Abstract
Genetic strategies to block expression of CCR5, the major co-receptor of human immunodeficiency virus type 1 (HIV-1), are being developed as anti-HIV therapies. For example, human hematopoietic stem/precursor cells (HSPC) can be modified by the transient expression of CCR5-targeted zinc finger nucleases (ZFNs) to generate CCR5-negative cells, which could then give rise to HIV-resistant mature CD4+ T cells following transplantation into patients. The safety and anti-HIV effects of such treatments can be evaluated by transplanting ZFN-treated HSPC into immunodeficient mice, where the extent of human cell engraftment, lineage differentiation and anti-HIV activity arising from the engineered HSPC can be examined. In this way, humanized mice are providing a powerful small animal model for pre-clinical studies of novel anti-HIV therapies.
Keywords: HIV, CCR5, hematopoietic stem cells, zinc finger nucleases, humanized mice
RATIONALE FOR ENGINEERING AN HIV RESISTANT IMMUNE SYSTEM BY CCR5 KNOCKOUT
Human immunodeficiency virus type 1 (HIV-1) infects and kills CD4+ T cells, causing the gross deregulation of the immune system that leads to AIDS. This loss of T cells can be partially compensated for by the regenerative powers of the hematopoietic system, differentiating new T cells from stem and precursor cells, or promoting the peripheral expansion of mature T cells [1]. However, these processes do not occur indefinitely and, in the absence of any intervention, CD4+ T-cell levels will eventually decline to a critical level that triggers immune collapse.
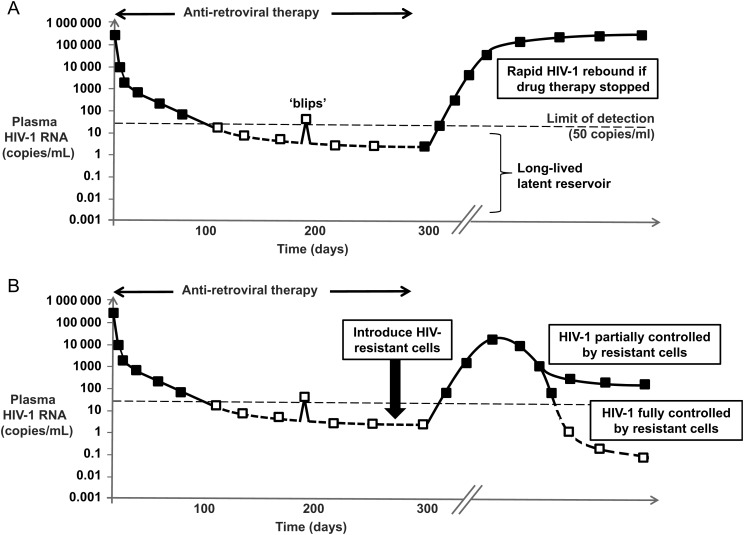
In contrast, patients receiving antiretroviral therapy frequently suppress HIV-1 to almost undetectable levels. But even the most potent drug combinations do not result in the elimination of HIV-1 from the body, and the persistence of a reservoir of latently infected cells that can restart an infection should drug therapy be withdrawn remains a limitation for all current drug treatments [2] (Figure 1A). Consequently, one strategy being pursued to treat HIV-1 infection and its consequences is to engineer HIV-resistant versions of a patient's own T cells and thereby preserve a functional immune system. One aspect of such “functional cure” strategies is that because HIV-resistant cells are expected to be selected for by the actions of the virus itself, any ongoing low-level HIV replication that occurs even in the presence of antiretroviral drugs should help to select for such a resistant population. Additionally, intentional transient breaks from therapy could be used to promote the establishment of such a resistant cell population [3], although the criteria to decide if and when to implement treatment interruptions are unclear at the moment. Either way, the presence of HIV-resistant cells could lead to partial or complete control of HIV-1 replication (Figure 1B).
Figure 1.
A, Schematic showing levels of human immunodeficiency virus type 1 (HIV-1) in blood and a typical response to the initiation of antiretroviral therapy, where virus levels are often suppressed to below the limit of detection in standard assays (shown as open squares). Occasional “blips” of virus replication above this threshold can occur, and a long-lived latent reservoir of uncertain composition persists, that is not cleared by the drug therapy. Upon interruption of therapy, HIV-1 levels rapidly rebound due to this persisting reservoir. B, The introduction of HIV-resistant cells is hypothesized to be able to reduce or even fully control/eradicate HIV-1. Shown is a scenario where antiretroviral drugs are withdrawn as part of a planned treatment interruption to promote the selection of such resistant cells [3], where it is expected that HIV-1 levels could initially increase, until a resistant population is selected that is sufficiently large to exert control.
The creation of HIV-resistant CD4+ T cells requires the selection of a genetic engineering strategy to make the cells resistant, as well as the decision to engineer either mature CD4+ T cells, or the precursor hematopoietic stem/progenitor cells (HSPC) that give rise to them. One approach being pursued for both cell populations is to prevent expression of the CCR5 protein, because this molecule is a major entry coreceptor for HIV-1 [4]. Furthermore, CCR5-negative individuals are highly resistant to HIV-1 [5], while exhibiting minimal negative side effects [6, 7]. CCR5-negative cells can be generated using various techniques, including RNA interference to reduce expression of the protein [8], or site-specific nucleases such as zinc finger nucleases (ZFNs), to knockout the gene's open-reading frame [9]. A striking proof of concept for these approaches has been provided by the case of the “Berlin patient”—an HIV-infected individual who received an HSPC transplant from a CCR5-negative donor following chemotherapy for leukemia and has remained cured of HIV-1 since that event [10–12]. Work in our group and others is building on this clinical finding to disrupt the CCR5 gene in human HSPC using engineered zinc finger nucleases [13–16].
HUMANIZED MICE AS A MODEL SYSTEM TO STUDY HIV-1 INFECTION AND EVALUATE NOVEL GENETIC THERAPIES
HIV-1 is a human disease with limited available animal models, such as challenge of Rhesus macaques or other primates with simian immunodeficiency virus (SIV), or chimeric SIV/HIV (SHIV) viruses. Consequently, immunodeficient mice that contain human CD4+ T cells and support authentic HIV-1 infection are increasingly being used in HIV-1 research (reviewed in [17–19]). A major advantage is that actual clinical reagents can be used in preclinical studies, without requiring modified simian or SIV-specific versions to be created in parallel.
To generate humanized mice suitable for HIV-1 research, human T cells from peripheral blood lymphocytes (PBLs) can be directly introduced into the mice, or they can be derived in vivo following transplantation of human HSPC. The human HSPC are typically obtained from hematopoietic sources such as fetal liver tissue or umbilical cord blood and are isolated based on expression of the CD34 antigen. In general, HSPC based mouse models are more long-lived and less susceptible to graft versus host complications than the PBL models, and robust HIV-1 infections from both CXCR4 and CCR5-tropic viruses can be established and maintained long-term in the mice [14, 20, 21].
Humanized mice also have the advantage of easily providing experimental replicates, because multiple mice can be created from the same donor source of human HSPC. Furthermore, the mice can be engrafted with human HSPC isolated from different hematopoietic sources that are used clinically (cord blood, bone marrow, or mobilized peripheral blood) [22]. Because of these characteristics, humanized mice are especially suited to preclinical modeling of anti-HIV gene or cell based therapies, and recent reports evaluating the efficacy of specific approaches have included a chimeric aptamer-siRNA combination [23], the combination of shRNAs against both CCR5 and HIV [24], HIV-neutralizing antibodies [25, 26], and the triple combination of a trans-activating response element (TAR) decoy, CCR5 shRNA, and a modified cellular restriction factor [27].
HUMANIZED MICE AS A MODEL SYSTEM TO EVALUATE HUMAN HSPC FUNCTION
HSPC are a difficult cell type to culture and maintain as stem cells ex vivo. Although various assays are available to evaluate HSPC function in vitro, transplantation of the cells into immunodeficient mice provides the most rigorous way to do this. In HSPC transplanted mice, human stem cell function can be evaluated both by measuring the extent of engraftment obtained (absolute numbers of human cells), as well as by characterizing the different hematopoietic lineages that develop. Because of this, humanized mice can be especially useful when evaluating the safety of genetic engineering or ex vivo manipulations performed on HSPC, because the animals can also be used to screen for unwanted long-term consequences, such as an impaired ability to support hematopoiesis or the development or tumors. However, it is worth noting that the predictive value of these preclinical mouse models for clinical outcome is not yet entirely clear. Some effort has been made to correlate human HSPC engraftment levels in mice and humans [28, 29], but inter-individual variability between mice receiving cells from different and even from the same donor might limit the usefulness of such correlations [30]. In addition, transplantation studies of baboon CD34+ cells into mice have indicated that distinct cell populations of cells repopulate NOD/SCID mice compared to baboons [31], particularly in respect to long-time engraftment of gene-modified CD34+ cells [32]. The newer humanized mouse models with higher, more robust engraftment of human cells, such as NOD/SCID/IL2rγnull (NSG) mice [33] might perform better as preclinical predictors than the NOD/SCID models, but this remains to be determined.
ESTABLISHING A MOUSE MODEL TO EVALUATE ZFN ENGINEERING OF HSPC ISOLATED FROM MOBILIZED PERIPHERAL BLOOD
We are conducting preclinical studies to evaluate the safety and efficacy of an anti-HIV gene therapy based on CCR5-targeted ZFNs to disrupt the CCR5 gene in mobilized peripheral blood HSPC. These cells represent the adult stem cell population that would be used in a clinical setting. The study builds on observations we initially made with engineered HSPC isolated from cord blood and electroporated with plasmid DNA expressing CCR5-specific ZFNs, followed by transplantation into neonatal NSG mice [14]. This treatment resulted in HSPC with a mean level of CCR5 gene disruption of 17%, and transplantation of the cells into neonatal NSG mice by facial vein injection proved to be an effective system that supported robust CD4+ T cell development from 8 to 12 weeks of age and allowed subsequent HIV-1 infection.
Using the neonatal NSG mouse model, we were able to demonstrate both safety and efficacy of the ZFN treatment. We found that ZFN-engineered cord blood HSPC were unchanged in their ability to engraft NSG mice compared to control HSPC, and the engineered cells had no obvious defects or skewing in their ability to differentiate into multiple lineages of the human immune system. Furthermore, engraftment of the mice with even a minority of CCR5-negative HSPC was able to reduce the replication of CCR5-tropic HIV-1 and ultimately preserve normal human CD4+ T-cell numbers in the animals' blood and tissues [14]. Initially, HIV-1 replicated to high levels in both control and ZFN-treated cohorts, which likely contributed to the selection of CCR5-negative CD4+ cells from the initial mixed population. However, HIV-1 levels in the blood subsequently dropped in the mice receiving ZFN-treated HSPC at later time points, and HIV-1 RNA was undetectable in the gut lymphoid tissue of the mice from the ZFN arm of the experiment when necropsied 10–12 weeks after infection. In addition, almost all of the human T cells present in the gut tissue following HIV-1 infection were found to be CCR5-negative, by both FACS and sequence analysis [14]. Importantly, the CCR5 gene knockout signatures present in the T cells that persisted after HIV-1 infection were highly diverse, indicating that a heterogeneous population had been selected for by the HIV-1 infection. This supports the idea that multiple uniquely modified HSPC had been generated and engrafted into the mice, and that this population was giving rise to the mature CD4+ T cells.
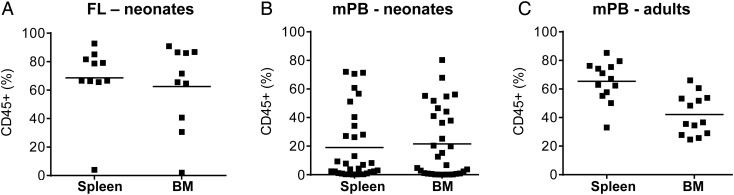
More recently, we have been developing methods to deliver ZFNs to mobilized blood HSPC. An important part of these preclinical studies is to use humanized mice to evaluate whether different ZFN delivery systems impact HSPC biology. To do this, we needed to establish a relevant humanized mouse model with which to acquire information about the baseline properties of unmodified mobilized HSPC. We first tested the protocols we had successfully used with cells isolated from cord blood and fetal liver sources, using facial vein or intrahepatic injection of up to 1 million CD34+ cells into neonatal NSG mice within 4 hours of receiving sublethal (150 cGy) radiation [14]. In contrast to the success of this protocol when using fetal liver HSPC (Figure 2A), we found that adult mobilized blood HSPC did not efficiently engraft the neonatal mice, with less than half of the mice becoming engrafted at levels that gave >1% human CD45+ leucocytes in the blood at any time point, and only very low levels of human cells were observed in hematopoietic tissues at necropsy (Figure 2B). However, we found that intravenous injection of mobilized blood HSPC into adult (8 week-old) NSG mice using a slightly higher irradiation dose (250 cGy) was very successful, giving engraftment rates approaching 100% (Figure 2C), and grafts of human cells that lasted >6 months. The adult NSG mouse transplanted with mobilized HSPC is therefore a suitable model for preclinical studies to evaluate the function of this clinically relevant adult stem cell population.
Figure 2.
Frequency of human CD45+ leucocytes in spleen and bone marrow (BM) of NSG mice transplanted with 1 million human CD34+ HSPC and necropsied at 20 weeks postengraftment. A, Mice transplanted with fetal liver (FL) HSPC as 1-day-old neonates (n = 10). B, Mice transplanted with mobilized peripheral blood (mPB) HSPC as 1-day-old neonates (n = 33). C, Mice transplanted with mPB HSPC as 8-week-old adults (n = 13). Mean levels of engraftment are indicated by a straight line. Abbreviations: HSPC, hematopoietic stem/precursor cells; NSG, NOD/SCID/IL2rγnull.
The availability of this humanized mouse model is allowing us to perform rigorous evaluations of the functionality of ZFN-treated, CCR5-disrupted mobilized blood HSPC. Specifically, we can address whether any aspect of the engineering process, including the ex vivo culture conditions, the ZFN delivery system, expression of the ZFNs, or loss of CCR5 function, has any effect on the ability of the HSPC to engraft, survive, and differentiate in the mice. By comparing the rates of CCR5 disruption present in the input HSPC with the rates detected in the mature human progeny that grow out in the mouse, we can also indirectly assess whether the CCR5-negative progeny, including mature T cells, are produced at the expected frequencies. Using this model, we are evaluating alternate methods to deliver ZFNs to mobilized blood HSPC, including the use of ZFN-expressing adenoviral vectors that are currently being used in clinical trials to modify autologous ex vivo expanded T cells [13, 15]. We determined that pretreating the HSPC with protein kinase C activators increased CCR5 distribution rates to >25%, while still supporting the engraftment of adult NSG mice [16]. However, adenoviral vectors cause toxicity when used at higher levels, so that fine-tuning is needed to obtain the correct balance between CCR5 gene disruption rates and HSPC viability and function. We are currently working with other systems, including mRNA electroporation, as alternate platforms to deliver ZFNs to mobilized blood HSPC.
Because HSPC engrafted NSG mice also develop human CD4+ T cells, they present an opportunity to challenge the animals with HIV-1 and thereby demonstrate antiviral effects within a complex, in vivo environment. However, the relatively longer period of time to develop mature CD4+ T cells, and the lower overall levels obtained when compared to fetal liver or cord blood engrafted mice, make the mobilized blood HSPC/adult NSG mouse model less robust for such efficacy studies [16], which may be better suited to neonatal mice transplanted with cord blood or fetal liver HSPC [14].
Finally, we have found that the high engraftment and survival rate, and long-term persistence of the human graft, makes mobilized HSPC engrafted adult NSG mice suitable for long-term safety studies to assess whether any skewing or restriction of blood lineage development occurs [16], or to monitor for any monoclonal proliferations or tumors that could arise from the transplanted human cells. The polyclonal CCR5-disruption pattern that was observed in our studies using engineered cord blood HSPC suggests that this will not be the case [14], but larger studies are needed to determine a detailed safety profile.
SUMMARY
Immunodeficient mouse strains such as NSG can be readily transplanted with human HSPC and provide both a small animal model that supports HIV-1 replication, as well as a rigorous way to evaluate the function of genetically modified human stem cells. Humanized mice are therefore a useful way to evaluate human HSPC engineered to create an HIV-resistant immune system and a possible functional cure. The different sources of CD34+ HSPC that are available for such preclinical studies (cord blood, fetal liver, and adult mobilized blood) have different characteristics when transplanted into mice, and this should be considered when designing appropriate preclinical studies. In particular, mobilized blood HSPC do not efficiently engraft neonatal NSG mice, and a better outcome is observed with adult recipients. Finally, as is the case in general for animal models, the predictive power of HSPC-engrafted humanized mice remains speculative until matched data are available from patients receiving similarly modified HSPC.
Notes
Financial support. This work was supported by Disease team grant DR1–01490 from the California Institute for Regenerative Medicine, and the National Institutes of Health research grant HL073104. U. H. was supported by a fellowship from the Swiss National Science Foundation, C. E. was supported by a fellowship from the California HIV/AIDS Research Program, and O. M. was supported by a fellowship from the California Institute for Regenerative Medicine.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Boyman O, Purton JF, Surh CD, Sprent J. Cytokines and T-cell homeostasis. Curr Opin Immunol. 2007;19:320–6. doi: 10.1016/j.coi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Finzi D, Blankson J, Siciliano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–7. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 3.Mitsuyasu RT, Merigan TC, Carr A, et al. Phase 2 gene therapy trial of an anti-HIV ribozyme in autologous CD34+ cells. Nat Med. 2009;15:285–92. doi: 10.1038/nm.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu L, Gerard NP, Wyatt R, et al. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;6605:179–83. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 5.Samson M, Libert F, Doranz BJ, et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;6593:722–5. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 6.Glass WG, McDermott DH, Lim JK, et al. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J Exp Med. 2006;203:35–40. doi: 10.1084/jem.20051970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossol M, et al. Negative association of the chemokine receptor CCR5Δ32 polymorphism with systemic inflammatory response, extra-articular symptoms and joint erosion in rheumatoid arthritis. Arthritis Res Ther. 2009;11:91–8. doi: 10.1186/ar2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu S, Hong P, Arumugan B, et al. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood. 2010;115:1534–44. doi: 10.1182/blood-2009-04-215855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urnov FD, Miller CJ, Lee Y-L, et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435:646–51. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]
- 10.Hutter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–8. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 11.Allers K, Hutter G, Hofmann J, Loddenkemper C, Rieger K, Theil E, Schneider T. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood. 2010;10:2791–9. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 12.Yukl SA, Boritz E, Busch M, et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 2013;9:e1003347. doi: 10.1371/journal.ppat.1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez EE, Wang J, Miller JC, et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26:808–16. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holt N, Wang J, Kim K, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28:839–47. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cannon P, June C. Chemokine receptor 5 knockout strategies. Curr OpinHIV AIDS. 2011;6:74–9. doi: 10.1097/COH.0b013e32834122d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Krymskaya L, Wang J, et al. Genomic editing of the HIV co-receptor CCR5 in adult hematopoietic stem and progenitor cells using zinc finger nucleases. Mol Ther. 2013;21:1259–69. doi: 10.1038/mt.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berges BF, Rowan MR. The utility of the new generation of humanized mice to study HIV-1 infection: transmission, prevention, pathogenesis, and treatment. Retrovirology. 2011;8:65. doi: 10.1186/1742-4690-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Su L. HIV-1 immunopathogenesis in humanized mouse models. Cell Mol Immunol. 2012;9:237–44. doi: 10.1038/cmi.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akkina R. New generation humanized mice for virus research: comparative aspects and future prospects. Virology. 2013;435:14–28. doi: 10.1016/j.virol.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baenziger S, Tussiwand R, Schlaepfer L, et al. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2-/-gamma c-/- mice. Proc Natl Acad Sci U S A. 2006;103:15951–6. doi: 10.1073/pnas.0604493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe S, Terashima K, Ohta S, et al. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rgamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood. 2007;109:212–8. doi: 10.1182/blood-2006-04-017681. [DOI] [PubMed] [Google Scholar]
- 22.Lepus CM, Gibson TF, Gerber SA, et al. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/[gamma]c-/-, Balb/c-Rag1-/-[gamma]c-/-, and C.B-17-scid/bg immunodeficient mice. Human Immunol. 2009;70:790–802. doi: 10.1016/j.humimm.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neff CP, Zhou J, Remling L, et al. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4+ T cell decline in humanized mice. Sci Tranl Med. 2011;3 doi: 10.1126/scitranslmed.3001581. 66ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ringpis GE, Shimuzu S, Arokium H, et al. Engineering HIV-1-resistant T-cells from short-hairpin RNA-expressing hematopoietic stem/progenitor cells in humanized BLT mice. PLoS One. 2012;7:e53492. doi: 10.1371/journal.pone.0053492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joseph A, Zheng J, Chien K, et al. Inhibition of in vivo HIV infection in humanized mice by gene therapy of human hematopoietic stem cells with a lentiviral vector encoding a broadly neutralizing anti-HIV antibody. J Virol. 2010;84:6645–53. doi: 10.1128/JVI.02339-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hur EM, Patel SN, Shimuzu S, et al. Inhibitory effect of HIV-specific neutralizing IgA on mucosal transmission of HIV in humanized mice. Blood. 2012;120:4571–82. doi: 10.1182/blood-2012-04-422303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker JE, Chen RX, McGee J, et al. Generation of an HIV-1-resistant immune system with CD34+ hematopoietic stem cells transduced with a triple-combination anti-HIV lentiviral vector. J Virol. 2012;86:5719–29. doi: 10.1128/JVI.06300-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung W, Ramirez M, Novelli EM, Civin CI. In vivo engraftment potential of clinical hematopoietic grafts. J Investig Med. 1998;46:303–11. [PubMed] [Google Scholar]
- 29.Angelopoulou MK, Rinder H, Wang C, Burtness B, Cooper DL, Krause DS. A preclinical xenotransplantation animal model to assess human hematopoietic stem cell engraftment. Transfusion. 2010;44:555–66. doi: 10.1111/j.1537-2995.2004.03285.x. [DOI] [PubMed] [Google Scholar]
- 30.Ballen KK, Valinski H, Greiner D, et al. Variables to predict engraftment of umbilical cord blood into immunodeficient mice: usefulness of the non-obese diabetic—severe combined immunodeficient assay. Br J Haematol. 2001;114:211–8. doi: 10.1046/j.1365-2141.2001.02904.x. [DOI] [PubMed] [Google Scholar]
- 31.Horn PA, Thomasson BM, Wood BL, Andrews RG, Morris JC, Kiem H-P. Distinct hematopoietic stem/progenitor cell populations are responsible for repopulating NOD/SCID mice compared with nonhuman primates. Blood. 2003;102:4329–35. doi: 10.1182/blood-2003-01-0082. [DOI] [PubMed] [Google Scholar]
- 32.Mezquita P, Beard BC, Kiem H-P. NOD/SCID repopulating cells contribute only to short-term repopulation in the baboon. Gene Ther. 2008;15:1460–2. doi: 10.1038/gt.2008.108. [DOI] [PubMed] [Google Scholar]
- 33.Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hematopoietic stem cells. J Immunol. 2005;174:6477–89. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]