Abstract
Syndecan-4 is a membrane-bound heparan sulfate proteoglycan that participates in cell–cell and cell–matrix interactions and modulates adhesion and migration of many cell types. Through its extracellular domain, syndecan-4 cooperates with adhesion molecules and binds matrix components relevant for cell migration. Importantly, syndecan-4 is a substrate of extracellular proteases, however the biological significance of this cleavage has not been elucidated. Here, we show that the secreted metalloprotease ADAMTS1, involved in angiogenesis and inflammatory processes, cleaves the ectodomain of syndecan-4. We further showed that this cleavage results in altered distribution of cytoskeleton components, functional loss of adhesion, and gain of migratory capacities. Using syndecan-4 null cells, we observed that ADAMTS1 proteolytic action mimics the outcome of genetic deletion of this proteoglycan with regards to focal adhesion. Our findings suggest that the shedding of syndecan-4 by ADAMTS1 disrupts cell adhesion and promotes cell migration.
Keywords: Extracellular proteolysis, Proteoglycan, Metalloprotease, Endothelial cell
1. Introduction
Syndecans consist of a family of four transmembrane proteo glycans that are widely expressed by mammalian cells (Couchman, 2003; Tkachenko et al., 2005). By virtue of their capacity to integrate cells with the extracellular environment, syndecans are involved in a large number of biological processes including growth, migration, and adhesion (Couchman, 2003). Although the mechanisms by which syndecans function in these events have already been outlined, recent research has revealed new and biologically relevant features of these transmembrane molecules. In addition to the extensively studied role of glycosaminoglycan chains (GAGs) as interacting molecules with extracellular matrix (ECM) components, adhesion receptors, proteases, and growth factors, more recent findings suggest a definitive function to heparan sulfates in complex processes such as tissue injury, inflammation, cancer and development (Alexopoulou et al., 2007; Sasisekharan et al., 2002; Bishop et al., 2007; Hacker et al., 2005).
Importantly, all syndecans have been found to be cleaved by extracellular proteases (Kim et al., 1994). This specialized type of limited proteolysis, known as ectodomain shedding, occurs in a variety of cell surface molecules and can be induced by multiple signals. Ectodomain shedding has been observed in vivo with recognized relevance to various physiological and pathological events such as wound healing, host defense, and neurological disorders (Arribas and Borroto, 2002). In the case of syndecans, it is described that ectodomain proteolysis releases a soluble extracellular fragment (Kim et al., 1994) which then functions to regulate the activities of proteases and growth factors (Kainulainen et al., 1998; Subramanian et al., 1997).
Relevant studies already demonstrated that a cell surface-associated metalloproteinase is responsible for syndecan-1 and syndecan-4 shedding (Fitzgerald et al., 2000). Later investigations showed that the proteases responsible for syndecan-1 shedding include MMP-7 and MT1-MMP (Endo et al., 2003; Li et al., 2002), but no studies to date have identified the enzymes that cleave syndecan-4 in vivo. In addition to MMPs, the family of ADAM proteases have also been implicated in ectodomain shedding (Arribas and Borroto, 2002). Recent studies guided us to specifically consider ADAMTS proteases as potential sheddases for syndecan-4 (Krampert et al., 2005; Rodriguez-Manzaneque et al, 2002). The ADAMTS family includes 19 secreted proteases whose reported catalytic activities are limited to matrix and other extracellular components (reviewed in Porter et al., 2005).
Here we found that ADAMTS family members are syndecan-4 sheddases. Further, we show that syndecan-4 shedding by ADAMTS has relevant consequences for cellular adhesion and migration. In line with the described roles of syndecans during wound healing, inflammation, and malignant transformation, our findings stress the potential importance of this post-translational activity in such processes.
2. Materials and methods
2.1. Expression vectors
Full-length human ADAMTS1 and inactive ADAMTS1 expression vectors were described previously (Rodriguez-Manzaneque et al., 2002). To obtain the full-length mouse ADAMTS4, a 3′ cDNA fragment was first cloned into pCMV-SPORT6 at SalI and NotI sites from a mESTclone (dbEST ID 6206786; ResGEN, Invitrogen Corporation). The remaining 5′ region (1153 kb) was obtained by amplification from total RNA extracted from the limbs of day 9 mouse embryos, using the specific primers: forward, 5′-GCCTTCCACA GTTAGGGTG-3′, and reverse, 5′-CCAGTTC ATGAGCAGCAG-3′. This fragment was placed into pCRII TOPO TA cloning vector (Invitrogen Corporation) and was then cloned into the pCMV-SPORT6 vector at RcaI and EcoRV sites to generate ADAMTS4 EST-pCMV-SPORT6. The ADAM9, ADAM10, ADAM15, ADAM17, and MT1-MMP expression vectors were a generous gift from Dr. Arribas (Vall d'Hebron University Hospital Research Institute, Barcelona, Spain). The MMP7 expression vector was a generous gift from Dr. Matrisian (Vanderbilt University, Nashville, TN, USA). The HA-tagged full-length rat syndecan-4 expression vector was previously described (Horowitz et al., 2002). (Δ140–150)HA-syndecan-4 construct was generated to produce a form that lacks the juxtamembrane region. (Δ32-67)HA-syndecan-4 construct was generated by introducing new PshAI site with the primer: 5′-GACTGAGGTCGGAGATCTAGATGACACG-3′. (Δ59-67)HA-syndecan-4 vector was generated by introducing new EcoNI site with the primer: 5′-CCTTGAGCAGGGAGATCT AGATGACACG-3′. (Δ71-89)HA-syndecan-4 vector was generated by introducing new BgIII site with the primer: 5′-CTAGATCTA GATAACCACATC-3′. All these truncated syndecan-4 forms are represented in Fig. 2A. GST-Syndecan-4 plasmid was a generous gift from Dr. Rapraeger (University of Wisconsin, Madison, WI, USA) (McFall and Rapraeger, 1997).
Fig. 2.

Determination of ADAMTS1 cleavage site in syndecan-4. (A) Diagram of the full length syndecan-4 protein and all the deletion-mutant forms analyzed here. Numbers indicate the aa residue according to rat sequence (P34901, Swiss-Prot Database). GAG-attachment sites and thrombin/plasmin reported cleavage sites are indicated. (B) 293T cells were co-transfected with ADAMTS1 and the indicated syndecan-4 construct. Equal amounts of CL protein were heparinase-digested, resolved, and WB analysis was performed with HA and S4 Cyto Abs. Black stars/white border point to uncleaved syndecan-4 forms; white stars/black border point to cleaved syndecan-4 forms (just detected in lower panel). TM: transmembrane domain; Cyto: cytoplasmic domain.
2.2. Cell culture
Parental and modified 293T cell lines were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS). Transient and stable transfections were performed with FuGENE 6 Reagent (Roche Molecular Biochemicals) according to manufacturer's directions. For treatments, cells were rinsed and incubated with serum-free medium in the absence or presence of phorbol-12-myristate 13-acetate (PMA) (Calbiochem), methyl-β-cyclodextrin (MCD) (Sigma), thrombin (Sigma), or the protease inhibitor BB94. Chinese hamster ovary CHO-K1 and CHO-745 cells (a generous gift from Dr. S. Vilaró, University of Barcelona, Spain) were grown in DMEM/F-12 with 10% FCS. Mouse lung endothelial cells from wild-type and syndecan-4 knockout mice were isolated and characterized as described (Lee et al., 2006).
2.3. Protein extraction and immunoblot analysis
Cells were lysed with RIPA buffer (20 mM Tris–HCl pH 8, 150 mM NaCl, 0.1% SDS, 1% NP40, 1 mM EDTA, 1 mM phenyl-methyl-sulfonyl-fluoride (PMSF), and 1 μg/ml aprotinin). Debris was removed by centrifugation for 20 min at 16,000 × g, and total protein was quantified using Bio-Rad DC Protein Assay (Bio-Rad Laboratories). For digestion with heparinases, extracts were precipitated with 95% ethanol for 2 h at −20 °C. The precipitates were resuspended in 100 mM Tris–HCl pH 8.0, 0.1% Triton X-100, 5 mM EDTA, 1 mM PMSF. Samples were incubated with 10 mU/ml Heparinase III (Sigma) and 25 mU/ml Chondroitin sulfate lyase ABC (Sigma) for 4 h at 37°C. Samples were resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Schleicher & Schuell). Membranes were blocked with PBS containing 5% defatted milk and then incubated with polyclonal antibodies to ADAMTS1 (Rodriguez-Manzaneque et al., 2000), extracellular and cytosolic domains of syndecan-4 (Shworak et al., 1994), or monoclonal antibodies to HA epitope, RhoA (26C4, Santa Cruz Biotechnology Inc.), or actin. After incubation with the appropriate peroxidase-conjugated secondary antibody, signal was detected by chemiluminescence (SuperSignal Detection Kit, Pierce Biotechnology Inc.).
2.4. Immunofluorescence assays
For adhesion assays, coverslips were previously coated with human fibronectin (10 μg/ml) (Becton Dickinson). Cells were rinsed with PBS three times, fixed with 3.7% paraformaldehyde in PBS containing 0.1% Tween-20 for 20 min at 4 °C, and washed with PBS for 10 min. Prior to incubation with specific primary antibodies, samples were blocked with 5% BSA in PBS for 30 min at room temperature. Primary antibodies used were monoclonal ADAMTS1 (clone 3E4C6B4 (Rodriguez-Manzaneque et al., 2002)), polyclonal rabbit HA and monoclonal vinculin (clone hVIN-1) (Sigma), paxillin, and polyclonal cytosolic domain and ectodomain of syndecan-4 (Shworak et al., 1994). When indicated, cells were stained with Phalloidin-TRITC (Sigma). Images were obtained and analyzed in a confocal microscope LeicaTCS-NT, with the assistance of personnel from the UCTS (Vall d'Hebron University Hospital Research Institute).
2.5. RhoA activation assays
This protocol is based in the specific binding of active Rho with Rhotekin, as previously described (Ren et al., 1999). Briefly, cells were washed with ice-cold PBS and lysed with 50 mM Tris pH 7.2, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 500 mM NaCl, 10 mM MgCl2, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM PMSF. The lysate was cleared by centrifugation at 13,000 × g for 1–2 min at 4 °C. Total protein (0.8–1 mg) was incubated with Rhotekin Rho-binding domain beads (GST-TRBD) (a generous gift from Dr. del Pozo, CNIC, Madrid, Spain) for 90 min at 4 °C. Beads were then washed three times with 50 mM Tris pH 7.2, 1% Triton X-100, 150 mM NaCl, 10 mM MgCl2, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 0.1 mM PMSF. Washed beads were resuspended with SDS sample buffer containing DTT, heated to 95 °C for 10 min, and bound proteins were resolved on 12% SDS-PAGE gels. As a loading control, 80–100 μg total protein was directly resolved on 12% SDS-PAGE gels.
2.6. Migration assays
50,000 cells in 100 μl of growth media containing 1% FCS were placed on top of an 8 μm pore size Transwell (Corning Life Sciences) that was previously coated with 10% FCS-containing growth media. The lower chamber contained 600 μl 10% FCS-containing growth media. After 16 h, Transwells were rinsed twice with PBS, and cells were fixed with 3.7% paraformaldehyde containing 0.1% Tween-20 for 30 min at 4 °C. Again, Transwells were rinsed twice with PBS, and cells were stained with DAPI. Cells on top of the Transwell were gently removed, and the cells that migrated to the other side of the Transwell were counted under a fluorescence microscope.
2.7. FACS assays
CHO-K1 cells were detached from plates with PBS 10 mM EDTA and equilibrated with PBS for incubations with the primary antibodies: rabbit S4 Ectodomain (Shworak et al., 1994) and monoclonal 10E4 (USBiological) that recognizes heparan sulfate chains. Proper secondary antibodies were used according to common protocols and cells were finally analyzed in a FacsCalibur Citometer (Becton Dickinson).
2.8. In vitro digestions
GST-Syndecan4 protein was purified from bacteria using Glutathione Sepharose beads as described (McFall and Rapraeger, 1997). Bound protein was washed with PBS and equilibrated with reaction buffer (20 mM Tris pH 7.4, 100 mM NaCl, 10 mM CaCl2). Purified recombinant human ADAMTS1 (Rodriguez-Manzaneque et al, 2002) and thrombin were added and digestions were performed at 37 °C for 16 h. To analyze the Glutathione-bound fraction, Sepharose beads were centrifugated and washed with PBS, resolved by SDS-PAGE and analyzed by Western blot with GST (Sigma) and S4 Ectodomain antibodies.
3. Results
3.1. ADAMTS proteases, but not other related proteases, cleave the syndecan-4 ectodomain
To identify the protease responsible for syndecan-4 shedding we transiently co-transfected 293T cells with several metallopro teases, and a syndecan-4 construct that contains a HA-epitope at the N-terminus. These proteases were selected because of their recognized abilities to cleave cell surface proteins and included MMPs (MMP7 and MT1-MMP), ADAMs (ADAM9, ADAM10, ADAM15, and ADAM17), and ADAMTSs (ADAMTS1 and ADAMTS4). Western blot analysis of the cell lysates with an antibody that recognizes the cytoplasmic fragment of syndecan-4 (S4 Cyto Ab) showed the presence of the full-length syndecan-4 molecule (FL Syn4) in each case. In addition, a cleavage product (Cl Syn4) appeared exclusively in the presence of the proteases ADAMTS1 and ADAMTS4 (Fig. 1A). To confirm the specificity of this cleavage event, we used a catalytically inactive mutant form of ADAMTS1 (Rodriguez-Manzaneque et al, 2002). As showed in Fig. 1B, this inactive form was efficiently overexpressed (lower panel), but it did not mediate the cleavage of syndecan-4 (upper panel).
Fig. 1.
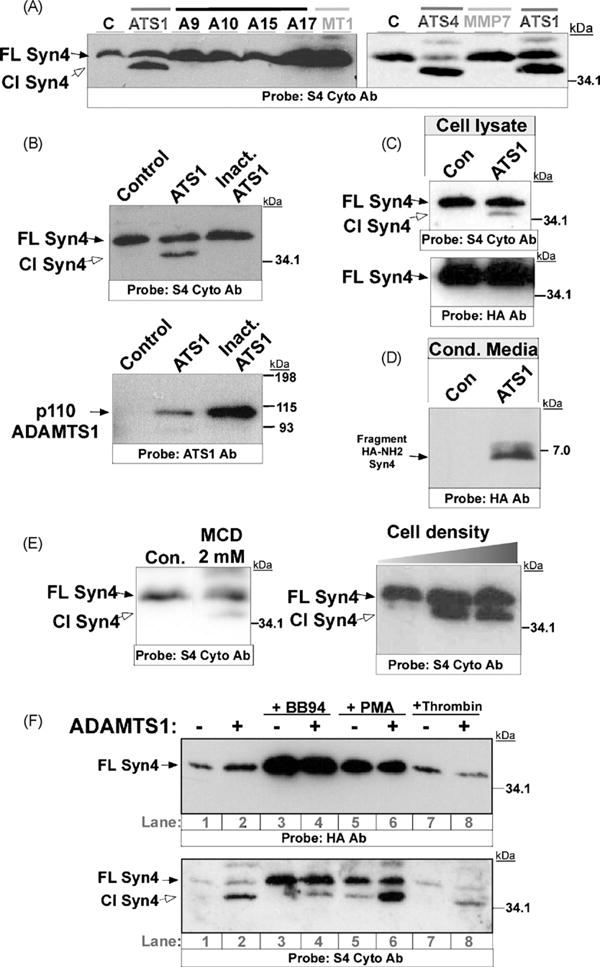
Cleavage of syndecan-4 by ADAMTS proteases. (A) 293T cells were co-transfected with syndecan-4 and the indicated protease or a plasmid control. 48 h after transfection, equal amounts of CL were heparinase-digested and resolved by SDS-PAGE. WB analysis was performed with a specific antibody for the cytosolic fragment of syndecan-4 (S4 Cyto Ab). (B) 293T cells were co-transfected with syndecan-4 and ADAMTS1 or an inactive form of ADAMTS1, and evaluated as in A with S4 Cyto Ab (upper panel) or an antibody recognizing ADAMTS1 (lower panel). (C) CL from cells transfected as in B were heparinase-digested, resolved, and WB analysis was then performed with a S4 Cyto and an HA antibody. (D) CM from cells transfected as in B were concentrated and resolved on 16% Tricine gels. WB analysis was performed with an HA antibody. (E) 293T cells were transfected with syndecan-4. For first panel, cells were treated with MCD 24 h after transfection in the absence of serum, and lysed after 24 h of treatment. For second panel, an increasing number of cells (first lane: 2 × 105, second lane: 6 × 105, third lane: 12 × 105) was lysed 48 h after transfection. In all cases, equal amounts of CL were heparinase-digested, resolved, and WB analysis was performed with the S4 Cyto Ab. (F) 293T cells were co-transfected with syndecan-4 and ADAMTS1 as indicated. 24 h after transfection, cells were treated with BB94, PMA, or thrombin in the absence of serum, and lysed after 24 h of treatment. Then, CL were heparinase-digested, resolved, and WB analysis was performed with HA and S4 Cyto Abs. A9, A10, A15, A17: ADAM9, 10, 15 and 17; ATS1, ATS4: ADAMTS1 and 4; MMP7: matrix metalloproteinase 7; MT1, membrane type 1-matrix metalloproteinase; C: control; FL Syn4: full length syndecan-4; Cl Syn4: Cleaved syndecan-4.
The presence of an N-terminal HA epitope in the syndecan-4 construct allowed us to estimate the location of the cleavage site. While the analysis of cell lysates with the S4 Cyto Ab showed two specific bands that corresponded to the full-length and cleaved syndecan-4, the HA antibody only recognized the uncleaved form (Fig. 1C). The hypothesis that the N-terminal fragment containing the HA tag is released to the extracellular milieu was tested by Western blot analysis of concentrated conditioned media. As predicted, a soluble fragment (approximately 6–7 kDa) of syndecan-4 that harbors the N-terminal HA tag was detected only in the conditioned media of cells expressing an active form of ADAMTS1 when compared to control cells (Fig. 1D).
Interestingly, a similar pattern of syndecan-4 proteolysis was observed when 293T cells overexpressing syndecan-4 were exposed to recognized shedding agonists, such as the cholesterol-depletion agent methyl-β-cyclodextrin (MCD) (Fig. 1E), and phorbol esters (PMA), shown to induce ADAMTS1 expression (Torres-Collado et al, 2006). Importantly, the effect of PMA together with the overexpression of ADAMTS1 appeared to have a cooperative effect (Fig. 1F). In addition, cell density facilitates this shedding event (Fig. 1E). This observation supports the potential role of cell–cell contact in regulating ectodomain shedding of syndecan-4. To further determine the nature of this cleavage process, we studied the action of a metalloprotease inhibitor (BB94) which partial inhibitory action over ADAMTS1 has been demonstrated (Torres-Collado et al, 2006). The addition of BB94 provokes an accumulation of full-length syndecan-4, and a partial inhibition of the proteolysis due to ADAMTS1 (Fig. 1F). Finally, we tested the action of thrombin as a potential cleavage agent of syndecan-4 (Schmidt et al., 2005) but we did not get any evidences of its action in this in vivo model (Fig. 1F).
3.2. Cleavage of syndecan-4 by ADAMTS1 occurs near its N-terminal end
Our findings indicated that the released syndecan-4 fragment corresponded to the extracellular N-terminal domain. However, the resulting molecular size (approximately 6–7 kDa) was not in agreement with a cleavage event at the juxtamembrane region that will give rise to a 16–20 kDa fragment. This finding was surprising because previous reports suggested that, like syndecan-1, the cleavage site of syndecan-4 would be located at the juxtamembrane region (Fitzgerald et al, 2000). To further verify our observations, we generated a truncated form of syndecan-4 with a deletion in the juxtamembrane domain (Δ140–150)HA-Syn4 (Fig. 2A). Transfection of ADAMTS1 resulted in cleavage of this truncated form of syndecan-4 in a similar manner to that observed for the full-length molecule (Fig. 2B, lanes 3–4). Indeed, this form appeared more sensitive to proteolysis even in the absence of ADAMTS1. In our approach, we associated this endogenous activity to induction by cell density, as reported above. To better define the cleavage area surrounding the attachment region for heparan sulfate chains (Shworak et al., 1994), we generated additional deletion-mutants, represented in Fig. 2A. Such deletions included residues 71–89: (Δ71–89)HA-Syn4, 59–67: (Δ59–67)HA-Syn4, and 32–67: (Δ32–67)HA-Syn4. The analysis of the effect of ADAMTS1 in this series of deletion-mutants clearly restricted the cleavage sequence to the region surrounding the first HS-attachment site (residue 44, rat sequence). All the generated forms, excepting (Δ32–67)HA-Syn4, underwent a similar pattern of proteolysis when compared with unmodified full length syndecan-4 (Fig. 2B). Together with the visualization of the full length version of all these forms, using an HA antibody (pointed out with a black star-white border in Fig. 2B), we observed the respective cleaved molecules with the S4 Cyto Ab (pointed out with a white star-black border in Fig. 2B), except (Δ32–67)HA-Syn4 construct that was not cleaved (lanes 9–10, Fig. 2B).
To further demonstrate that this cleavage event is directly performed by ADAMTS1, we took advantage of a GST-syndecan-4 chimera construct that contained the N-terminal extracellular domain of mouse syndecan-4 (aas 24–145) fused to GST (McFall and Rapraeger, 1997). The purified chimeric protein was incubated with human recombinant ADAMTS1 and thrombin at 37 °C for 16 h and analyzed by Western blot with S4 Ecto and GST antibodies. The incubation resulted in the generation of a lower fragment that corresponded to GST and the NH2-terminal end of syndecan-4, slightly higher than GST fragment generated by thrombin (Supplemental Fig. 1).
3.3. GAG and spatial requirements for the cleavage of syndecan-4 by ADAMTS1
Similarly to all heparan sulfate proteoglycans, syndecans are highly modified by GAG chains, whose binding properties are fundamental for proteoglycan functions (Iozzo and San Antonio, 2001). Interestingly, ADAMTS1 was observed to possess high affinity to heparan sulfate molecules, although the necessity of this binding for catalytic activity has not been directly addressed as for related proteases (Tortorella et al., 2000). In addition to the deletion-mutant constructs presented above, we wanted to determine the overall requirement of heparan sulfate chains to syndecan-4 cleavage by ADAMTS1. With this purpose we used CHO-745 cells, which display defective xylosyltransferase activity rendering them unable to synthesize GAG chains (Esko et al., 1985), and control CHO-K1 cells. We observed that the cleavage of syndecan-4 by ADAMTS1 occurs in both CHO-K1 and CHO-745 cell lines (Fig. 3A), what indicates that ADAMTS1 cleavage of syndecan-4 occurs independently of the presence of GAG chains. The apparent higher activity in CHO-K1 cells (observed by a increased proportion of cleaved syndecan-4) could be related with the higher levels of p87-ADAMTS1 in this cell line (Fig. 3A and B).
Fig. 3.
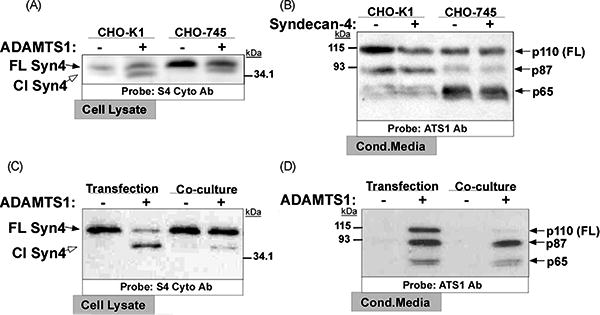
Cleavage of syndecan-4 by ADAMTS1 under different conditions. (A and B) Control and ADAMTS1-overexpressing CHO-K1 and CHO-745 cells were transfected with syndecan-4. After 24 h of serum-deprivation, CL (A) and CM (B) were harvested, heparinase-digested, resolved, and analyzed by WB with the indicated antibodies. (C and D) For co-transfection approach (two first lanes), equal number of 293T cells were transfected with syndecan-4 and ADAMTS1 expression vectors as indicated; for co-culture approach (two last lanes), same number of cells (control or ADAMTS1 overexpressing cells) were co-cultured with syndecan-4 overexpressing cells. 24 h after co-transfection or co-culture, cells were serum-deprived for 24 h, and then CL (C) and CM (D) were harvested, heparinase-digested, resolved, and analyzed by WB with the indicated antibodies. p110, p87 and p65 represent different ADAMTS1 forms.
We next explored the requirements of cell proximity and/or co-expression of syndecan-4 and ADAMTS1 for the shedding event. In addition to the aforementioned co-transfection assays which illustrate a potential cis-cleavage and autocrine mode of action of ADAMTS1, we performed experiments with syndecan-4 expressing cells co-cultured with control or ADAMTS1 cells (Fig. 3C). Although the most efficient syndecan-4 cleavage was noted in cis, by the co-transfection approach, we also observed a degree of syndecan-4 shedding in the co-culture condition with ADAMTS1 expressing cells (Fig. 3C, last lane). The analysis of ADAMTS1 in the conditioned media of co-cultured cells confirmed the presence of both active forms of ADAMTS1, p87 and p65 (Fig. 3D).
3.4. Cleavage of syndecan-4 by ADAMTS1 alters actin distribution and cell adhesion properties
Consistent with previous reports that demonstrate the participation of syndecan-4 in focal adhesion assembly and actin stress fiber formation (Couchman, 2003), we observed changes in cell shape when both ADAMTS1 and syndecan-4 proteins were co-expressed. Individual transfection of ADAMTS1 or syndecan-4 did not result in major alterations in actin fiber distribution (Fig. 4A and B). However, the co-expression of both protease and substrate resulted in cell rounding and a clear redistribution of actin bundles to the periphery of the cell, as a pattern that is associated with a decrease in adhesion area (Fig. 4C1 and C2). The decrease of full length HA-syndecan-4 levels in the presence of ADAMTS1 was further demonstrated (Supplemental Fig. 2).
Fig. 4.
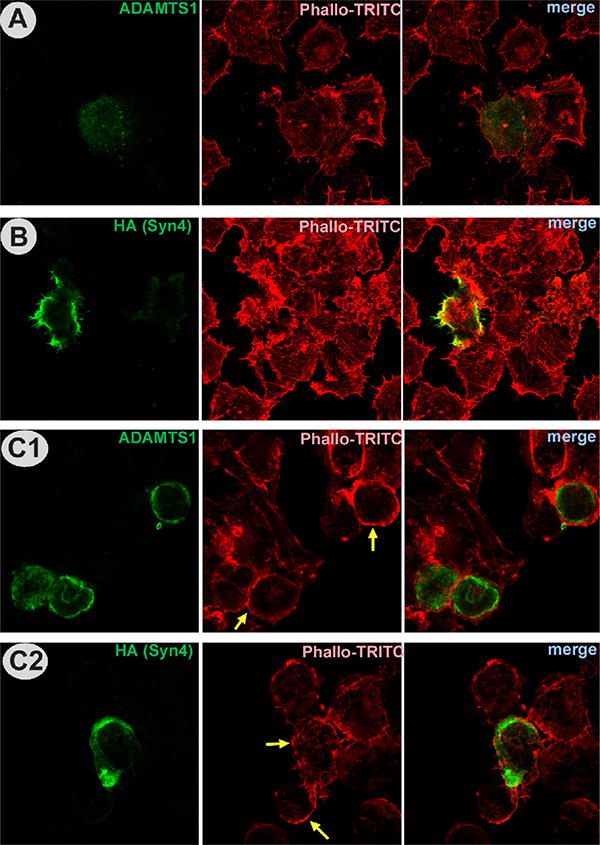
Immunocytochemical analysis of actin in cells transfected with ADAMTS1 and syndecan-4. (A) 293T cells transfected with ADAMTS1 were fixed and double stained with Phalloidin-TRITC (red) and ADAMTS1 antibody (green). (B) 293T cells transfected with syndecan-4 were fixed and double stained with HA antibody (green) and Phalloidin-TRITC. (C) 293T cells co-transfected with ADAMTS1 and syndecan-4 were fixed and double stained with Phalloidin-TRITC and ADAMTS1 antibody (C1) or HA antibody (C2). Yellow arrows point to representative cells that undergo major actin reorganization. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
To gain additional mechanistic information, we performed similar assays using stable 293T clones expressing (i) a parental vector control, (ii) ADAMTS1, and (iii) mutant inactive ADAMTS1. The number of suspended cells was low (ranging from 0.25 to 1.25%) and did not differ significantly between clones. Transfection of syndecan-4 increased slightly the percentage of suspended cells in all of the groups. However, a rather substantial increase in suspended cells was found in the presence of active ADAMTS1 and syndecan-4 (Fig. 5A). Western blot analysis of an equivalent number of attached and suspended cells confirmed the presence of syndecan-4 in both populations. Importantly, the population of cells in suspension contained higher levels of syndecan-4, and it also showed the cleaved form more predominantly (Fig. 5B).
Fig. 5.
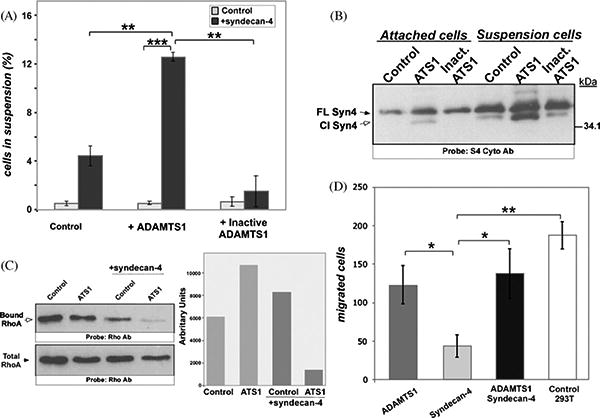
Analysis of 293T cells in the presence of ADAMTS1 and syndecan-4. (A) Control cells, as well as ADAMTS1 and inactive ADAMTS1 overexpressing 293T cells, were transfected with control or syndecan-4 expression vectors. The number of suspended cells was quantified 36 h after transfection. These experiments were performed in triplicate. Graph shows the percentage of suspended cells compared with total number of cells. (B) Equal numbers of attached and suspended cells (20,000) from A were lysed, heparinase-digested, and resolved by SDS-PAGE. WB analysis was performed with S4 Cyto Ab. (C) Control and ADAMTS1 overexpressing 293T cells were transfected with control or syndecan-4 vector. Cells were lysed 36 h after transfection. To determine RhoA activity, equal amounts of protein were incubated with Rhotekin Rho-binding domain beads and then resolved (upper panel). For total RhoA determination, equal amount of protein was directly resolved and analyzed by WB (lower panel). The side panel shows the quantification of these WB, normalized versus total RhoA. (D) Equal numbers of control 293T cells and stable clones overexpressing ADAMTS1 and/or syndecan-4 were placed on top of 8 μm pore size Transwells. After 16 h, migrated cells were fixed, DAPI-stained, and counted. The graph represents the number of migrated cells per field in each group. These numbers are the result of counting four different fields per experimental group, and experiments were performed in triplicate. *p < 0.05; **p < 0.005; ***p < 0.0005.
The small GTPase RhoA is a key regulator of actin fiber formation, and its functional interaction with syndecan-4 has been previously shown (Saoncella et al., 1999; Wilcox-Adelman et al., 2002a). The alterations that we observed in actin patterns in the presence of syndecan-4 shedding prompted us to evaluate RhoA activity, by determination of levels of active RhoA bound to Rhotekin (Ren et al., 1999), in the presence or absence of ADAMTS1 and its substrate syndecan-4. The individual expression of these proteins did not significantly affect the levels of active RhoA when compared to untransfected cells (Fig. 5C). In contrast, the co-expression of both ADAMTS1 and syndecan-4 with the subsequent cleavage of syndecan-4 caused a decrease of active RhoA (Fig. 5C, last lane). This finding correlates well with the morphological alterations described, in particular the reduction of stress fibers (Burridge and Wennerberg, 2004).
Considering the changes in cell adhesion and actin cytoskele ton, we investigated the functional consequences of syndecan-4 shedding on cell migration. In this assay, the overexpression of syndecan-4 resulted in a significant inhibition in cell migration (up to 75%), consistent with the pro-adhesive and anti-migratory properties previously reported for this transmembrane proteo glycan (Longley et al., 1999). Co-expression of both ADAMTS1 and syndecan-4 rescued their migration capacity, with the cells reaching levels similar to those of ADAMTS1 overexpressing cells (Fig. 5D).
3.5. Cleavage of endogenous syndecan-4 affects focal adhesions and resembles genetic ablation of this proteoglycan
To ascertain the effect of ADAMTS1 on syndecan-4 processing in cells with wild-type levels of the proteoglycan, we evaluated by flow cytometry its presence at the cell surface in CHO-K1 cells in the absence or presence of ADAMTS1 (Fig. 6A). The use of two different antibodies, S4Ecto that recognizes syndecan-4 ectodomain, and 10E4 that detects heparan sulfate chains, showed that the presence of ADAMTS1 diminished the intensity of intact syndecan-4 at the cell surface, including the attached heparan sulfate modifications. These same cells were used to evaluate the formation of focal adhesions on fibronectin-coated plates, previously reported to be syndecan-4 dependent (Mahalingam et al., 2007; Thodeti et al., 2003). Although cells were able to adhere, vinculin-positive focal adhesions were significantly reduced in ADAMTS1-expressing CHO cells (Fig. 6B). The co-staining of these cells with phalloidin also showed the decrease of the number of stress fibers, as previously demonstrated (Fig. 4).
Fig. 6.
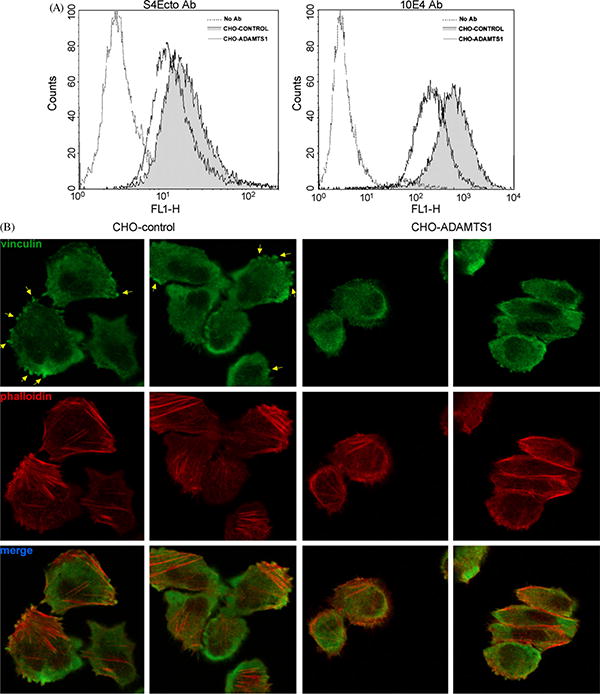
Analysis of cell surface syndecan-4 and focal adhesions in CHO-K1 cells. (A) Parental and ADAMTS1 CHO-K1 cells were labeled with S4Ecto and 10E4 antibodies and fluorescence-intensity was analyzed by flow cytometry. (B) Same cells as A were plated in fibronectin-coated coverslips and fixed after 2 h. Focal adhesions (yellow arrows) and stress fibers were visualized by staining with a vinculin antibody and Phalloidin-TRITC, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Finally, the biological relevance of syndecan cleavage was explored using primary cultures of murine lung endothelial cells. Exposure of these cells to ADAMTS1 resulted in a reduction in the number of focal adhesions, as assessed by paxillin visualization (Fig. 7). Interestingly the effect of ADAMTS1 resembled the pattern and frequency of focal adhesions found in syndecan-4 knockout endothelial cells (Fig. 7). Together these findings suggest that proteolytic processing of syndecan-4 allows cells to effectively diminish focal adhesions and, consequently, alters their migration properties.
Fig. 7.
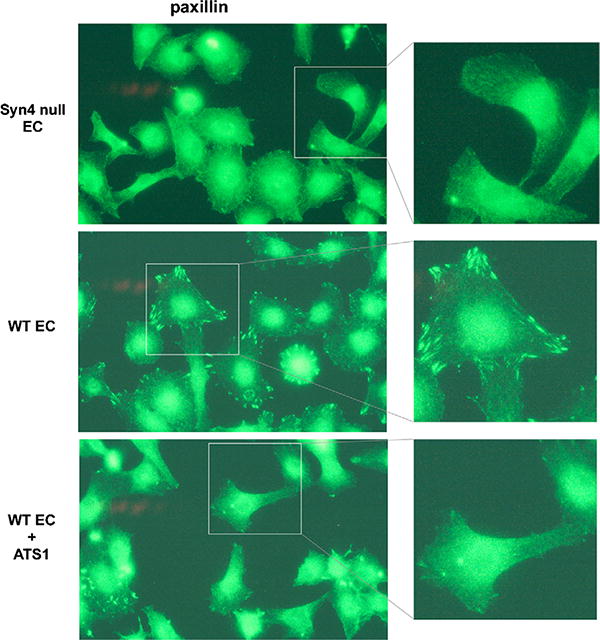
Analysis of focal adhesions in lung murine endothelial cells. EC isolated from wild-type and syndecan-4 knockout mice were plated on fibronectin-coated plates and treated with purified rhADAMTS1 as indicated. Cells were fixed after 16 h and focal adhesions were visualized by staining with a paxillin antibody. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
4. Discussion
In this study we demonstrated that, among a set of recognized shedding proteases, ADAMTS1 and ADAMTS4 were uniquely able to cleave the transmembrane proteoglycan syndecan-4. This cleavage event occurs in a manner similar to that shown by shedding agonists and is induced by increasing cell density. Ectodomain shedding of transmembrane proteins appears to help maintain extracellular matrix homeostasis, to provide a source of physiologically active soluble factors, and to induce significant changes in cell behavior. Recent reports have attributed the capacity to shed syndecan-1 to the matrix metalloproteases MMP7 and MT1-MMP (Endo et al., 2003; Li et al., 2002). However, we have shown here that these enzymes were unable to cleave syndecan-4. The finding that ADAMTS proteases act as sheddases of syndecan-4 uncovers a role previously limited to proteases of the MMP and ADAM families (Arribas and Borroto, 2002; Werb, 1997).
The affinity of ADAMTSs to the extracellular matrix and cell surface components appears important for their activity. For ADAMTS1, its binding to the cell surface and the extracellular matrix is mediated by its Thrombospondin Repeat (TSR) domains and spacer region which display high affinities for heparan sulfates and other surface molecules (Kuno and Matsushima, 1998; Rodriguez-Manzaneque et al., 2000). Interestingly, we observed that the expression profile of the ADAMTS1 processed forms differed in the wild-type CHO-K1 cells and the GAG-deficient CHO-745. The increased proportion of p87-ADAMTS1 in wild-type cells could be due to the stronger heparan sulfate affinity of this form, so it increases its stability, and it translates in a higher catalytic activity than the C-terminally truncated p65 form (Rodriguez-Manzaneque et al., 2000). Consistently, a more efficient cleavage of syndecan-4 was detected in CHO-K1 cells (Fig. 3A). In addition, co-transfection and co-culture analysis support an autocrine mode of action for ADAMTS1 and emphasize the relevance of co-localization of enzyme and substrate within a specific cellular microenvironment. Albeit less efficient, our results also provide evidence that a juxtacrine and/or paracrine action of ADAMTS1 is possible. Given the extracellular properties of this protease, it is relevant to consider its action on targets of different cell types, independently of its endogenous expression of ADAMTS1.
The only syndecan for which the cleavage site by metallopro teases has been determined is human syndecan-1, where this site is located at the juxtamembrane region (Endo et al., 2003, Fitzgerald et al., 2000). According to our results with ADAMTS1, ADAMTS4, and the shedding agonists tested, neither the band pattern of syndecan-4 forms in the cell layer nor the released fragment in the conditioned media correspond to a cleavage site in the juxtamembrane region of syndecan-4. Rather, our data delimits an alternative cleavage location at the N-terminus, near the first GAG-attachment site. This region appears highly conserved among rat, mouse and human syndecan-4 sequences (Supplemental Fig. 1).
The biological relevance of the cleavage event described here is first supported by the fact that the shedding of syndecan-4 by ADAMTS1 occurred in a pattern similar to that induced by recognized shedding agonists, such as phorbol esters, cholesterol-depleting agents, and increasing cell density (Asher et al., 2005). Importantly, the expression of both ADAMTS1 and ADAMTS4 proteases are induced by phorbol esters (Torres-Collado et al., 2006; Worley et al., 2003).
Assessment of the biological significance of the cleavage event provided evidence for changes in cytoskeleton and focal adhesions. While the single expression of either ADAMTS1 or syndecan-4 molecules did not cause phenotypic alterations, significant changes where noted when the increased levels of syndecan-4 were accompanied by overexpression of ADAMTS1. The observed effects of syndecan-4 shedding included the redistribution of actin stress fibers, cell rounding, and a significant decrease of active RhoA. Syndecan-4 has been widely reported as a relevant player in cell adhesion, mainly due to its presence in focal adhesions in several cell types (Couchman, 2003). The use of CHO-K1 and endothelial cell primary cultures allowed us to confirm the alterations in focal adhesions by ADAMTS1. More significantly, a reduction in the number of focal adhesions similar to the one we observed in cells accompanied by ADAMTS1 was found to occur in endothelial cells from syndecan-4 knockout mice. Ongoing studies demonstrate also that the cleavage of endogenous syndecan-4 by ADAMTS1 impairs angiogenesis (D. Carpizo, J.C. Rodríguez-Manzaneque, M.L. Iruela-Arispe, manuscript in preparation).
Complementing our data on adhesion, we observed that the overexpression of syndecan-4 lead to inhibition of migration, in agreement with previous reports (Longley et al., 1999). As reviewed by Wilcox-Adelman et al. (2002b), either excess or complete lack of cell surface syndecan-4 can have negative effects on cellular migration. Here, we provide an example of negative regulation by an excess of syndecan-4. More importantly, the inhibition of migration by syndecan-4 was fully reverted by the co-expression of the protease ADAMTS1 and the subsequent shedding of the syndecan-4 ectodomain. Interestingly, a recent report has provided evidence for pro-metastatic activity of full-length ADAMTS1 (Liu et al., 2006). Our migration results suggested that the shedding of syndecan-4 by ADAMTS1 might be involved as an important pathway to consider in further studies regarding the role of these proteases in metastasis.
Studies on the regulation of syndecan-4 function by shedding have not been largely explored. Syndecan-4 is directly implicated in various signaling pathways associated with cellular stress. It is becoming clear that these pathways induce the cleavage of syndecan-4. Importantly, the relevance of ADAMTS1 in wound healing has been demonstrated (Krampert et al., 2005). Our results suggest that the shedding of syndecan-4 is a potential mechanism by which ADAMTS1 could act in this process. This hypothesis is supported by the defects in wound healing observed in syndecan-4 null mice (Echtermeyer et al., 2001) where migration and adhesion properties of fibroblasts were impaired. Other studies with syndecan-4 null mice confirmed a close relationship of this proteoglycan with the vascular system (Ishiguro et al., 2000) and inflammatory episodes (Ishiguro et al., 2001a,b). Similarly, ADAMTS1 is involved in angiogenesis and inflammation, suggesting a possible link between this molecule and syndecan-4 shedding in these processes.
Supplementary Material
Acknowledgments
We thank Dr. Arribas (Vall d'Hebron University Hospital Research Institute, Barcelona, Spain), Dr. Matrisian (Vanderbilt University, Nashville, TN, USA), Dr. Vilaró (University of Barcelona, Spain), Dr. Rapraeger (University of Wisconsin, Madison, WI, USA) and Dr. del Pozo (CNIC, Madrid, Spain) for providing us with various reagents. This work was supported by grants from Ministerio de Educación y Ciencia (SAF2006-04019) and Fundació La Marató de TV3 (052510) to JCRM.
Abbreviations
- ADAMTS
a disintegrin-like and metalloprotease with throm bospondin type I motifs
- CL
cell lysate
- CM
conditioned media
- ECM
extracellular matrix
- HA
hemagglutinin epitope
- MMP
matrix metalloproteinase
- PAGE
polyacrylamide gel electrophoresis
- PMA
phorbol-12-myristate-13-acetate
- WB
Western blot
Footnotes
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.biocel.2008.08.014.
References
- Alexopoulou AN, Multhaupt HA, Couchman JR. Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol. 2007;39:505–28. doi: 10.1016/j.biocel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Arribas J, Borroto A. Protein ectodomain shedding. Chem Rev. 2002;102:4627–38. doi: 10.1021/cr010202t. [DOI] [PubMed] [Google Scholar]
- Asher RA, Morgenstern DA, Properzi F, Nishiyama A, Levine JM, Fawcett JW. Two separate metalloproteinase activities are responsible for the shedding and processing of the NG2 proteoglycan in vitro. Mol Cell Neurosci. 2005;29:82–96. doi: 10.1016/j.mcn.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–7. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Couchman JR. Syndecans: proteoglycan regulators of cell-surface microdomains? Nat Rev Mol Cell Biol. 2003;4:926–37. doi: 10.1038/nrm1257. [DOI] [PubMed] [Google Scholar]
- Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, et al. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest. 2001;107:R9–14. doi: 10.1172/JCI10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, et al. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J Biol Chem. 2003;278:40764–70. doi: 10.1074/jbc.M306736200. [DOI] [PubMed] [Google Scholar]
- Esko JD, Stewart TE, Taylor WH. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci USA. 1985;82:3197–201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol. 2000;148:811–24. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530–41. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- Horowitz A, Tkachenko E, Simons M. Fibroblast growth factor-specific modulation of cellular response by syndecan-4. J Cell Biol. 2002;157:715–25. doi: 10.1083/jcb.200112145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angio genesis arena. J Clin Invest. 2001;108:349–55. doi: 10.1172/JCI13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Iwase M, Yoshikai Y, et al. Syndecan-4 deficiency leads to high mortality of lipopolysaccharide-injected mice. J Biol Chem. 2001a;276:47483–8. doi: 10.1074/jbc.M106268200. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Matsuo S, Kusugami K, et al. Syndecan-4 deficiency increases susceptibility to kappa-carrageenan-induced renal damage. Lab Invest. 2001b;81:509–16. doi: 10.1038/labinvest.3780259. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Nakamura E, Ito M, et al. Syndecan-4 deficiency impairs the fetal vessels in the placental labyrinth. Dev Dyn. 2000;219:539–44. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1081>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Kainulainen V, Wang H, Schick C, Bernfield M. Syndecans, heparan sulfate proteo glycans, maintain the proteolytic balance of acute wound fluids. J Biol Chem. 1998;273:11563–9. doi: 10.1074/jbc.273.19.11563. [DOI] [PubMed] [Google Scholar]
- Kim CW, Goldberger OA, Gallo RL, Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol Biol Cell. 1994;5:797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampert M, Kuenzle S, Thai SN, Lee N, Iruela-Arispe ML, Werner S. ADAMTS1 proteinase is up-regulated in wounded skin and regulates migration of fibroblasts and endothelial cells. J Biol Chem. 2005;280:23844–52. doi: 10.1074/jbc.M412212200. [DOI] [PubMed] [Google Scholar]
- Kuno K, Matsushima K. ADAMTS-1 protein anchors at the extracellular matrix through the thrombospondin type I motifs and its spacing region. J Biol Chem. 1998;273:13912–7. doi: 10.1074/jbc.273.22.13912. [DOI] [PubMed] [Google Scholar]
- Lee NV, Sato M, Annis DS, Loo JA, Wu L, Mosher DF, et al. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. EMBO J. 2006;25:5270–83. doi: 10.1038/sj.emboj.7601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–46. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- Liu YJ, Xu Y, Yu Q. Full-length ADAMTS-1 and the ADAMTS-1 fragments display pro-and antimetastatic activity, respectively. Oncogene. 2006;25:2452–67. doi: 10.1038/sj.onc.1209287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longley RL, Woods A, Fleetwood A, Cowling GJ, Gallagher JT, Couchman JR. Control of morphology, cytoskeleton and migration by syndecan-4. J Cell Sci. 1999;112:3421–31. doi: 10.1242/jcs.112.20.3421. [DOI] [PubMed] [Google Scholar]
- Mahalingam Y, Gallagher JT, Couchman JR. Cellular adhesion responses to the heparin-binding (HepII) domain of fibronectin require heparan sulfate with specific properties. J Biol Chem. 2007;282:3221–30. doi: 10.1074/jbc.M604938200. [DOI] [PubMed] [Google Scholar]
- McFall AJ, Rapraeger AC. Identification of an adhesion site within the syndecan-4 extracellular protein domain. J Biol Chem. 1997;272:12901–4. doi: 10.1074/jbc.272.20.12901. [DOI] [PubMed] [Google Scholar]
- Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–85. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque JC, Milchanowski AB, Dufour EK, Leduc R, Iruela-Arispe ML. Characterization of METH-1/ADAMTS1 processing reveals two distinct active forms. J Biol Chem. 2000;275:33471–9. doi: 10.1074/jbc.M002599200. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque JC, Westling J, Thai SN, Luque A, Knauper V, Murphy G, et al. ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun. 2002;293:501–8. doi: 10.1016/S0006-291X(02)00254-1. [DOI] [PubMed] [Google Scholar]
- Saoncella S, Echtermeyer F, Denhez F, Nowlen JK, Mosher DF, Robinson SD, et al. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc Natl Acad Sci USA. 1999;96:2805–10. doi: 10.1073/pnas.96.6.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasisekharan R, Shriver Z, Venkataraman G, Narayanasami U. Roles of heparan-sulphate glycosaminoglycans in cancer. Nat Rev Cancer. 2002;2:521–8. doi: 10.1038/nrc842. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Echtermeyer F, Alozie A, Brands K, Buddecke E. Plasmin- and thrombin-accelerated shedding of syndecan-4 ectodomain generates cleavage sites at Lys(114)-Arg(115) and Lys(129)-Val(130) bonds. J Biol Chem. 2005;280:34441–6. doi: 10.1074/jbc.M501903200. [DOI] [PubMed] [Google Scholar]
- Shworak NW, Shirakawa M, Mulligan RC, Rosenberg RD. Characterization of ryudo can glycosaminoglycan acceptor sites. J Biol Chem. 1994;269:21204–14. [PubMed] [Google Scholar]
- Subramanian SV, Fitzgerald ML, Bernfield M. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor receptor activation. J Biol Chem. 1997;272:14713–20. doi: 10.1074/jbc.272.23.14713. [DOI] [PubMed] [Google Scholar]
- Thodeti CK, Albrechtsen R, Grauslund M, Asmar M, Larsson C, Takada Y, et al. ADAM12/syndecan-4 signaling promotes beta 1 integrin-dependent cell spreading through protein kinase Calpha and RhoA. J Biol Chem. 2003;278:9576–84. doi: 10.1074/jbc.M208937200. [DOI] [PubMed] [Google Scholar]
- Tkachenko E, Rhodes JM, Simons M. Syndecans: new kids on the signaling block. Circ Res. 2005;96:488–500. doi: 10.1161/01.RES.0000159708.71142.c8. [DOI] [PubMed] [Google Scholar]
- Torres-Collado AX, Kisiel W, Iruela-Arispe ML, Rodriguez-Manzaneque JC. ADAMTS1 interacts with, cleaves, and modifies the extracellular location of the matrix inhibitor tissue factor pathway inhibitor-2. J Biol Chem. 2006;281:17827–37. doi: 10.1074/jbc.M513465200. [DOI] [PubMed] [Google Scholar]
- Tortorella M, Pratta M, Liu RQ, Abbaszade I, Ross H, Burn T, et al. The thrombospondin motif of aggrecanase-1 (ADAMTS-4) is critical for aggrecan substrate recognition and cleavage. J Biol Chem. 2000;275:25791–7. doi: 10.1074/jbc.M001065200. [DOI] [PubMed] [Google Scholar]
- Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–42. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- Wilcox-Adelman SA, Denhez F, Goetinck PF. Syndecan-4 modulates focal adhesion kinase phosphorylation. J Biol Chem. 2002a;277:32970–7. doi: 10.1074/jbc.M201283200. [DOI] [PubMed] [Google Scholar]
- Wilcox-Adelman SA, Denhez F, Iwabuchi T, Saoncella S, Calautti E, Goetinck PF. Syndecan-4: dispensable or indispensable? Glycoconj J. 2002b;19:305–13. doi: 10.1023/A:1025304602057. [DOI] [PubMed] [Google Scholar]
- Worley JR, Baugh MD, Hughes DA, Edwards DR, Hogan A, Sampson MJ, et al. Metalloproteinase expression in PMA-stimulated THP-1 cells Effects of peroxisome proliferator-activated receptor-gamma (PPAR gamma) agonists and 9-cis-retinoic acid. J Biol Chem. 2003;278:51340–6. doi: 10.1074/jbc.M310865200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


