Abstract
Background:
Ayurvedic system of medicine holds a number of drugs that improves the immunity. Āmalaki (Emblica officinalis) is one such drug. Researches with crude extracts of Āmalaki have proven the antioxidant and immunomodulatory activities. But, works on Āmalaki Rasāyana are not found reported.
Aims:
Considering this, two samples of Āmalaki Rasāyana (AR7 and AR21) were studied to evaluate comparative immunomodulatory activity against the cyclophosphamide immunosuppression in rats.
Materials and Methods:
Test drugs were prepared by following classical guidelines. Wistar strain albino rats of either sex were used in the study.
Statistical Analysis:
For comparison of data from cyclophosphamide control group with remaining cyclophosphamide plus test drug administered groups one way ANOVA with Dunnett's multiple t-test (DMTT) was employed.
Results and Conclusions:
Āmalaki Rasāyana possesses significant immunostimulant activity and moderate cytoprotective activity. AR21 was found to have better activity profile in terms of both immunostimulant as well as cytoprotective activity.
KEY WORDS: Āmalaki, cyclophosphamide, Ghrita, humoral immunity, Madhu, Rasāyana
INTRODUCTION
Ayurvedic system of medicine has suggested means to increase the body's natural immunity to disease, the principles of which have been emphasized under the heading of Vyādhi Kṣamatva.[1] A number of medicinal plants individually or in combinations have been claimed to possess immunomodulatory activity. Āmalaki (Emblica officnalis Gaertn), one of such plants is mentioned as a Rasāyana drug in classics.[2] Certain researches of the recent past also revalidate the antioxidant and immunomodulatory activities[3] of crude extract of E. officinalis fruit. Recent study reported the in vivo effects of “Āmalaki Rasāyana” in Drosophila melanogaster on median life span, developmental time, starvation tolerance, etc.[4] Different types of Āmalaki Rasāyana Prayoga have been mentioned in various Ayurvedic classics. In the present study, two Āmalaki Rasāyana prepared by 7 Bhāvanas[5] (levigations) and 21 Bhāvanas[6] with Āmalaki Swarasa (Fresh juice of Āmalaki fruit) were considered to evaluate their comparative immunomodulatory activity against the cyclophosphamide immunosuppression in rats.
MATERIALS AND METHODS
Test formulations
The two test formulations were prepared in the Department of Rasa Shastra and Bhaishajya Kalpana, Institute for Post Graduate Teaching and Research in Ayurveda (IPGT and RA), Gujarat Ayurved University, Jamnagar. Āmalaki fruits were procured from the local markets of Jamnagar. Fine powder (100 #) was prepared out of it. The fruits and the powder were authenticated in the Pharmacognosy Laboratory of the Institute.
Āmalaki Swarasa (Juice of Āmalaki fruit) was prepared with a juice extractor and was blended with Āmalaki powder. The blend was levigated until a doughy mass is formed and allowed to become dry. The process of levigation was repeated six times to obtain Āmalaki Rasāyana (7) and 20 times to obtain Āmalaki Rasāyana (21). The final formulations were packed in sealed containers and stored under ambient conditions. They have been coded as AR 7 (Āmalaki Rasāyana prepared by 7 Bhāvanas) and AR 21 (Āmalaki Rasāyana prepared by 21 Bhāvanas) respectively.
Animals and husbandry conditions
Wistar strain albino rats of either sex weighing 200 ± 35 g were used in the experimental study. Animals were obtained from the animal house attached to the Pharmacology Laboratory of IPGT and RA. Animals were exposed to natural day and night cycles with ideal laboratory conditions in terms of ambient temperature (22 ± 2°C) and humidity (50-60%). They were fed with Amrut brand rat pellet feed supplied by Pranav Agro Industries and tap water given ad libitum. All the experiments were carried out after obtaining permission from “Institutional Animal Ethics Committee” (Approval number: IAEC/09/11/21).
Dose fixation
The human dose Āmalaki Rasāyana is 2 g,[6] which should be administered after addition of honey and ghee. The human dose of the trial drug including adjuvant was 5 g. The animal dose fixation was carried out on the basis of body surface area ratio using the table of Paget and Barnes (1964)[7] and thus obtained dose was 450 mg/kg rat.
Experimental design
The study was carried out as described as per the established methods[8] with slight modifications. The selected animals were divided into different groups [Table 1] randomly, each group comprising of six animals with three male and three female and were housed separately. The test formulations and vehicles were administered to respective groups for nine consecutive days by oral route with the help of suitable sized gastric catheter no. 6 sleeved on to a syringe. On the 3rd day of drug administration, all animals were sensitized with 20% sheep red blood cells (SRBC) subcutaneously in the nape of the neck. For this purpose, the sheep blood was collected from city slaughter-house in a sterilized bottle containing Alsever's solution (2% dextrose, 0.8% sodium citrate, 0.5% citric acid and 0.42% sodium chloride) aseptically to avoid coagulation of blood. The collected sheep blood was thoroughly washed with sterile normal saline through repeated centrifugation until the supernatant fluid became colorless and made to 20% SRBC solution. This sensitizing agent was injected subcutaneously in the dose of 0.5 ml/100 g of body weight to the rats. The immune system was further challenged with two doses of Cyclophosphamide (80 mg/kg), first on the 4th day and the second on the 6th day to 2nd to 5th groups of animals. On the 10th day, the body weight of the animals was recorded. Blood was collected in two separate sets of tubes by puncturing supraorbital plexus by capillary tubes under ether anesthesia for estimation of hematological and biochemical parameters as well as antibody titer estimation. Then, the animals were sacrificed by over dose of ether anesthesia. Thymus, spleen, lymph nodes, testis and kidney were dissected out from the animals, cleaned out, pressed between the folds of filter paper for moisture absorption and then weighed in a digital balance (excluding lymph nodes and testis) and transferred to 10% formaldehyde solution for histopathological studies. The histopathological slides were prepared by referring standard procedure.[9] The slides were viewed under binocular research Carl-Zeiss's microscope (Germany) at various magnifications to note down the changes in the microscopic features of the tissues studied.
Table 1.
Animal groupings
Hematological parameters
To estimate hematological parameters, 0.08 ml blood was mixed with 0.02 ml of ethylenediaminetetraacetic acid (33.33 mg/ml) and fed to the auto analyzer (Sismes KX-21, Trans Asia) and parameters such as hemoglobin content, total red blood cells count, total white blood cells (WBC) count, differential WBC count and packed cell volume were estimated.
Serum biochemical parameters
For estimation of biochemical parameters, serum was separated from collected blood and requisite quantity of serum was fed to the auto analyzer (fully automated Biochemical Random Access Analyzer, BS-200; Lilac Medicare Pvt. Ltd., Mumbai), which was automatically drawn in to the instrument for estimating different parameters such as blood urea,[10] serum creatinine,[11] serum alkaline phosphatase[12] and serum glutamate pyruvate transaminase.[13] References given in the kit literature mentioning the basis of the methods on which test procedures were carried out.
Estimation of hemagglutination titer
Serum separated from the blood and the complements were inactivated by incubating for 30 min at 56°C in a serological water bath. Antibody levels were determined by the hemagglutination technique.[14] The microtiter plate was filled with 0.1 ml sterile normal saline and serial two fold dilutions of 0.1 ml of the serum in sterile saline solution were made in the microtiter plate. A total volume of 0.1 ml of thrice saline washed 3% SRBC was added to each well of the microtiter plate. SRBC from the same animal (sheep) was used for both sensitization and to determine antibody titer. The plate was allowed to settle down in the refrigerator overnight until the control wells showed unequivocally negative pattern (a small button formation). The value of the highest serum dilution showing visible hemagglutination was taken as antibody titer and was converted to log2 values for easy comparison.
Statistical analysis
For comparison of normal control group to cyclophosphamide control group student's unpaired t-test has been employed. For comparison of data from cyclophosphamide control group with remaining cyclophosphamide plus test drug administered groups one- way ANOVA with DMTT was employed. A ‘P’ value less than 0.05 was considered as statistically significant.
RESULTS AND DISCUSSION
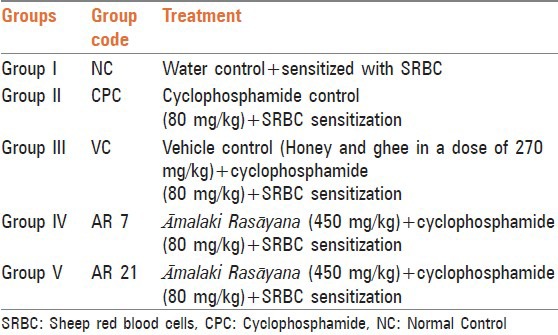
Reduction in body weight of cyclophosphamide treated rats after 10 days of exposure is indicative of impending toxicity. This was expected because of its tendency to produce cytotoxicity, especially in fast multiplying cells. The cytotoxicity in the gut may interfere with the absorption of nutrients. Treatment with vehicle and AR7 failed to attenuate toxicant induced weight change while treatment with AR21 remarkably attenuated cyclophosphamide induced weight loss, i.e., almost 50% reduction in the toxicant induced weight loss [Table 2], but the reversal did not reach statistically significant level.
Table 2.
Effect on body weight
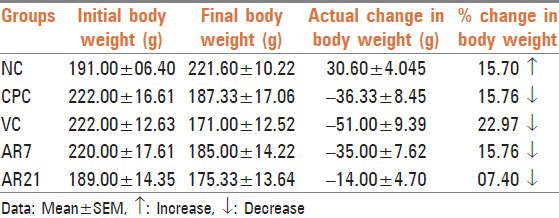
Administration of cyclophosphamide non-significantly decreased the weight of spleen and thymus [Table 3]. The observed decrease may be due to toxic effect of cyclophosphamide as reported earlier.[15] Treatment with vehicle and AR7 failed to attenuate cyclophosphamide induced weight change in different organs while treatment with AR21 apparently attenuated the weight loss observed in thymus, kidney and spleen. The observed attenuation in the thymus and kidney weight loss was found to be statistically non-significant. The observed protection in thymus may be due to stimulation of the gland activity. Recovery in kidney weight indicates nephroprotective activity of this formulation against the cyclophosphamide induced injury. These observations were further supported by histopathological examination.
Table 3.
Effect on weight of thymus, spleen and kidney
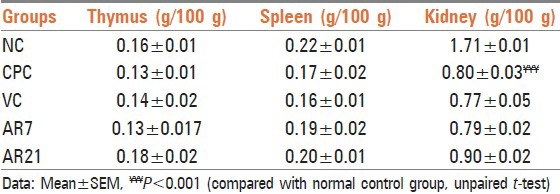
The antibody titer in vehicle and drug treated groups were found to be higher than those observed with cyclophosphamide control group. However, only the increase observed in the vehicle group was found to be statistically significant [Table 4]. It has been well-established fact that both Madhu[16–18] and Ghrita[19,20] have immune potentiating properties. This may be the reason for the attenuation of the immunosuppression effect of cyclophosphamide.
Table 4.
Effect on hemagglutination titer
Administration of cyclophosphamide leads to a significant decrease in the counts of total WBC, neutrophils, lymphocytes, RBC and platelets [Table 5]. This confirms the reported myelosuppressive activity of the toxicant.[21,22] Treatment with vehicle and test formulations non-significantly attenuated almost all the cyclophosphamide induced changes in hematological parameters.
Table 5.
Effect on hematological parameters
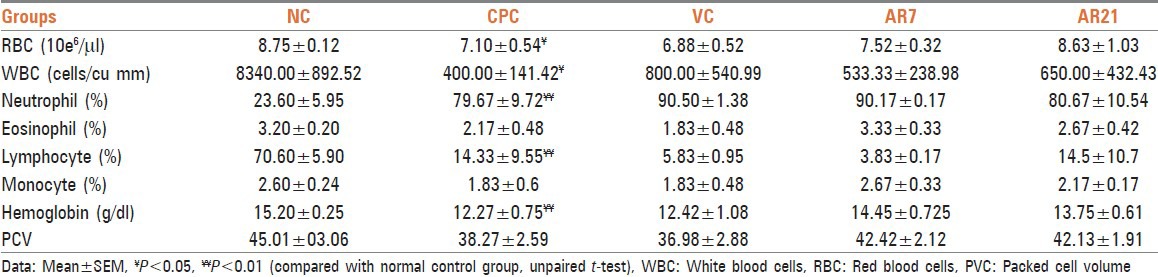
Administration of cyclophosphamide leads to significant elevation of blood urea and serum glutamic-pyruvic transaminase (SGPT) activity in comparison with the control group [Table 6]. Administration of vehicle, AR7 and AR21 non-significantly attenuated these parameters. Further cyclophosphamide administration leads to non-significant decrease in alkaline phosphatase activity. Treatment with vehicle and AR7 non-significantly attenuated this parameter while AR21 significantly attenuated alkaline phosphatase activity, elevated level of SGPT and decreased alkaline phosphatase activity may be attributed to acute hepatotoxicity induced by cyclophosphamide. It is evident by results that AR21 drug is able to reverse the hepatotoxicity produced by cyclophosphamide.
Table 6.
Effect on serum biochemical parameters
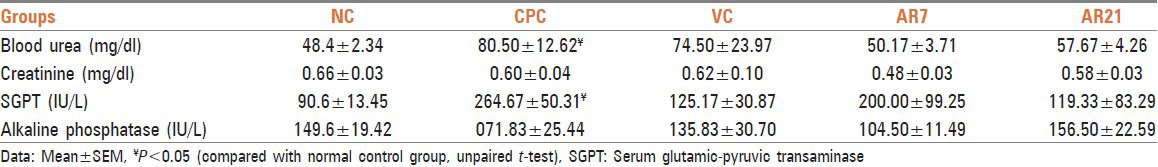
Administration of cyclophosphamide led to marked cell depletion in lymph nodes. Treatment with vehicle failed to reverse cyclophosphamide induced pathological changes while AR7 and AR21 markedly attenuated toxicant induced pathological changes [Figure 1]. Administration of cyclophosphamide leads to cell depletion and decreased white cell proportions in the spleen. Treatment with vehicle showed mild attenuation of cyclophosphamide induced pathological changes while treatment with AR7 remarkably and AR21 mildly reversed the cyclophosphamide induced pathological changes in splenic sections [Figure 2]. Kidney sections from the cyclophosphamide administered control groups showed mild fatty changes and glomerular congestion while sections of kidney treated with vehicle and all test drugs shows normal cytoarchitecture [Figure 3]. Administration of cyclophosphamide leads to the marked decrease in spermatogenesis in cyclophosphamide control group. Treatment with vehicle shows moderate attenuation of cyclophosphamide induced decreased spermatogenesis while treatment with both test formulations showed marked attenuation of cyclophosphamide induced changes [Figure 4]. Analysis of data pertaining to histopathological study from treated groups shows AR7 and AR21 is having marked cytoprotective activity by attenuating pathological changes in almost all the organs studied. Ghrita-Madhu, the vehicle, which showed excellent immune-stimulating activity in immunosuppressed rats failed to show significant cytoprotective activity of immunological organs. However, it proved to be protective against cyclophosphamide induced nephrotoxicity and testicular injury.
Figure 1.
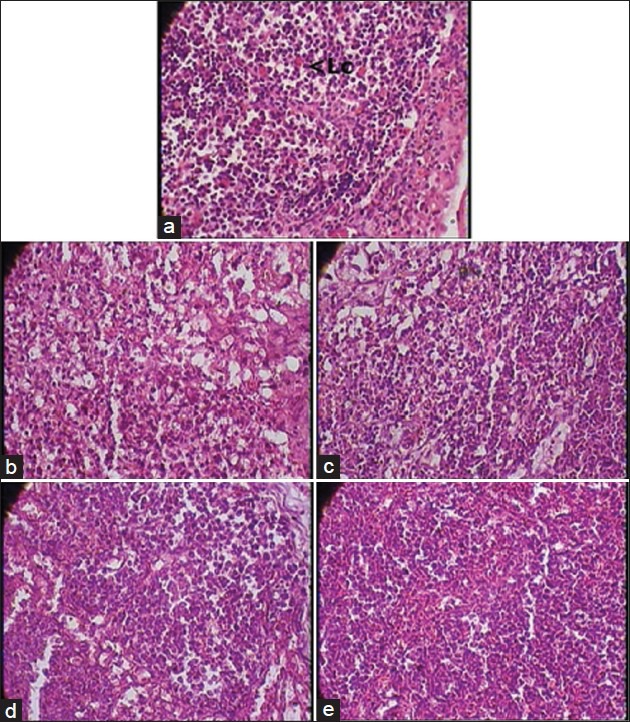
(a) Lymph node - Normal control group (1 × 400 magnification) Lc - Lymphocytes. Note: Normal cytoarchitecture. (b) Lymph node - Cyclophosphamide control group (1 × 400 magnification). Note: Marked cell depletion. (c) Lymph node - Vehicle plus cyclophosphamide group (1 × 400 magnification). Note: Marked cell depletion. (d) Lymph node - AR7 plus cyclophosphamide group (1 × 400 magnification). Note: Marked attenuation of cyclophosphamide induced changes. (e) Lymph node - AR21 plus cyclophosphamide group (1 × 400 magnification). Note: Marked attenuation of cyclophosphamide induced changes
Figure 2.
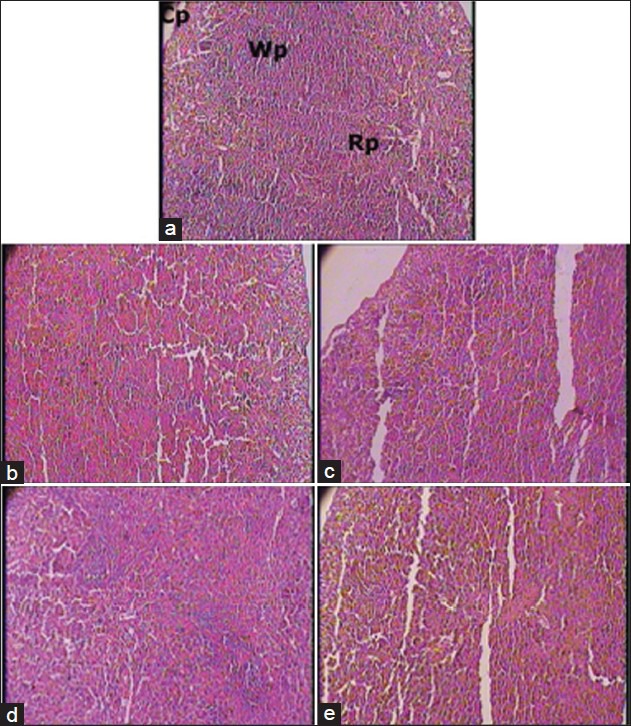
(a) Spleen - Normal control group (1 × 100 magnification). Rp - Red pulp; Wp - White pulp; Cp apsule. Note: Normal cytoarchitecture. (b) Spleen - Cyclophosphamide control group (1 × 100 magnification). Note: Cell depletion and decrease in white pulp proportion. (c) Spleen - Vehicle plus cyclophosphamide group (1 × 100 magnification). Note: Mild attenuation of cyclophosphamide induced changes. (d) Spleen - AR7 plus cyclophosphamide group (1 × 100 magnification). Note: Marked attenuation of cyclophosphamide induced changes. (e) Spleen - AR21 plus cyclophosphamide group (1 × 100 magnification). Note: Mild attenuation of cyclophosphamide induced changes
Figure 3.
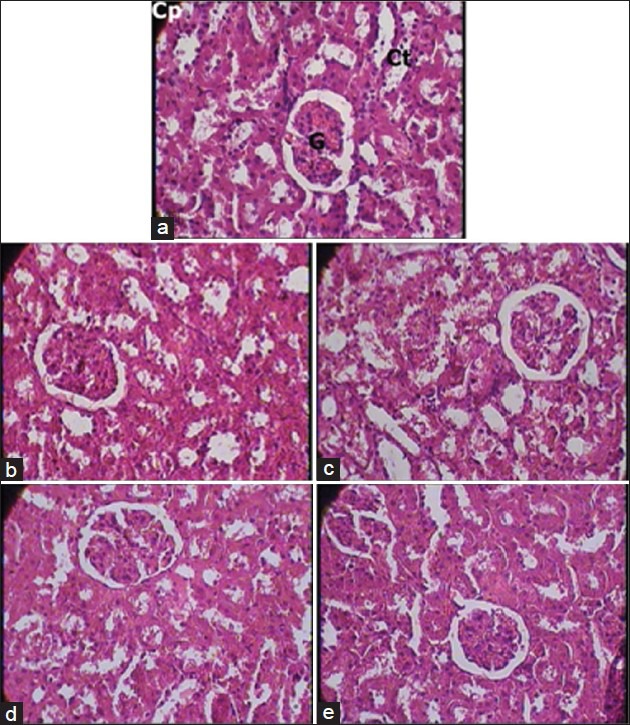
(a) Kidney - Normal control group (1 × 400 magnification). Cp - Capsule; G - Glomerulus; Ct - Convoluted tubule. Note: Normal cytoarchitecture. (b) Kidney - Cyclophosphamide control group (1 × 400 magnification). Note: Mild fatty changes and glomerular congestion. (c) Kidney - Vehicle plus cyclophosphamide group (1 × 400 magnification). Note: Normal cytoarchitecture. (d) Kidney - AR7 plus cyclophosphamide group (1 × 400 magnification). Note: Normal cytoarchitecture. (e) Kidney - AR21 plus cyclophosphamide group (1 × 400 magnification). Note: Normal cytoarchitecture
Figure 4.
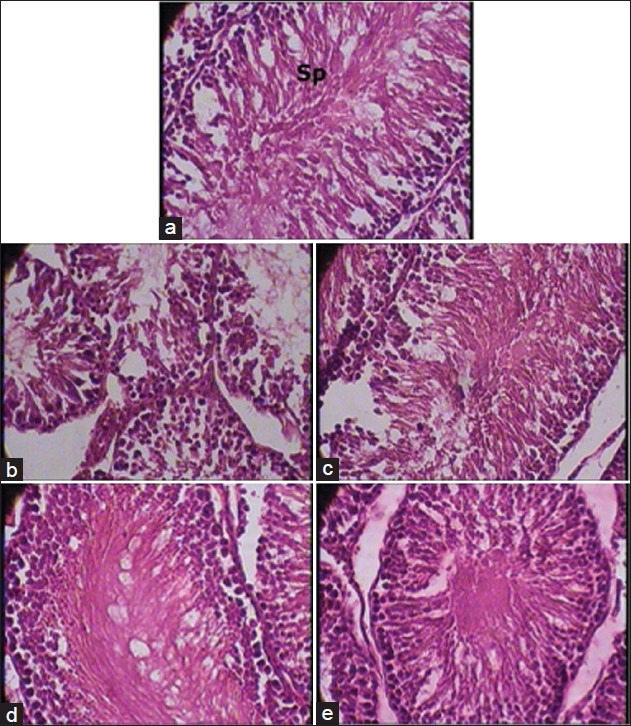
(a) Testis - Normal control group (1 × 400 magnification). Sp - Sperms. Note: Normal cytoarchitecture. (b) Testis - Cyclophosphamide control group (1 × 400 magnification). Note: Marked decrease in spermatogenesis. (c) Testis - Vehicle plus cyclophosphamide group (1 × 400 magnification). Note: Mild attenuation of cyclophosphamide induced changes. (d) Testis - AR7 plus cyclophosphamide group (1 × 400 magnification). Note: Marked attenuation of cyclophosphamide induced changes. (e) Testis - AR21 plus cyclophosphamide group (1 × 400 magnification). Note: Marked attenuation of cyclophosphamide induced changes
The results obtained show that as cytoprotective agent, the test formulation showed much better effect in comparison with the improvement noted in vehicle treated given group. This is evident from the analysis of histopathological investigations and changes in the biochemical parameters. However, with respect to immunoprotection the adjuvants given to vehicle control group showed better effect in comparison to the test formulation. The exact reason is not known; however, it can be suggested that immune-modulation is mediated by different sets of cytokines, which are significantly influenced the contents of the adjuvants given to the vehicle control group. This confirms the earlier reports of presence of significant immune-modulation effect in Ghrita based preparations. The cytoprotective activity may be due to modulation of the activity of different set of cytokines like tumor necrosis factor-α that may have a major role in causing tissue degeneration.
CONCLUSION
Āmalaki Rasāyana possesses significant immunostimulant activity and moderate cytoprotective activity. Among AR7 and AR21 formulations; AR21 was found to have better activity profile in terms of both immunostimulant as well as cytoprotective activity. Hence, it can be concluded that it is better to give 21 Bhāvanas to get desired pharmacological activity.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Singh G. New Delhi: 1999. Immunity (Vyādhikshamatva) potentiation effect of Rāsayana drugs. In: Proceedings of National Seminar on Rasāyana, 8-10 March 1999, CCRAS, Dept. of AYUSH, Ministry of Health and Family Welfare; p. 113. [Google Scholar]
- 2.Bhavamishra . Bhavaprakasha Nighantu. Haritakyadi Varga-39. In: Mishra BS, Vaisya RL, editors. 10th ed. Varanasi: Chaukhambha Sanskrit Sansthan; 2002. p. 10. [Google Scholar]
- 3.Biswas S, Talukder G, Sharma A. Protection against cytotoxic effects of arsenic by dietary supplementation with crude extract of Emblica officinalis fruit. Phytother Res. 1999;13:513–6. doi: 10.1002/(sici)1099-1573(199909)13:6<513::aid-ptr525>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Dwivedi V, Anandan EM, Mony RS, Muraleedharan TS, Valiathan MS, Mutsuddi M, et al. In vivo effects of traditional Ayurvedic formulations in Drosophila melanogaster model relate with therapeutic applications. PLoS One. 2012;7:e37113. doi: 10.1371/journal.pone.0037113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nischalakara . Chakradatta-Ratnaprabha. In: Sharma PV, editor. 1st ed. Jaipur: Swami Jayaramdas Ramprakash Trust; 1993. pp. 791–2. [Google Scholar]
- 6.11th ed. Kaleda: Krishna Gopal Ayurved Bhavan; 2006. Rasatantra Sara Siddhaprayoga Samgraha, Rasāyana; p. 570. [Google Scholar]
- 7.Paget GE, Barnes JM. Evaluation of drug activities. In: Lawrence DR, Bacharach AL, editors. Pharmacometrics. Vol. 1. New York: Academic Press; 1964. p. 161. [Google Scholar]
- 8.De P, Dasgupta SC, Gomes A. Immunopotentiating activity of immune-21-A polyherbal product. Indian J Pharmacol. 1998;30:163–8. [Google Scholar]
- 9.Raghuramulu N, Nair KM, Kalyanasundaram S. Hyderabad, India: National Institute of Nutrition; 1983. A Manual of Laboratory Techniques; pp. 246–53. [Google Scholar]
- 10.Tiffany, Talke HN, Schubert GE. Influence of non-enzymatic antioxidants on antioxidation status in acute haemorrhagic Necrotizing pancreatitis in rat. Bull Vet Inst Pulawy. 2005;49:133–9. [Google Scholar]
- 11.Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17:381–7. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson JH, Boutwell JH, Winsten S. Evaluation of a new system for the kinetic measurement of serum alkaline phosphatase. Clin Chem. 1969;15:487–95. [PubMed] [Google Scholar]
- 13.Burtis CA, Ashwood ER. Tietz Textbook of Clinical Chemisry. In: Moss DW, Henderson AR, editors. 3rd ed. Philadelphia, PA: W.B. Saunders Company; 1999. p. 652. [Google Scholar]
- 14.Khan T, Tatke P, Gabhe SY. Immunological studies on the aerial roots of the Indian banyan. Indian J Pharm Sci. 2008;70:287–91. doi: 10.4103/0250-474X.42970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pratheesh K, Kuttan G. Cardiospermum halicacabum inhibits cyclophosphamide induced immunosupression and oxidative stress in mice and also regulates iNOS and COX-2 gene expression in LPS Stimulated macrophages. Asian Pac J Cancer Prev. 2010;11:1245–52. [PubMed] [Google Scholar]
- 16.Marcucci MC. Propolis; chemical composition, biological properties and therapeutical utility. Apidologie. 1995;26:83–9. [Google Scholar]
- 17.Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Alvarez JA. Functional properties of honey, propolis, and royal jelly. J Food Sci. 2008;73:R117–24. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 18.Kassim M, Mansor M, Achoui M, Yan OS, Devi S, Yusoff KM. Honey as an immunomodulator during sepsis in animal model. Sepsis. 2009;13:16. [Google Scholar]
- 19.Ashok BK. Jamnagar, Gujarat: Gujarat Ayurved University; 2009. A detailed investigation on the gender and formulation influences on the expression of pharmacological activity in Guduchi (Tinospora cordifolia (Willd.) Miers). PhD Thesis. [Google Scholar]
- 20.Rajagopala S, Ashok BK, Ravishankar B. Immunomodulatory activity of Vachadhatryadi avaleha in albino rats. Ayu. 2011;32:275–8. doi: 10.4103/0974-8520.92546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bone marrow suppression. [Retrieved on 2011 Mar 05]. Available from: http://www.medical dictionary.thefreedictionary.com/bone+marrow+suppression .
- 22.Xie RF. Effect of cyclophosphamide on the bone marrow hematopoiesis in the mouse. Zhonghua Zhong Liu Za Zhi. 1985;7:435–7. [PubMed] [Google Scholar]


