Abstract
Humanized mouse models have, over the past few years, seen dramatic improvements, including the colonization of both lymphoid and nonlymphoid tissues with all major immune cell lineages, the development of T cells with human major histocompatibility complex restriction, and the ability to mount functional adaptive immune responses to human pathogens, as documented in some instances. This has greatly increased the range of questions related to the biology of human immunodeficiency virus (HIV) infection that can be usefully addressed through experimental approaches utilizing small animal models. Among these approaches is in vivo imaging, and specifically multiphoton intravital microscopy (MP-IVM), which allows for the investigation of dynamic biological processes at cellular and subcellular resolution in the tissues of live animals.
We have recently begun to use MP-IVM in lymph nodes of humanized mice in order to examine HIV infectious spread in vivo at the tissue and cellular level. Here, we provide a short perspective on the close link between the patterns of immune cell migration and the mechanisms of viral dissemination, and summarize the results of our initial studies.
Keywords: HIV-1, lymph node, multiphoton intravital microscopy, T-cell trafficking
The human acquired immunodeficiency syndrome (AIDS) is caused by a retrovirus that primarily infects and induces a dramatic decline of the CD4+ T-cell population. This has led to the hypothesis that T-cell tropism may contribute to viral success by facilitating the evasion of antiviral adaptive immune responses. However, the related simian immunodeficiency viruses (SIV) can successfully establish generally nonpathogenic, chronic infections and are endemic in various species of African nonhuman primates [1]. In these natural SIV hosts, CD4+ T-cell depletion is generally not as profound [2], at least unless expansion of coreceptor tropism occurs [3]. This suggests that CD4 entry receptor usage provides other benefits to primate lentiviruses in addition to or instead of immune evasion. One property of T cells that possibly makes them attractive retroviral targets is the dynamic regulation of their activation state. While activation through antigen or interferons makes them permissive for productive infection and viral gene expression, the subsequent return to a resting state enables infected T cells to survive for a very long time while harboring latent provirus. This facilitates the maintenance of stable viral reservoirs, which can be expanded upon renewed T-cell activation [4].
Here, we propose an additional reason why utilization of the CD4 molecule as the primary entry receptor makes primate lentiviruses so successful at establishing systemic infections: the constitutively migratory lifestyle of some of their target cells, most prominently CD4+ T cells, facilitates efficient viral dissemination in the host. We will briefly discuss how the dynamic organization of the immune system makes it a suitable target for human immunodeficiency virus (HIV) (and other pathogens that cause systemic infections), considering the pathways by which HIV can be transferred between susceptible T cells. Then, we will discuss our recent multiphoton intravital microscopy (MP-IVM)–based observations on the behavior of infected T cells in a humanized model of HIV infection in order to provide a perspective on how a closer examination of viral and cellular dynamics at the tissue and organism level may enhance our understanding of the biology of HIV infection.
MIGRATORY ROUTES OF T CELLS AND THEIR INTERACTIONS WITH ANTIGEN-PRESENTING CELLS
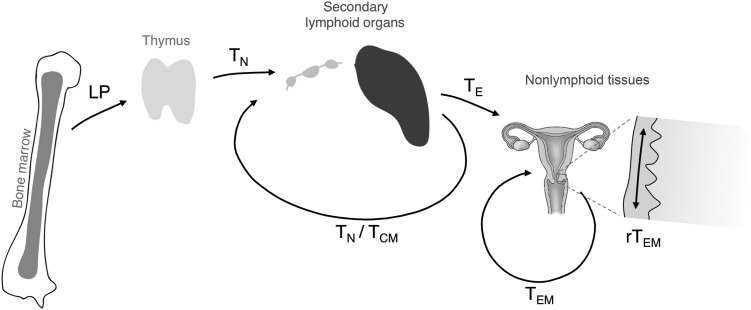
In order to understand why lymphocytes, and specifically T cells, are such attractive targets for viruses that cause systemic infections, some aspects of their physiological behavior should be considered (Figure 1). Conventional αβT cells emerge from the thymus as small clonal populations after a series of selection and lineage specification processes, whose outcome depends on the ability of their newly generated antigen receptors to interact at low affinity with major histocompatibility complex (MHC) molecules presenting self-antigen-derived peptides in the thymus [5]. Outside of the thymus, encounters with their cognate high-affinity antigenic ligands presented by professional antigen-presenting cells (APCs) lead to cellular activation [6]. Under appropriate circumstances, this can induce their differentiation into effector T cells, characterized by the acquisition of various cellular effector functions [7]. These importantly contribute to maintaining homeostasis at our interfaces with the microbial world, such as the epithelial barrier tissues of skin, lung, and gastrointestinal and genitourinary tracts.
Figure 1.
Migratory pathways of naive and antigen-experienced T cells. Lymphoid progenitors (LP) derived from bone marrow–resident hematopoietic stem cells colonize the thymus. Naive T cells (TN) emerge from thymic selection based on the function of their newly acquired T-cell receptors and recirculate through secondary lymphoid organs (SLOs) via the blood and the lymph. Encounters with cognate antigen can induce their differentiation into effector T cells (TE) that are capable of entering inflamed nonlymphoid tissues. Upon contraction of the T-cell response, various populations of memory T cells are left behind, including central memory T cells (TCM) that again recirculate through SLOs, effector memory T cells (TEM) that also recirculate though nonlymphoid tissues, and resident effector memory T cells (rTEM) that do not recirculate but remain local and survey the epithelia of the skin and mucosal tissues.
Due to the small number of T cells specific for any particular antigenic epitope (on the order of a few thousand in humans), finding its rare cognate antigen in the vast expanse of our body represents a “needle-in-the-haystack” problem for an individual T cell [8]. Naive T cells (TN) meet this challenge by restricting their surveillance to secondary lymphoid organs (SLOs), such as lymph nodes, where tissue-derived, lymph-borne antigens are concentrated and presented by dendritic cells (DCs) and other APCs [8]. Lymphocytes enter lymph nodes from the bloodstream via high endothelial venules, and survey it through continuous migration along the fibroblastic reticular cell network and by scanning the surface of DCs, which preferentially reside on the branching points of this network [9, 10]. Dynamic imaging studies in explanted lymph nodes have suggested that 1 DC interacts with on the order of 500–5000 T cells per hour [11, 12]. After typically 6–18 hours [13, 14], T cells again exit the lymph node and, via the lymph vasculature, travel either to the next in a chain of lymph nodes, or enter the venous circulation in order to access the next SLO after a brief transit through the bloodstream. Assuming that a single T cell spends most of its time in SLOs, it will interact with many thousands of DCs each day, and meanwhile likely form an even larger number of transient contacts with other T cells.
Encounters of TN with DCs presenting their cognate antigen can, when additional signals such as costimulation or cytokines report the likely presence of an acute infection, induce their clonal expansion and differentiation into effector cells (TE) [15, 16]. These cells facilitate immune control and containment or elimination of invading infectious agents. Subsequently, these clonally expanded TE populations contract, but different populations of memory cells are left behind [17]. These provide enhanced local and systemic protection against the reemergence of residual pathogens and against subsequent exposures [18]. So-called central memory T cells (TCM) resume the migration patterns of their naive ancestors, and continue to survey SLOs [19]. In contrast, effector memory T cells (TEM) develop the capacity to enter and recirculate through nonlymphoid, including epithelial tissues, such as the skin and genital and intestinal mucosa [18–21]. Here, they survey APCs preferentially in subepithelial compartments before exiting through efferent lymphatics and draining lymph nodes to again reach the bloodstream [17, 22, 23]. Resident effector memory T cells (rTEM) are a subset of TEM that stably populate nonlymphoid tissues, preferentially the epithelial layers of previously infected environmental interfaces [18, 24–27]. rTEM appear to not recirculate, but they also actively survey the local epithelium for their cognate antigen through migration and probing behavior [25, 28]. Little information is available on the interactions of these memory T-cell populations with various APCs in nonlymphoid tissues, but based on the density of macrophages and DCs in epithelial organs [29], they can be predicted to occur at high frequency.
Collectively, the T-cell lineage represents a dynamic antigen surveillance network, which patrols virtually all tissues of the body, and whose function relies on the maximized motility of its cellular units and their continuous interactions with specialized populations of APCs that are equipped to scavenge pathogens and present their antigens to T cells.
MECHANISMS OF VIRUS TRANSFER BETWEEN CELLS
How do the migratory properties of T cells, as outlined above, make them attractive targets for infection by HIV and other primate lentiviruses? One possible explanation comes from considering the natural obstacles to viral spread in infected hosts.
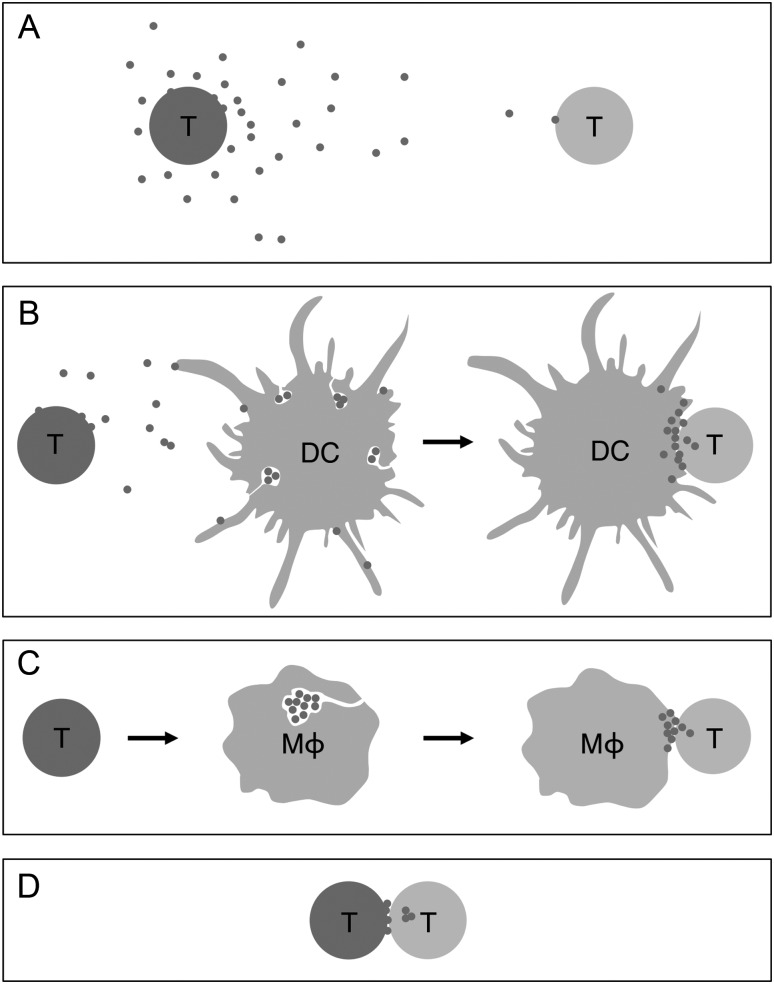
In order for HIV infection to spread from an infected donor cell to an uninfected target T cell, the donor cell must assemble and release viral particles with the capacity to interact and fuse with target cells, followed by the release of viral proteins and RNA into the cytosol for reverse transcription and genomic integration. The oldest (and experimentally most frequently reproduced) model by which this can occur is release of free virions into the extracellular space, where they distribute through the forces of fluid-phase diffusion and convection until, at random, they encounter a target cell (Figure 2A). As a result of their dilution with increasing distance from the donor cells, only very rarely will multiple virions come into simultaneous contact with the same target cell [30]. The efficiency of productive virus transfer through this pathway is low and determined by the intrinsic infectivity of the individual viral particle and by the likelihood that its target cell encounter leads to interaction with both the entry receptor and coreceptor [31]. In addition to the effects of dilution and the low probability of encountering an entry receptor, this mode of virus transfer is further complicated in vivo by (1) the fact that in tissue fluids, virions are exposed to soluble antiviral factors, such as antibodies and complement [32]; (2) the variable, and in some tissues, low spatial density of target cells; and (3) that in order to disseminate within and between organs, the virus has to overcome epi- and endothelial barriers.
Figure 2.
Putative pathways of HIV transfer between T cells in vivo. A, Transfer via release of free extracellular virions. B, Transfer via trans-presentation by uninfected DCs (or macrophages). C, Transfer via delivery from virion stores generated in infected macrophages by budding into deep membrane invaginations. D, Transfer via direct T cell–T cell contact. Green color in A–D indicates productive cellular infection. Abbreviations: DCs, dendritic cells; HIV, human immunodeficiency virus; Mφ, macrophages; T, CD4+ T cells.
A variety of cell contact–mediated mechanisms have been identified that greatly enhance the otherwise low efficiency of HIV spread among T cells in vitro [33]. These mechanisms share the common principles that the concentration of infectious virus at the molecularly structured interfaces between cells increases the viral “payload” per target cell, and that physiological mechanisms of intercellular communication, such as adhesion, cell polarization, and secretion, can be exploited to facilitate virus transfer. Such interfaces have been described between DCs and T cells [34], macrophages and T cells [35, 36], and between T cells [37–39], and have been named virological synapses (VSs), in analogy to the organized molecular assemblies for information transfer between neurons (“neural synapses”) and between lymphocytes and antigen-presenting cells (“immunological synapses”).
Befitting their role as pathogen scavengers, DCs can bind HIV virions via cell surface receptors such as DC-SIGN [40, 41] or Siglec-1 [42] and, without being infected themselves, present these virions to CD4+ T cells during noncognate interactions in a way that recruits HIV entry receptors and coreceptors to the VS [34, 43] (Figure 2B). Macrophages may possess a similar capacity for such trans-presentation to and infection of T cells [44, 45]. In addition, they can store the virions they themselves produce upon productive infection within cell surface invaginations [46–48], and deliver them to the interface with interacting T cells [35] (Figure 2C). By accumulating on the outside of infected and uninfected APCs, HIV may therefore be able to take advantage of the natural propensity of T cells to constantly interact with these APCs in both lymphoid and nonlymphoid tissues, in order to be presented to its target cells in a form that maximizes the chance of productive infection, while being shielded from the perils of the extracellular space.
While T-cell interactions with APCs, such as DCs and macrophages, are well characterized, and the ability of HIV to exploit these interactions seems intuitive, a more recently described type of VS formed between infected and uninfected T cells is more surprising. Here, viral envelop protein (Env) expressed on the surface of HIV-infected T cells triggers rapid recruitment of CD4, CXCR4 or CCR5, and integrin (LFA)–1 on interacting target cells and of Env-Gag coclusters in the donor cell, to an adhesive T cell–T cell contact, followed by transfer of viral Gag into the target cell [37] (Figure 2D). Videomicroscopy analysis supports an endocytotic mechanism of virion uptake by target T cells [49], which can be followed by viral fusion from within the endosome [50]. Because CD4+ T cells constantly interact with each other in SLOs during random encounters, HIV-infected T cells will, if they maintain their physiological migratory behavior, likely be exposed to numerous potential target cells, which may facilitate virus transfer through T-cell VSs.
Finally, as described above, the various T-cell populations collectively recirculate through the entire body, using the blood- and the lymph-stream as transit routes, have the capacity to migrate over large distances, and to overcome physical boundaries within and between tissues, such as the endothelial linings of blood vessels and epithelial barriers. This may include donor-derived infected T cells in the seminal fluid that have been suggested to transmigrate the host genital mucosa during sexual transmission [51]. By infecting migratory T cells, primate lentiviruses likely have found a way to overcome important limitations that restrict their capacity to enter and colonize practically all tissues of the body and thus generate a systemic infection.
In summary, HIV may exploit the physiological behavior of T cells to travel through virtually all organs and to constantly interact with each other and with various APCs, in order to disseminate through the numerous cellular interfaces of this dynamic immune surveillance network.
MULTIPHOTON INTRAVITAL MICROSCOPY TO STUDY IMMUNE CELL MIGRATION IN HUMANIZED MICE
What would be adequate approaches to test the in vivo relevance of free virions compared to cell contact–mediated virus transfer in cis and in trans, for the dissemination of HIV from a mucosal site of entry to the draining lymph node, and from there to remote lymphoid and nonlymphoid tissues, as well as for the perpetuation of infection with these tissues?
Cell contact–mediated virus transfer favors a high multiplicity of infection [30, 52]. Observations in human tissues of T cells that have integrated multiple different HIV genomes, accompanied by extensive viral recombination [53, 54], have consequently been seen as evidence for cell contact–mediated spread in vivo. This principle could be exploited experimentally in humanized mice by tracking the coinfection of T cells with distinguishable reporter viruses [52], which may provide one opportunity to delineate the collective role of various forms of homotypic and heterotypic T-cell interactions for infectious spread in vivo. Second, if unique molecular requirements for the different pathways of viral transfer were known (eg, a specific scavenger receptor critically needed for trans-infection by DCs), genetic approaches could be used to test their role (eg, through RNAi-mediated in vivo silencing of relevant genes in animal models) [55]. However, no truly unique requirement for a single host or viral molecular mechanisms, which could be disabled to examine the significance of the different pathways, has been identified. Alternatively, direct visualization of virion behavior and of infectious spread between cells in tissues may provide valuable clues for a better understanding of how primate lentiviruses propagate in vivo.
About 10 years ago, immunologists started to make use of the capabilities of multiphoton microscopy [56, 57]. This imaging modality permits prolonged observations of dynamic cellular processes deep within the light-scattering tissues of live animals and exploration of the migratory behavior and the interplay of different immune cells in the complex environments of lymphoid and nonlymphoid organs [58]. Immunological imaging studies, with a central focus on T-cell migration and their interactions with APCs, have since then generated a new understanding of how immune responses are induced and dynamically regulated in complex tissue environments [59]. Given the new opportunities provided by recent improvements of humanized mouse models [60–62], we therefore considered that MP-IVM may also be a useful tool to test some of the basic premises of cell contact–mediated viral spread in an animal model of HIV infection.
MIGRATORY PROPERTIES OF HIV-INFECTED T CELLS
Live-cell imaging studies have described HIV transfer through VSs between T cells to occur during stable cell-cell interactions that typically last for several hours in vitro. During these interactions, green fluorescent protein (GFP)–labeled Gag molecules, including those incorporating into virions, accumulate in infected cells at the cellular contact interface to form large clusters (so called “buttons”), which are eventually taken up by target cells through a process resembling endocytosis [49]. However, the observation of such prolonged, stationary T cell–T cell contacts in this study contrasted with our own observations and those of others that, in the absence of their cognate antigen, T cells continually migrate, both in lymph nodes [11, 63–65] and in epithelial tissues [25], and form only dynamic contacts with each other that last not more than a few minutes [66]. We therefore hypothesized that for VSs to form, HIV may alter the properties of infected cells in ways that favors the formation of stable contacts with target T cells.
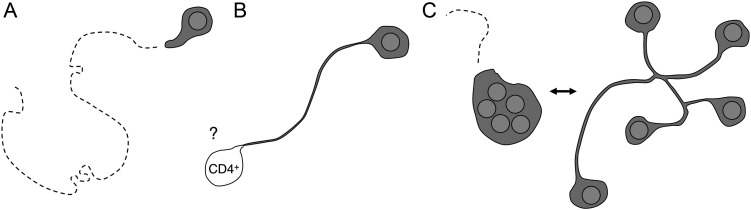
To test this hypothesis, we conducted intravital microscopy studies in lymph nodes of bone marrow-liver-thymus (BLT)–humanized mice generated on the nonobese diabetic/severe combined immunodeficient (NOD/SCID) background (see below for a discussion of this mouse model) [67]. An HIVNL4-3-derived reporter virus that expresses GFP from a Nef–internal ribosome entry site (IRES)–GFP bicistronic transcript [68] was rendered R5-tropic by replacing parts of the V3 loop region of the X4-tropic envelop of HIVNL4-3 with that of HIVBAL. Footpad injection of this reporter virus allowed us to track productively infected cells in the draining popliteal lymph nodes of BLT mice [69]. Two days after subcutaneous inoculation of a large number of virions in a way that likely delivers them directly to the lymph node, practically all detectably infected cells were T cells, comprising a mixture of both CCR7high and CCR7low, CD45RO+, antigen-experienced phenotypes. Surprisingly, we found that while the average migration speed of infected T cells was reduced, most of the cells migrated with otherwise similar characteristics as their uninfected counterparts, and efficiently roamed the lymph node cortex (Figure 3A).
Figure 3.
Behavior of HIV-infected T cells in lymph nodes of BLT-humanized mice. A, Infected T cells migrate at reduced speeds, but effectively roam the lymph node cortex. B, Env-dependent adhesion causes tethering to unidentified CD4+ lymph node cells. C, Motile multinucleated syncytia alternate between coordinated and uncoordinated migration. The latter leads to the transient formation of long membrane processes that connect the individual nuclei. Abbreviations: BLT, bone marrow-liver-thymus; HIV, human immunodeficiency virus.
Expression of GFP is expected not to begin until at least 8–12 hours after the initial contact between a target cell with a productively infecting virion [70, 71]. Robust migratory activity of GFP-expressing cells therefore suggests that after virus uptake, subsequently infected T cells have ample opportunity to egress from the lymph node and, following their natural recirculation patterns, traffic to other lymphoid or nonlymphoid tissue before beginning to assemble and release the next generation of virions. To test the hypothesis that the trafficking of infected T cells contributes to systemic viral spread, we pharmacologically prevented T-cell egress from lymph nodes through treatment of mice with the S1P-receptor functional antagonist FTY720. We started treatment either before subcutaneous infection, or when a systemic infection had already been established. While delayed FTY720 treatment had no effect on preexisting plasma viremia, treatment before infection prevented viremia for several weeks, likely reflecting inefficient systemic spread. Importantly, this treatment did not eliminate the virus, because viremia rapidly developed once the drug was discontinued [69]. We concluded from this that an FTY720-sensitive, recirculating cell population likely serves as a carrier for the virus between tissues, and suggest that infected T cells are likely candidates, although we have so far not ruled out alternative mechanisms, such as transport through recirculating B cells that adsorb extracellular virions [72].
INTERACTIONS OF INFECTED T CELLS IN LYMPH NODES
While most infected T cells in lymph nodes migrated at reduced speed but otherwise appeared phenotypically normal, a small fraction of cells exhibited abnormally (up to 200 µm) long, membranous extensions, typically oriented away from their direction of movement [69]. These extensions fully depended on the ability of HIV Env to interact with CD4 proteins, which led us to hypothesize that they might result from adhesive interactions with uninfected CD4+ T cells (Figure 3B). Adhesive interactions of T cells through integrins are normally regulated through chemokine- or antigen-receptor signaling, and are coordinated with their actin-driven motility [73]. Env-mediated adhesive interactions, on the other hand, may not be regulated. Furthermore, although binding by Env appears to trigger a migratory stop signal in CD4-expressing T cells [74], a stop signal has not been described in the Env-expressing donor T cells. This suggests a scenario in which a T cell that has begun to express and expose Env proteins on its cytoplasmic membrane will firmly bind to adjacent CD4-expressing cells. Unlike in the culture dish, however, it will continue to migrate in the chemokine-rich environment of the lymph node, leading to the formation of a long, uropodial membrane tether. Indeed, we did occasionally observe that the apparently blind-ending tethers of infected T cells (putative CD4+ target cells were not visualized) remained fixed in one place for several minutes, but then suddenly retracted, possibly reflecting detachment from a point of adhesion.
Determining if these HIV-induced tethering events represent an in vivo correlate of the T cell–T cell VS described in vitro will require the development and implementation of new fluorescent reporters in MP-IVM studies, including for the visualization of budding HIV virions as well as of target cells. Interestingly, a recent MP-IVM study of the murine γ-retrovirus Friend murine leukemia virus showed that GFP-tagged Gag molecules were polarized in an Env-dependent manner in a subpopulation of infected B cells that stopped migrating, presumably as a result of interactions with target cells expressing the entry receptor murine cationic amino acid transporter (mCAT)-1 [75]. Future MP-IVM studies will hopefully determine if HIV virions behave similarly, and how efficiently they are transferred via direct T cell–T cell contact or via trans-presenting APCs in ways that support infectious spread.
Surprisingly, however, when we visualized the nuclei of HIV-infected T cells, we discovered that the vast majority of elongated cells (which comprised 10%–20% of all GFP-expressing cells) were in fact multinucleated syncytia (Figure 3C) [69]. This was unexpected, because syncytium formation is rarely observed in primary T cells infected by R5-tropic strains of HIV in vitro, and it is thought that the immature Gag lattice [76, 77] as well as the incorporation of tetraspanin molecules in the viral membrane during budding [78, 79] antagonize the fusion process. Syncytium formation was not due to particular properties specific to the HIV-1 strain NL4-3, and did not result from artificially introducing parts of the V3 loop region of the R5 strain HIVBAL into the X4-tropic HIVNL4-3 strain, because we made similar observations with the naturally R5-tropic strain SF162. However, we will need to test whether nonlab-adapted R5 reporter strains generated from isolates of early stages of human HIV infection exhibit the same fusogenic properties in vivo. Also, it will be interesting to investigate if syncytia are merely viral “accidents” of cell contact–mediated spread, or if they provide a potential benefit to the virus.
CURRENT LIMITATIONS OF THE BLT-HUMANIZED MOUSE MODEL
The strengths of the BLT-humanized model [67, 80] (reviewed in [62]) are that (1) human immune cells robustly colonize not only lymphoid but also nonlymphoid tissues, including the genital [81] and gastrointestinal tract [67]; (2) due to their development in the human thymus organoid, human T cells are restricted by human MHC molecules and T-cell responses are therefore predicted to target the same antigenic determinants as in humans; and (3) these animals have been reported to mount both humoral and cellular adaptive immune responses [82], which at least in the case of the cytotoxic T-lymphocyte response, have been shown to be functional in that they exert similar immune selection pressure on HIV-1 as observed in humans [83].
However, this model also exhibits several limitations, which are important to consider in the planning and interpretation of experiments. Notable among these is the xenogeneic graft versus host disease (GvHD)–like syndrome that begins to develop in NOD/SCID recipients in the weeks following full human immune reconstitution [84]. Because hair loss is a sensitive indicator of GvHD in BLT mice (M. Deruaz, A. D. Luster, personal communication), affected animals can be eliminated from studies to avoid the confounding effects of this condition on the course of HIV infection. However, the duration of studies is thereby limited to a few weeks. GvHD is significantly delayed in NOD/SCID animals in which the common cytokine γ-chain has been deleted [84, 85]. This makes longer-term studies feasible [69, 86], but it comes at the expense that skin draining and many other lymph nodes (eg, the iliac lymph nodes draining the female genital tract) are absent or severely hypoplastic in these animals, limiting intravital microscopy studies to other tissues.
A second shortcoming is that it is largely unknown to what extent the manifold molecular interactions between hematopoietic and nonhematopoietic tissue elements are intact in BLT mice [87]. For the purpose of cell migration studies, this issue pertains especially to adhesion and chemoattractant receptors and their ligands. Human T cells are capable of CCR7-dependent lymph-borne migration from skin to the draining lymph node, and their interstitial migration in the lymph node appears to be intact [69]. However, the efficiency of human CD4+ T-cell entry into lymph nodes from the blood is reduced by about half compared to mouse T cells [69], suggesting that either the mechanisms mediating leukocyte rolling (physiologically facilitated by L-selectin) or firm adhesion (mediated by LFA-1) are only partially functional.
A striking histological feature is the incomplete organization of lymphoid tissues in BLT mice. While the stromal architecture of the T-cell area in lymph nodes of BLT mice appears largely normal, and supports T-cell migratory behavior similar to what is observed in mouse lymph nodes [69, 88], primary B-cell follicles are lacking and B-cell numbers are low in lymph nodes [82]. The latter may at least in part result from insufficient activity of the murine B-cell survival factor BLyS on human B cells [89].
These examples highlight that each new experimental study first requires testing the extent to which the relevant interactions between mouse and human tissue are preserved in the BLT-humanized model, and that observations need to be interpreted within the boundaries of these limitations.
CONCLUSION
Initial evidence suggests that primate lentiviruses have evolved to exploit the migratory and interactive behavior of CD4+ T cells to disseminate in their hosts and generate systemic infections. We propose that imaging studies in humanized mice will be useful to characterize the role of various pathways of cell contact–mediated viral spread as it occurs in the tissues of living animals. Many caveats need to be considered, given the chimeric nature of these humanized model systems, and the molecular compatibility of the murine–human tissue interface must be tested for each experimental purpose. Ultimately, understanding the mechanisms of viral spread will increase our insight into a variety of events, on which the mechanism of virus transfer has an impact, including the antiviral activities of drugs and antibodies, or the role of viral recombination to the genetic diversity and collective “fitness” of the viral quasispecies [90].
Notes
Financial support. This work was supported by the National Institutes of Health (NIH) grants P01 AI0178897, R01 AI097052, and P30 AI060354 (to T. R. M.); and by a Mass General Hospital (MGH) Executive Committee on Research (ECOR) Tosteson Postdoctoral Fellowship Award, NIH training grant T32 AI007387, Harvard University CFAR grant NIH 5P30AI060354–09, and a pilot grant from the Center for Human Immunology NIH U19 AI082630 (to T. T. M.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
References
- 1.Paiardini M, Pandrea I, Apetrei C, Silvestri G. Lessons learned from the natural hosts of HIV-related viruses. Annu Rev Med. 2009;60:485–95. doi: 10.1146/annurev.med.60.041807.123753. [DOI] [PubMed] [Google Scholar]
- 2.Silvestri G, Paiardini M, Pandrea I, Lederman MM, Sodora DL. Understanding the benign nature of SIV infection in natural hosts. J Clin Invest. 2007;117:3148–54. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milush JM, Reeves JD, Gordon SN, et al. Virally induced CD4+ T cell depletion is not sufficient to induce AIDS in a natural host. J Immunol. 2007;179:3047–56. doi: 10.4049/jimmunol.179.5.3047. [DOI] [PubMed] [Google Scholar]
- 4.Siliciano RF, Greene WC. HIV latency. Cold Spring Harb Perspect Med. 2011;1:a007096. doi: 10.1101/cshperspect.a007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–76. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 6.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nature Reviews Immunology. 2003;3:867–78. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 9.Bajenoff M, Egen JG, Koo LY, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mempel TR, Junt T, von Andrian UH. Rulers over randomness: stroma cells guide lymphocyte migration in lymph nodes. Immunity. 2006;25:867–9. doi: 10.1016/j.immuni.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Bousso P, Robey E. Dynamics of CD8(+) T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–85. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 12.Miller MJ, Hejazi AS, Wei SH, Cahalan MD, Parker I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc Natl Acad Sci USA. 2004;101:998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grigorova IL, Panteleev M, Cyster JG. Lymph node cortical sinus organization and relationship to lymphocyte egress dynamics and antigen exposure. Proc Natl Acad Sci USA. 2010;107:20447–52. doi: 10.1073/pnas.1009968107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandl JN, Liou R, Klauschen F, et al. Quantification of lymph node transit times reveals differences in antigen surveillance strategies of naive CD4+ and CD8+ T cells. Proc Natl Acad Sci USA. 2012;109:18036–41. doi: 10.1073/pnas.1211717109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arens R, Schoenberger SP. Plasticity in programming of effector and memory CD8 T-cell formation. Immunol Rev. 2010;235:190–205. doi: 10.1111/j.0105-2896.2010.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sprent J, Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551–79. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 17.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–5. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 18.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2012;31:137–61. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 20.Sheridan BS, Lefrancois L. Regional and mucosal memory T cells. Nat Immunol. 2011;12:485–91. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gebhardt T, Mueller SN, Heath WR, Carbone FR. Peripheral tissue surveillance and residency by memory T cells. Trends Immunol. 2013;34:27–32. doi: 10.1016/j.it.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–7. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 23.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–31. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–30. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 25.Gebhardt T, Whitney PG, Zaid A, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–9. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 26.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA. 2010;107:17872–9. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–20. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 28.Ariotti S, Beltman JB, Chodaczek G, et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci USA. 2012;109:19739–44. doi: 10.1073/pnas.1208927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sigal A, Kim JT, Balazs AB, et al. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature. 2011;477:95–8. doi: 10.1038/nature10347. [DOI] [PubMed] [Google Scholar]
- 31.Zhong P, Agosto LM, Munro JB, Mothes W. Cell-to-cell transmission of viruses. Curr Opin Virol. 2013;3:44–50. doi: 10.1016/j.coviro.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin N, Sattentau Q. Cell-to-cell HIV-1 spread and its implications for immune evasion. Curr Opin HIV AIDS. 2009;4:143–9. doi: 10.1097/COH.0b013e328322f94a. [DOI] [PubMed] [Google Scholar]
- 33.Piguet V, Sattentau Q. Dangerous liaisons at the virological synapse. J Clin Invest. 2004;114:605–10. doi: 10.1172/JCI22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–7. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- 35.Groot F, Welsch S, Sattentau QJ. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood. 2008;111:4660–3. doi: 10.1182/blood-2007-12-130070. [DOI] [PubMed] [Google Scholar]
- 36.Gousset K, Ablan SD, Coren LV, et al. Real-time visualization of HIV-1 GAG trafficking in infected macrophages. PLoS Pathog. 2008;4:e1000015. doi: 10.1371/journal.ppat.1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004;199:283–93. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanco J, Bosch B, Fernandez-Figueras MT, Barretina J, Clotet B, Este JA. High level of coreceptor-independent HIV transfer induced by contacts between primary CD4 T cells. J Biol Chem. 2004;279:51305–14. doi: 10.1074/jbc.M408547200. [DOI] [PubMed] [Google Scholar]
- 39.Chen P, Hubner W, Spinelli MA, Chen BK. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J Virol. 2007;81:12582–95. doi: 10.1128/JVI.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geijtenbeek TB, Kwon DS, Torensma R, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–97. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 41.Kwon DS, Gregorio G, Bitton N, Hendrickson WA, Littman DR. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity. 2002;16:135–44. doi: 10.1016/s1074-7613(02)00259-5. [DOI] [PubMed] [Google Scholar]
- 42.Izquierdo-Useros N, Lorizate M, Puertas MC, et al. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol. 2012;10:e1001448. doi: 10.1371/journal.pbio.1001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Felts RL, Narayan K, Estes JD, et al. 3D visualization of HIV transfer at the virological synapse between dendritic cells and T cells. Proc Natl Acad Sci USA. 2010;107:13336–41. doi: 10.1073/pnas.1003040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carr JM, Hocking H, Li P, Burrell CJ. Rapid and efficient cell-to-cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology. 1999;265:319–29. doi: 10.1006/viro.1999.0047. [DOI] [PubMed] [Google Scholar]
- 45.Sharova N, Swingler C, Sharkey M, Stevenson M. Macrophages archive HIV-1 virions for dissemination in trans. Embo J. 2005;24:2481–9. doi: 10.1038/sj.emboj.7600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deneka M, Pelchen-Matthews A, Byland R, Ruiz-Mateos E, Marsh M. In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J Cell Biol. 2007;177:329–41. doi: 10.1083/jcb.200609050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welsch S, Keppler OT, Habermann A, Allespach I, Krijnse-Locker J, Kraeusslich HG. HIV-1 buds predominantly at the plasma membrane of primary human macrophages. PLoS Pathog. 2007;3:e36. doi: 10.1371/journal.ppat.0030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bennett AE, Narayan K, Shi D, et al. Ion-abrasion scanning electron microscopy reveals surface-connected tubular conduits in HIV-infected macrophages. PLoS Pathog. 2009;5:e1000591. doi: 10.1371/journal.ppat.1000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hubner W, McNerney GP, Chen P, et al. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science. 2009;323:1743–7. doi: 10.1126/science.1167525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dale BM, McNerney GP, Thompson DL, et al. Cell-to-cell transfer of HIV-1 via virological synapses leads to endosomal virion maturation that activates viral membrane fusion. Cell Host Microbe. 2011;10:551–62. doi: 10.1016/j.chom.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. 51–90. See the Supplementary data online. [Google Scholar]