, rIL-2 protein) or in mice infected with HSV-1 alone (Fig. 1A, HSV-1). Similar results were obtained when this series of experiments was repeated using the avirulent HSV-1 strain KOS (data not shown).
Fig 1.
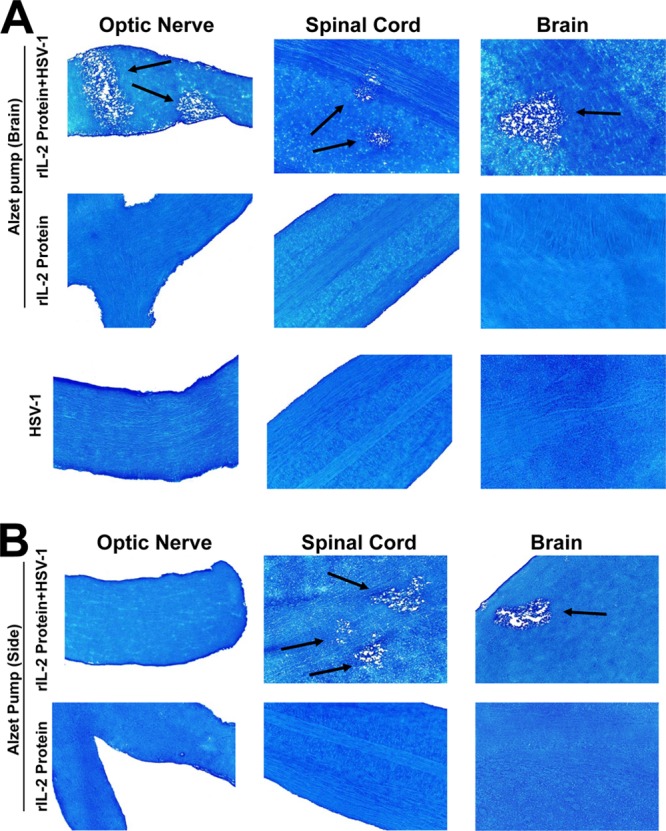
Histologic analyses of IL-2 infusion in HSV-1-infected mouse CNS. Female C57BL/6 mice were treated by continuous infusion of purified mIL-2 administered via an Alzet microosmotic pump (1 μg/24 h) over successive 14-day periods. The pumps were inserted i.c. into the back of the skull of the mice through a small skin incision, and IL-2 was delivered into the cerebral ventricles using the Alzet brain infusion kit 1. Wound edges were repaired with surgical clips. Most animals began to regain weight 3 to 4 days after surgery. Osmotic pumps were loaded under sterile conditions with 30 μg of mIL-2 (Peprotech) in 100 μl of PBS. Two weeks after IL-2 infusion, some mice were ocularly infected with 2 × 105 PFU/eye of HSV-1 strain McKrae. Control mice were mock infected with a similar volume of medium without HSV-1 infection. wt control mice were similarly infected with HSV-1. Mice were sacrificed at 14 days p.i., and CNS tissues were stained with LFB. Arrows, areas of demyelination. (A) Results obtained when the pump was inserted i.c.; (B) results obtained when the pump was inserted in the side.
In contrast, when microosmotic pumps were implanted subcutaneously in the side of the mice, 2/7 (28%) mice showed demyelination in their brain, 4/7 (57%) mice showed demyelination in their spinal cord, and none of the mice showed demyelination in their optic nerves following infection with wt HSV-1 strain McKrae (Fig. 1B and Table 1). Previously, it was shown that rIL-2/IL-2 monoclonal antibody (MAb) complexes increase the biological activity of preexisting rIL-2 (25). As the half-life of rIL-2 in the circulation is short, we compared the delivery of rIL-2 protein alone to the delivery of rIL-2 protein plus anti-IL-2 MAb (clone S4B6-1), which has been reported to increase the IL-2 half-life (25). However, we observed no differences in the severity or duration of demyelination between the two groups (not shown). As we did not observe any differences between mice injected with rIL-2 protein alone and mice injected with rIL-2 protein and anti-IL-2 MAb, all of the subsequent experiments described in this study used only rIL-2 protein without incubating it with anti-IL-2 MAb.
Table 1.
Demyelination in the CNS of mice that received rIL-2 by microosmotic pumps implanted in the sidea
| Treatment | No. of mice with demyelination/total no. of mice tested (%) |
||
|---|---|---|---|
| Brain | SC | ON | |
| rIL-2 protein + HSV-1 | 2/7 (28) | 4/7 (57) | 0/0 |
| rIL-2 protein | 0/0 | 0/0 | 0/0 |
| HSV-1 | 0/0 | 0/0 | 0/0 |
Microosmotic pumps delivering rIL-2 were implanted subcutaneously into the side of C57BL/6 mice, and 14 days later the mice were ocularly infected with HSV-1 strain McKrae. Demyelination was assessed at 14 days p.i.
Injection of mice with IL-2 DNA induces CNS demyelination following ocular HSV-1 infection.
It has been shown previously that DNA vaccine-encoded immunogens can elicit an immune response (26, 27). To test our hypothesis, we injected IL-2 DNA i.m. into female BALB/c mice to determine if this might induce demyelination following ocular infection with wt HSV-1. Injections were done at 21, 14, and 7 days before ocular infection with avirulent HSV-1 strain KOS. As a negative control, some mice were similarly injected with IL-4 DNA or vector DNA. Demyelination in the CNS of infected mice was measured on day 14 p.i. Demyelination was observed in the CNS of BALB/c mice injected with IL-2 DNA (Fig. 2, top). However, mice injected with IL-4 DNA did not display any CNS demyelination (Fig. 2, bottom). Similar results were obtained when C57BL/6 mice were injected with IL-2 DNA and ocularly infected with HSV-1 strain McKrae or when mice were injected once rather than three times with IL-2 DNA (data not shown). Thus, our results suggest that IL-2 DNA also can induce demyelination in the CNS of infected mice.
Fig 2.
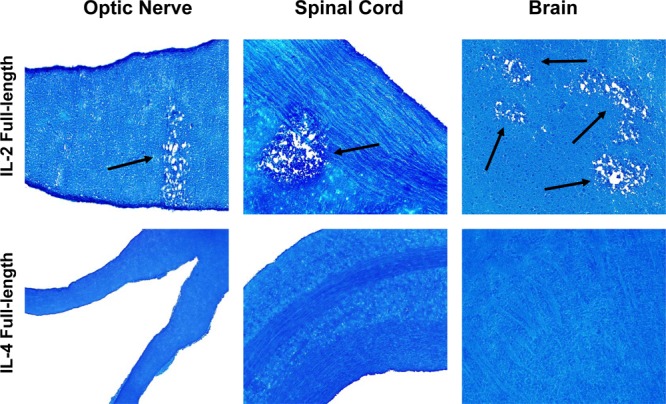
CNS demyelination in mice injected with IL-2 DNA and ocularly infected with wt HSV-1. The complete ORF of IL-2 or IL-4 was cloned into the VR-1055 vector as we described previously (22). Female BALB/c mice were injected 3 times with 100 μg of IL-2 or IL-4 DNA. Seven days after the 3rd immunization, mice were ocularly infected with 2 × 105 PFU/eye of HSV-1 strain KOS. Mice were sacrificed at 14 days after ocular infection, and CNS tissues were harvested and stained with LFB, as described in the legend to Fig. 1. Arrows, areas of demyelination.
To determine if a specific region of IL-2 rather than the full length of IL-2 is required for CNS demyelination, we initially made two IL-2 DNA constructs representing aa 1 to 84 and aa 85 to 169 of IL-2 DNA (Fig. 3). BALB/c mice were injected with each DNA fragment 3 times as described above and subsequently ocularly infected with HSV-1, and demyelination was monitored on day 14 p.i. Mice injected with IL-2A DNA (aa 1 to 84) developed CNS demyelination, whereas mice injected with IL-2B DNA (aa 85 to 169) did not develop demyelination (Fig. 3). As shown in Fig. 3, to further more finely map the demyelination region within the IL-2A DNA fragment, we made two additional fragments corresponding to aa 1 to 42 of IL-2A (called IL-2A1) and aa 43 to 84 of IL-2A (called IL-2A2). When mice were injected with either the IL-2A1 or IL-2A2 DNA fragment and ocularly infected with wt HSV-1 strain McKrae, demyelination was detected in the mice injected with the IL-2A1 fragment but not those injected with the IL-2A2 fragment (Fig. 3). Thus, our results suggest that the region of IL-2 that is involved in CNS demyelination resides within aa 1 to 42 of IL-2 and that full-length IL-2 is not required to induce CNS demyelination. Similar to our results with the IL-2A fragment, it was previously shown that this region of IL-2 is essential for its biological activity (28).
Fig 3.
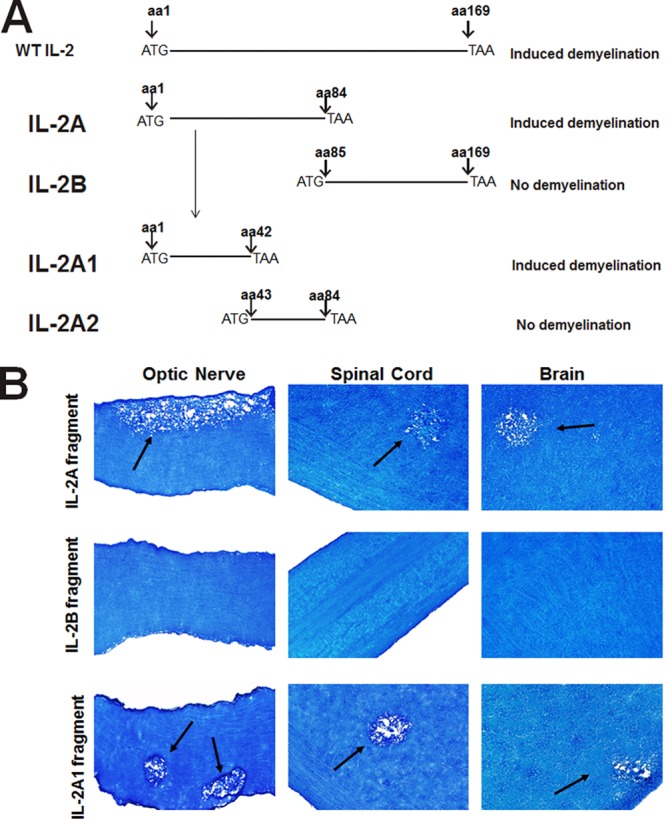
CNS demyelination in mice injected with different forms of IL-2 DNA. (A) Schematic representation of the IL-2 constructs used for DNA injection. Full-length IL-2 is shown at the top. IL-2A and IL-2B, the 1st and 2nd halves of full-length IL-2, respectively; IL-2A1, the 1st half of IL-2A; IL-2A2, the 2nd half of IL-2A. All constructs have an ATG and a termination codon (TAA) and were inserted into pVR-1055, as described in the legend to Fig. 2. (B) Demyelination in injected mice. Female C57BL/6 mice were injected 3 times with 100 μg of each construct. Seven days after the 3rd immunization, mice were ocularly infected with 2 × 105 PFU/eye of HSV-1 strain McKrae. Mice were sacrificed at 14 days p.i., and CNS tissues were harvested and stained with LFB, as described in the legend to Fig. 1. Arrows, areas of demyelination.
Fine mapping of the region of IL-2 that causes CNS pathology using IL-2 synthetic peptides.
The results presented above suggest that the rIL-2 protein and IL-2 DNA can cause CNS pathology in ocularly infected mice. Our DNA mapping results also suggested that full-length IL-2 is not required for CNS pathology. To further finely map whether a specific region of IL-2 is required for CNS pathology, we synthesized 32 peptides that cover the entire IL-2 sequence and overlapped by 10 aa. Mice were given 2 subcutaneous injections of each peptide or a mixture of peptides that was either coupled to keyhole limpet hemocyanin (KLH; Pierce Chemical) or left uncoupled. Full-length rIL-2 protein was used as a positive control. The first injection was in CFA, while the second injection was in IFA. Demyelination was determined at 14 days p.i. with HSV-1 strain McKrae. As summarized in Table 2 (corresponding photomicrographs are presented in Fig. 4), infected mice that received peptides which contained the STSSS sequence (aa 26 to 30) developed demyelination of the optic nerve, spinal cord, and brain, while other regions of IL-2 did not induce demyelination. We did not detect any differences in the level of demyelination between mice injected with peptides coupled to KLH and mice injected with peptides that were not coupled to KLH (data not shown).
Table 2.
Fine mapping of demyelination region of IL-2 using synthetic peptidesa
| Peptide injectedb | Demyelination |
||
|---|---|---|---|
| Brain | SC | ON | |
| Full-length IL-2 protein | Yes | Yes | Yes |
| Peptides 1–10 | Yes | Yes | Yes |
| Peptides 11–21 | No | No | No |
| Peptides 22–32 | No | No | No |
| Peptide 1 (MYSMQLASCVTLTLV) | No | No | No |
| Peptide 2 (LASCVTLTLVLLVNS) | No | No | No |
| Peptide 3 (TLTLVLLVNSAPTSS) | No | No | No |
| Peptide 4 (LLVNSAPTSSSTSSS) | Yes | Yes | Yes |
| Peptide 5 (APTSSSTSSSTAEAQ) | Yes | Yes | Yes |
| Peptide 6 (STSSSTAEAQQQQQQ) | Yes | Yes | Yes |
| Peptide 7 (TAEAQQQQQQQQQQQ) | No | No | No |
| Peptide 8 (QQQQQQQQQQQHLEQ) | No | No | No |
| Peptides 7–32 | No | No | No |
C57BL/6 mice were injected with 10 μg of each peptide at 7 days and 4 h before ocular infection with HSV-1 strain McKrae. The first injection included CFA, and the second injection included IFA. Demyelination was assessed at 14 days p.i.
Boldface amino acids indicate areas of overlap between the peptides.
Fig 4.
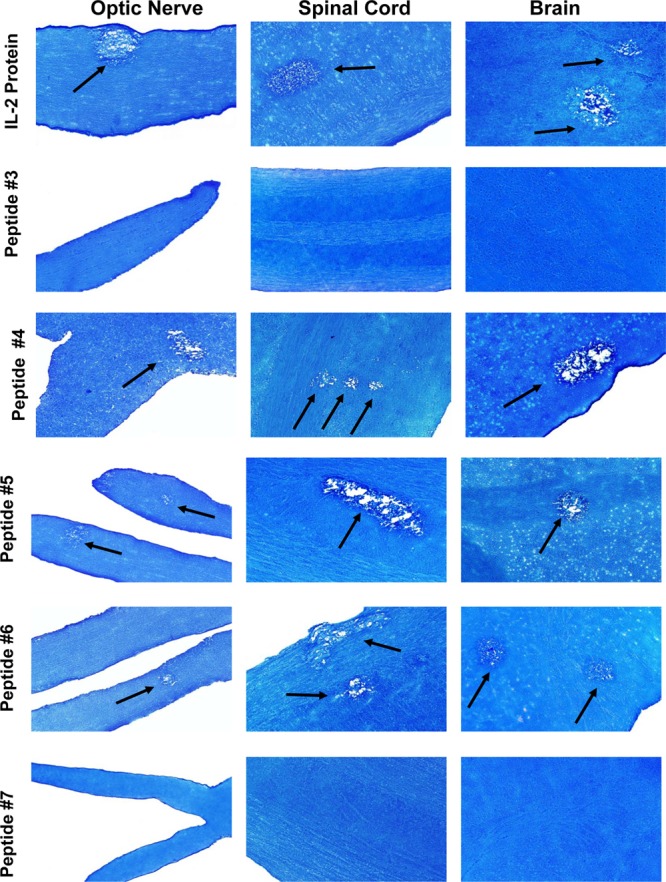
Injection of mice with IL-2 synthetic peptides. C57BL/6 mice were injected with 10 μg of rIL-2 protein or peptide 3, 4, 5, 6, or 7 at 7 days and 4 h before ocular infection with HSV-1 strain McKrae. The first injection included CFA, and the second injection included IFA. Demyelination in CNS tissues of infected mice was assessed at 14 days p.i. Arrows, areas of demyelination.
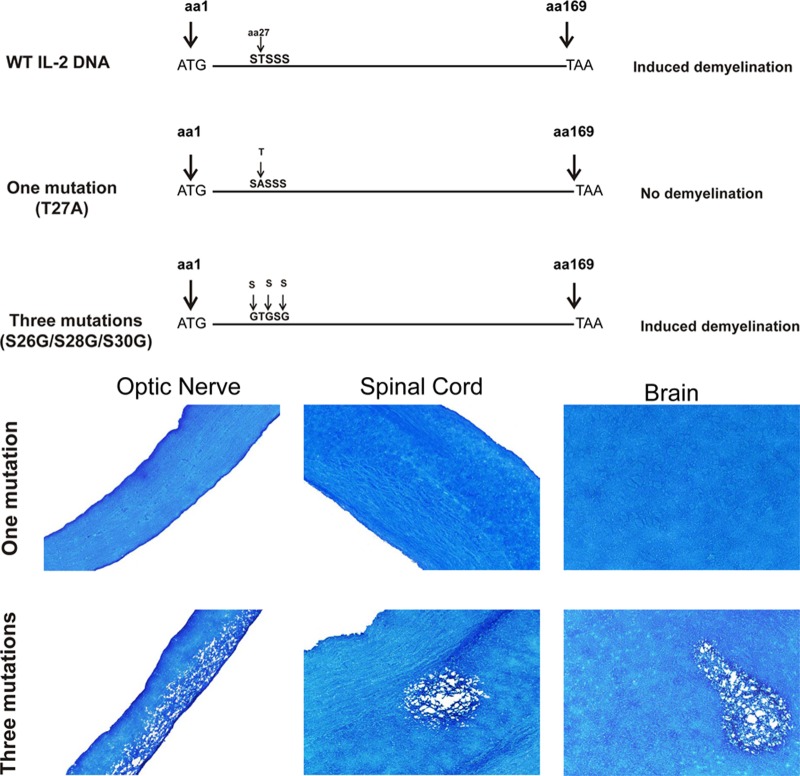
The mapping results described in Table 2 were consistent with those obtained using aa 1 to 42 of IL-2 DNA (Fig. 3A). Therefore, on the basis of the results obtained with the synthetic peptides and DNA fragments described in Table 2 and Fig. 3, the region of IL-2 corresponding to aa 26 to 30 was subjected to site-specific mutagenesis, producing two constructs: one with a mutation at aa 27 (T27A) and another with three mutations at aa 26, 28, and 30 (S26G, S28G, S30G) (Fig. 5). When mice were injected with these two DNA constructs and infected with wt HSV-1, mice that were injected with IL-2 T27A DNA showed no sign of demyelination (Fig. 5, one mutation), while mice that were injected with the IL-2 DNA construct with three mutations in aa 26, 28, and 30 showed demyelination in their CNS (Fig. 5, three mutations). Thus, our results suggest that a single mutation in aa 27 of IL-2 blocked CNS demyelination in infected mice.
Fig 5.
(Top) Fine mapping of the region of IL-2 involved in CNS demyelination. Full-length IL-2 DNA is shown on the top map. One mutation, a single mutation (T27A); three mutations, a construct with mutations in aa 26, 28, and 30 (S to G). All constructs had an ATG and a termination codon (TAA) and were inserted into pVR-1055. Female C57BL/6 mice were injected 3 times with 100 μg of each construct. At 7 days after the 3rd immunization, mice were ocularly infected with 2 × 105 PFU/eye of HSV-1 strain McKrae. Mice were sacrificed at 14 days p.i., and CNS tissues were harvested and stained with LFB (bottom), as described in the legend to Fig. 1. Arrows, areas of demyelination.
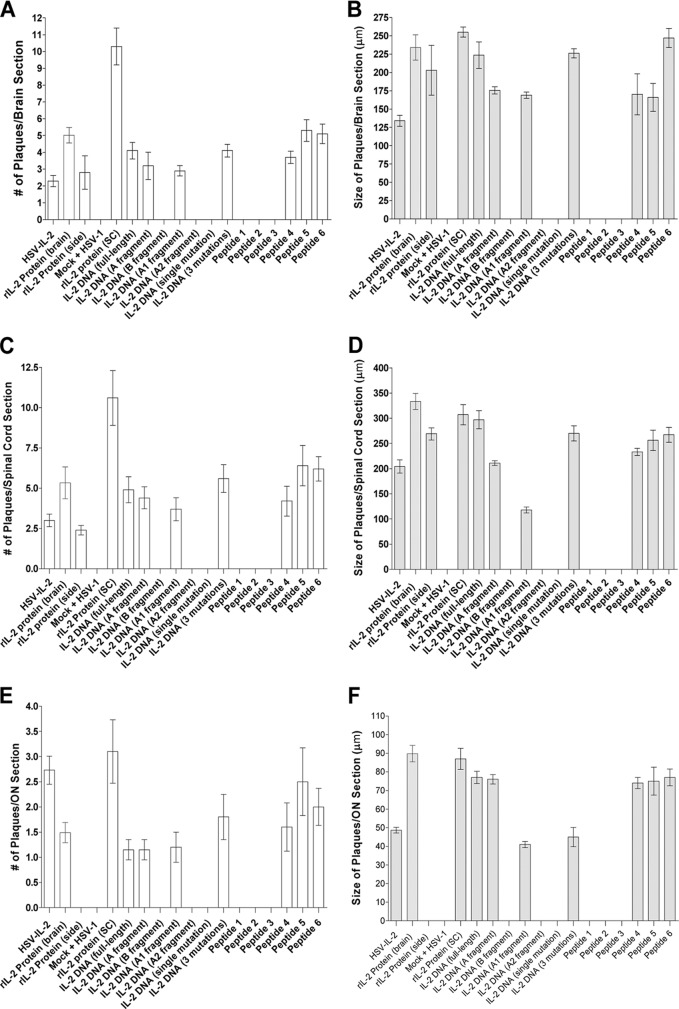
Quantitation of demyelination severity in infected mice.
We observed that the severity of the HSV–IL-2-induced demyelination appeared to be the same as that in mice that received rIL-2 protein by a pump, full-length IL-2 DNA, DNA fragments corresponding to the CNS demyelination region of IL-2 DNA, full-length rIL-2 protein, or IL-2 synthetic peptides containing the STSSS sequence. To determine if the number of plaques and the size of plaques were different between these different groups, we counted the number and size of the plaques observed in the brains, spinal cords, and optic nerves of infected mice. The data are shown as the number of plaques and the area of demyelination per total stained section from Fig. 1 to 5. The number of plaques for mice that were injected with full-length rIL-2 protein was higher than that for mice in the other groups (Fig. 6A, C, and E). We also quantitated the area of demyelination (the size of the observed plaques) in mice whose results are presented in Fig. 1 to 5. Mice injected with different forms of IL-2 also had similar areas of demyelination (Fig. 6B, D, and F). Thus, our results suggest that, similar to HSV–IL-2, injection of mice with different forms of IL-2 induces a similar level of demyelination in ocularly infected mice.
Fig 6.
Severity of CNS demyelination following injection with different forms of IL-2 and infection with WT HSV-1 or control HSV–IL-2. The brains, spinal cords, and optic nerves of 5 mice in each treatment group for which the results are presented in Fig. 1 to 5 were sectioned, 30 sections per mouse per tissue were stained, and the size and the number of the demyelination plaques in the entire sections of the brains, spinal cords, and optic nerves were determined, as described in Materials and Methods. Data are presented as the number of plaques per section or the demyelination area in each section per the total number of sections stained using a total of 150 sections from 5 mice per group. (A) Numbers of plaques per brain section; (B) sizes of plaques per brain section; (C) numbers of plaques per spinal cord section; (D) sizes of plaques per spinal cord section; (E) numbers of plaques per optic nerve section; (F) sizes of plaques per optic nerve section.
DISCUSSION
Demyelinating diseases represent a spectrum of immune reactions in which, myelin, the fatty covering of nerve cell fibers in the brain, optic nerve, and spinal cord, is destroyed (29). Destruction of the myelin sheath has been associated with a number of diseases (30, 31), with multiple sclerosis (MS) being the most common condition of CNS inflammatory demyelination. The World Health Organization (WHO) estimates that over 2.5 million people globally suffer from MS, and according to the National Multiple Sclerosis Society, approximately 400,000 Americans have MS. The total economic impact of MS in the United States is estimated to be more than $28 billion per year. The available therapies, however, are not effective in all patients with MS. Thus, the development of new therapeutic and/or prophylactic drugs to control MS is critical. The cause of MS remains elusive. One hypothesis is that autoimmunity to antigens of the CNS is triggered by environmental factors, such as viral infection in genetically susceptible individuals, leading to destruction of the myelin (29, 32, 33).
Several lines of evidence suggest that IL-2 may be involved in CNS demyelination in MS (1–10). The presence of IL-2 is also associated with the disease state in experimental autoimmune encephalitis (EAE) (34–36) and following ocular infection of mice with HSV-1 (37–41). These observations suggest that, if IL-2 is involved, it alone may not be sufficient to initiate the observed pathology. Although evidence has accumulated that infectious agents, particularly viral agents, may be involved (42, 43); this, however, remains controversial (44–46). Previously, to show the involvement of higher levels of IL-2 in combination with viral infection in CNS demyelination, we constructed a recombinant HSV-1 strain constitutively expressing mIL-2, referred to as HSV–IL-2 (13). We have shown that infection of different strains of mice with the HSV–IL-2 recombinant resulted in CNS demyelination (17, 18). We have also found that infection of mice with a recombinant HSV-1 expressing IL-12p70 or injection of mice with IL-12p70 DNA blocks the CNS demyelination induced by the HSV–IL-2 recombinant (19, 20).
The HSV–IL-2 recombinant that we constructed and that we have shown induces CNS demyelination in ocularly infected mice is not a naturally occurring virus. Thus, the results obtained with the HSV–IL-2 recombinant may not directly support the hypothesis that elevated IL-2 levels in conjunction with viral infection contribute to CNS demyelination. Consequently, to rule out this possible ambiguity, we directly tested the potential role of elevation of IL-2 following infection with wild-type HSV-1 rather than infection with a recombinant HSV-1 strain expressing IL-2 (HSV–IL-2) in CNS demyelination. Evidence for the involvement of elevation of IL-2 in demyelination was observed using wt HSV-1 rather than HSV–IL-2. Demyelination was detected in the CNS of mice that received IL-2 by using rIL-2 protein, IL-2 DNA, or IL-2 peptides. Similar to our findings in our previous study (18), we detected CNS demyelination in BALB/c and C57BL/6 mouse strains. In addition, CNS demyelination was detected in both strains of mice infected with virulent wt HSV-1 strain McKrae and avirulent wt HSV-1 strain KOS. Overall, the degree of demyelination in brain, spinal cord, and optic nerve of infected mice was similar following injection of mice with IL-2 protein, IL-2 DNA, or IL-2 peptides. Moreover, this demyelination was similar to that in HSV–IL-2-infected mice (17–20).
The CNS pathology detected here is consistent with the published data concerning histologic analyses of specimens obtained from patients with MS at autopsy (47, 48), demyelination induced by mouse hepatitis virus (MHV) (49), and the EAE model of MS (47, 50). Analyses of the mechanisms by which the combination of the enhanced levels of IL-2 and viral infection elicits CNS demyelination suggest the possible role of IL-2 and viral infection in CNS pathology. Furthermore, our results suggest that full-length IL-2 is not required to induce CNS demyelination. In addition, the region of IL-2 that is involved in CNS demyelination resides within aa 26 to 30 of IL-2, and a single mutation (T27A) blocks HSV-1-induced CNS demyelination. These results have broad implications in terms of understanding the mechanisms by which elevated IL-2, which is common in autoimmune patients, can interact with environmental factors (in this case, HSV-1) to promote autoimmunity. They also suggest that selective targeting of IL-2 function may lay the basis for the development of therapies to rapidly control CNS demyelination in some patients.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants EY13615, EY15557, and AI093941.
Footnotes
Published ahead of print 28 August 2013
REFERENCES
- 1.Lu CZ, Fredrikson S, Xiao BG, Link H. 1993. Interleukin-2 secreting cells in multiple sclerosis and controls. J. Neurol. Sci. 120:99–106 [DOI] [PubMed] [Google Scholar]
- 2.Gallo P, Piccinno M, Pagni S, Tavolato B. 1988. Interleukin-2 levels in serum and cerebrospinal fluid of multiple sclerosis patients. Ann. Neurol. 24:795–797 [DOI] [PubMed] [Google Scholar]
- 3.Gallo P, Piccinno MG, Pagni S, Argentiero V, Giometto B, Bozza F, Tavolato B. 1989. Immune activation in multiple sclerosis: study of IL-2, sIL-2R, and gamma-IFN levels in serum and cerebrospinal fluid. J. Neurol. Sci. 92:9–15 [DOI] [PubMed] [Google Scholar]
- 4.Trotter JL, Clifford DB, McInnis JE, Griffeth RC, Bruns KA, Perlmutter MS, Anderson CB, Collins KG, Banks G, Hicks BC. 1989. Correlation of immunological studies and disease progression in chronic progressive multiple sclerosis. Ann. Neurol. 25:172–178 [DOI] [PubMed] [Google Scholar]
- 5.Greenberg SJ, Marcon L, Hurwitz BJ, Waldmann TA, Nelson DL. 1988. Elevated levels of soluble interleukin-2 receptors in multiple sclerosis. N. Engl. J. Med. 319:1019–1020 [DOI] [PubMed] [Google Scholar]
- 6.Hartung HP, Hughes RA, Taylor WA, Heininger K, Reiners K, Toyka KV. 1990. T cell activation in Guillain-Barre syndrome and in MS: elevated serum levels of soluble IL-2 receptors. Neurology 40:215–218 [DOI] [PubMed] [Google Scholar]
- 7.Bansil S, Troiano R, Cook SD, Rohowsky-Kochan C. 1991. Serum soluble interleukin-2 receptor levels in chronic progressive, stable and steroid-treated multiple sclerosis. Acta Neurol. Scand. 84:282–285 [DOI] [PubMed] [Google Scholar]
- 8.Traugott U. 1987. Multiple sclerosis: relevance of class I and class II MHC-expressing cells to lesion development. J. Neuroimmunol. 16:283–302 [DOI] [PubMed] [Google Scholar]
- 9.Adachi K, Kumamoto T, Araki S. 1989. Interleukin-2 receptor levels indicating relapse in multiple sclerosis. Lancet i:559–560 [DOI] [PubMed] [Google Scholar]
- 10.Kittur SD, Kittur DS, Soncrant TT, Rapoport SI, Tourtellotte WW, Nagel JE, Adler WH. 1990. Soluble interleukin-2 receptors in cerebrospinal fluid from individuals with various neurological disorders. Ann. Neurol. 28:168–173 [DOI] [PubMed] [Google Scholar]
- 11.Selmaj K, Nowak Z, Tchorzewski H. 1988. Multiple sclerosis: effect of myelin basic protein on interleukin 1, interleukin 2 production and interleukin 2 receptor expression in vitro. Clin. Exp. Immunol. 72:428–433 [PMC free article] [PubMed] [Google Scholar]
- 12.Selmaj K, Nowak Z, Tchorzewski H. 1988. Interleukin-1 and interleukin-2 production by peripheral blood mononuclear cells in multiple sclerosis patients. J. Neurol. Sci. 85:67–76 [DOI] [PubMed] [Google Scholar]
- 13.Ghiasi H, Osorio Y, Perng GC, Nesburn AB, Wechsler SL. 2002. Overexpression of interleukin-2 by a recombinant herpes simplex virus type 1 attenuates pathogenicity and enhances antiviral immunity. J. Virol. 76:9069–9078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghiasi H, Osorio Y, Hedvat Y, Perng GC, Nesburn AB, Wechsler SL. 2002. Infection of BALB/c mice with a herpes simplex virus type 1 recombinant virus expressing IFN-gamma driven by the LAT promoter. Virology 302:144–154 [DOI] [PubMed] [Google Scholar]
- 15.Osorio Y, Sharifi BG, Perng G, Ghiasi NS, Ghiasi H. 2002. The role of T(H)1 and T(H)2 cytokines in HSV-1-induced corneal scarring. Ocular Immunol. Inflamm. 10:105–116 [DOI] [PubMed] [Google Scholar]
- 16.Ghiasi H, Osorio Y, Perng GC, Nesburn AB, Wechsler SL. 2001. Recombinant herpes simplex virus type 1 expressing murine interleukin-4 is less virulent than wild-type virus in mice. J. Virol. 75:9029–9036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osorio Y, La Point SF, Nusinowitz S, Hofman FM, Ghiasi H. 2005. CD8+-dependent CNS demyelination following ocular infection of mice with a recombinant HSV-1 expressing murine IL-2. Exp. Neurol. 193:1–18 [DOI] [PubMed] [Google Scholar]
- 18.Zandian M, Belisle R, Mott KR, Nusinowitz S, Hofman FM, Ghiasi H. 2009. Optic neuritis in different strains of mice by a recombinant HSV-1 expressing murine interleukin-2. Invest. Ophthalmol. Vis. Sci. 50:3275–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zandian M, Mott KR, Allen SJ, Chen S, Arditi M, Ghiasi H. 2011. IL-2 suppression of IL-12p70 by a recombinant HSV-1 expressing IL-2 induces T cells auto-reactivity and CNS demyelination. PLoS One 6:e16820. 10.1371/journal.pone.0016820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zandian M, Mott KR, Allen SJ, Dumitrascu O, Kuo JZ, Ghiasi H. 2011. Use of cytokine immunotherapy to block CNS demyelination induced by a recombinant HSV-1 expressing murine interleukin-2. Gene Ther. 18:734–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Research Council 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC [Google Scholar]
- 22.Osorio Y, Cohen J, Ghiasi H. 2004. Improved protection from primary ocular HSV-1 infection and establishment of latency using multigenic DNA vaccines. Invest. Ophthalmol. Vis. Sci. 45:506–514 [DOI] [PubMed] [Google Scholar]
- 23.Ghiasi H, Wechsler SL, Kaiwar R, Nesburn AB, Hofman FM. 1995. Local expression of tumor necrosis factor alpha and interleukin-2 correlates with protection against corneal scarring after ocular challenge of vaccinated mice with herpes simplex virus type 1. J. Virol. 69:334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mott KR, Gate D, Zandian M, Allen SJ, Rajasagi NK, Van Rooijen N, Chen SC, Arditi M, Rouse BT, Flavell RA, Town T, Ghiasi H. 2011. Macrophage IL-12p70 signaling prevents HSV-1-induced CNS autoimmunity triggered by autoaggressive CD4+ Tregs. Invest. Ophthalmol. Vis. Sci. 52:2321–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyman O, Surh CD, Sprent J. 2006. Potential use of IL-2/anti-IL-2 antibody immune complexes for the treatment of cancer and autoimmune disease. Expert Opin. Biol. Ther. 6:1323–1331 [DOI] [PubMed] [Google Scholar]
- 26.Giri M, Ugen KE, Weiner DB. 2004. DNA vaccines against human immunodeficiency virus type 1 in the past decade. Clin. Microbiol. Rev. 17:370–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heath WR, Carbone FR. 2001. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 19:47–64 [DOI] [PubMed] [Google Scholar]
- 28.Zurawski SM, Zurawski G. 1989. Mouse interleukin-2 structure-function studies: substitutions in the first alpha-helix can specifically inactivate p70 receptor binding and mutations in the fifth alpha-helix can specifically inactivate p55 receptor binding. EMBO J. 8:2583–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin R, McFarland HF, McFarlin DE. 1992. Immunological aspects of demyelinating diseases. Annu. Rev. Immunol. 10:153–187 [DOI] [PubMed] [Google Scholar]
- 30.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. 2000. Multiple sclerosis. N. Engl. J. Med. 343:938–952 [DOI] [PubMed] [Google Scholar]
- 31.Hunter SF, Weinshenker BG, Carter JL, Noseworthy JH. 1997. Rational clinical immunotherapy for multiple sclerosis. Mayo Clin. Proc. 72:765–780 [DOI] [PubMed] [Google Scholar]
- 32.Hafler DA. 2004. Multiple sclerosis. J. Clin. Invest. 113:788–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemmer B, Cepok S, Nessler S, Sommer N. 2002. Pathogenesis of multiple sclerosis: an update on immunology. Curr. Opin. Neurol. 15:227–231 [DOI] [PubMed] [Google Scholar]
- 34.Yang J, Lindsberg PJ, Hukkanen V, Seljelid R, Gahmberg CG, Meri S. 2002. Differential expression of cytokines (IL-2, IFN-gamma, IL-10) and adhesion molecules (VCAM-1, LFA-1, CD44) between spleen and lymph nodes associates with remission in chronic relapsing experimental autoimmune encephalomyelitis. Scand. J. Immunol. 56:286–293 [DOI] [PubMed] [Google Scholar]
- 35.Petitto JM, Streit WJ, Huang Z, Butfiloski E, Schiffenbauer J. 2000. Interleukin-2 gene deletion produces a robust reduction in susceptibility to experimental autoimmune encephalomyelitis in C57BL/6 mice. Neurosci. Lett. 285:66–70 [DOI] [PubMed] [Google Scholar]
- 36.McCombe PA, Nickson I, Pender MP. 1998. Cytokine expression by inflammatory cells obtained from the spinal cords of Lewis rats with experimental autoimmune encephalomyelitis induced by inoculation with myelin basic protein and adjuvants. J. Neuroimmunol. 88:30–38 [DOI] [PubMed] [Google Scholar]
- 37.Ghiasi H, Cai S, Slanina SM, Perng GC, Nesburn AB, Wechsler SL. 1999. The role of interleukin (IL)-2 and IL-4 in herpes simplex virus type 1 ocular replication and eye disease. J. Infect. Dis. 179:1086–1093 [DOI] [PubMed] [Google Scholar]
- 38.Tang Q, Chen W, Hendricks RL. 1997. Proinflammatory functions of IL-2 in herpes simplex virus corneal infection. J. Immunol. 158:1275–1283 [PubMed] [Google Scholar]
- 39.Hendricks RL, Tumpey TM, Finnegan A. 1992. IFN-gamma and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J. Immunol. 149:3023–3028 [PubMed] [Google Scholar]
- 40.Allen EM, Weir JP, Martin S, Mercadal C, Rouse BT. 1990. Role of coexpression of IL-2 and herpes simplex virus proteins in recombinant vaccinia virus vectors on levels of induced immunity. Viral Immunol. 3:207–215 [DOI] [PubMed] [Google Scholar]
- 41.Arrunategui-Correa V, Baltatzis S, Foster CS. 1999. The role of cytokines in experimental herpes simplex keratitis. Acta Virol. 43:325–329 [PubMed] [Google Scholar]
- 42.Challoner PB, Smith KT, Parker JD, MacLeod DL, Coulter SN, Rose TM, Schultz ER, Bennett JL, Garber RL, Chang M, Schad PA, Stewart PM, Nowinski RC, Brown JP, Burner GC. 1995. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 92:7440–7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedman JE, Lyons MJ, Cu G, Ablashl DV, Whitman JE, Edgar M, Koskiniemi M, Vaheri A, Zabriskie JB. 1999. The association of the human herpesvirus-6 and MS. Mult. Scler. 5:355–362 [DOI] [PubMed] [Google Scholar]
- 44.Boman J, Roblin PM, Sundstrom P, Sandstrom M, Hammerschlag MR. 2000. Failure to detect Chlamydia pneumoniae in the central nervous system of patients with MS. Neurology 54:265. [DOI] [PubMed] [Google Scholar]
- 45.Martin C, Enbom M, Soderstrom M, Fredrikson S, Dahl H, Lycke J, Bergstrom T, Linde A. 1997. Absence of seven human herpesviruses, including HHV-6, by polymerase chain reaction in CSF and blood from patients with multiple sclerosis and optic neuritis. Acta Neurol. Scand. 95:280–283 [DOI] [PubMed] [Google Scholar]
- 46.Mirandola P, Stefan A, Brambilla E, Campadelli-Fiume G, Grimaldi LM. 1999. Absence of human herpesvirus 6 and 7 from spinal fluid and serum of multiple sclerosis patients. Neurology 53:1367–1368 [DOI] [PubMed] [Google Scholar]
- 47.Steinman L. 2001. Myelin-specific CD8 T cells in the pathogenesis of experimental allergic encephalitis and multiple sclerosis. J. Exp. Med. 194:F27–F30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Traugott U, Reinherz EL, Raine CS. 1983. Multiple sclerosis. Distribution of T cells, T cell subsets and Ia-positive macrophages in lesions of different ages. J. Neuroimmunol. 4:201–221 [DOI] [PubMed] [Google Scholar]
- 49.Wu GF, Dandekar AA, Pewe L, Perlman S. 2000. CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination. J. Immunol. 165:2278–2286 [DOI] [PubMed] [Google Scholar]
- 50.Zamvil S, Nelson P, Trotter J, Mitchell D, Knobler R, Fritz R, Steinman L. 1985. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. Nature 317:355–358 [DOI] [PubMed] [Google Scholar]