Abstract
Introduction:
The present work was aimed at development and evaluation of zidovudin (AZT) loaded gelatin nanoparticles (GNPs) by simple desolvation method and further couple it with mannose.
Material and Methods:
Total seven batches of GNPs (A1-A7) were formulated by changing the concentration of polymer gelatin. Various parameters such as particle size, polydispersity index, zeta potential, % entrapment efficiency and in-vitro drug release of plain and mannosylated gelatin nanoparticles (M-GNPs) were studied.
Results:
Scanning electron microscopy (SEM) studies revealed that the average particle size of GNPs and M-GNPs were found to be 394 ± 3.21 and 797.2 ± 2.89 nm respectively (optimised batch A3). It was interesting to note that the average particle size of M-GNPs was more due to anchored mannose, whereas drug entrapment was lesser compared to plain GNPs. Studies have showed drug loading for GNPs and M-GNPs to be 66.56% and 58.85% respectively. Zeta potential studies demonstrated little reduction in solution stability of M-GNPs compared to GNPs. In-vitro drug release studies showed almost 80% release (bimodal) up to 24 h, following Korsmeyer-Peppas release kinetics model (GNPs, r = 0.9760; M-GNPs, r = 0.9712).
Conclusions:
Hence, it can be concluded that, development of GNPs and M-GNPs will pave the way for reticuloendothelial system uptake of AZT; thus, achieving targeted delivery, selectivity and reduction in associated side effect reduction in acquired immuno defficiency syndrome.
Keywords: Desolvation, mannosylation, particle size, release, reticuloendothelial system uptake, zidovudine nanoparticles.
INTRODUCTION
Zidovudine (AZT) is a drug recommended in the treatment of acquired immuno defficiency syndrome (AIDS) and associated conditions. AZT belongs to the class of antiviral agents known as dideoxynucleoside reverse transcriptase inhibitors and acts by inhibiting human immunodeficiency virus (HIV)-1 reverse transcriptase enzyme thus, interfering with the critical step in the viral life cycle.[1] AZT is rapidly metabolized in the liver to the inactive glucoronide form resulting in poor oral bioavailability.[2]
The most serious and frequent dose dependant side-effects noted with the use of AZT are haematological toxicity, anaemia, neutropenia and thrombocytopenia.[3] To overcome those, target specific particulate drug delivery can be one of the most promising approaches to achieve dose reduction, minimization of systemic toxicity and efficient therapy in the treatment of AIDS.
The reticuloendothelial system (RES) where macrophages are abundant acts as a major reservoir for HIV. Macrophages on their surface possess various receptors such as fucose, mannose, galactose etc.[4] Especially, mannose receptors are present at the surface of monocyte macrophages, alveolar macrophages, astrocytes in the brain and hepatocytes in liver.[5,6,7,8] Hence, using nanoparticulate systems, mannose receptors can be targeted, ultimately the reservoir of HIV.
Literature has revealed numerous attempts demonstrating preparation of AZT nanoparticles. AZT nanoparticles using Poly Lactic-co-Glycolic Acid (PLGA), Polylactic acid-Polyethylene glycol (PLA-PEG) and chitosan are reported.[9,10,11] Comparative studies on lamivudine-AZT nanoparticles for their selective uptake by macrophages have also been reported.[12] For improved anti-retroviral effect/therapeutic efficacy, AZT sustained release matrix tablets have also been prepared demonstrating the need extended release formulation.[13,14] However, attempt is missing to target RES, especially, macrophages, which are HIV reservoir. Targeting such reservoir can be achieved from understanding the fact about recognition of foreign particle having size larger than 200 nm by RES.[15,16] Keeping that in view, attempt has been made to prepare AZT loaded gelatin nanoparticles (GNPs) and mannosylated them to target RES. Gelatin being efficient carrier, aiming its biodegradable, biocompatible, non-toxic, ease of cross linking nature, considerable work on delivery of drugs and proteins is also been evidenced.[17] Considerable work has also been reported on nanoparticles formulation using glutaraldehyde as cross linking agent.[18,19]
The present work deals with formulation of AZT loaded GNPs, its coupling with mannose and characterization for surface topology and polydispersibility, zeta potential, entrapment efficiency (EE), size distribution and in-vitro release drug studies. Coupling of the mannose with AZT loaded GNPs has been confirmed by Fourier transform infrared spectroscopy (FTIR).
MATERIAL AND METHODS
Materials
AZT was provided as a gift sample from Cipla Pvt., Ltd. (Mumbai, India). Sephadex G-50 was procured from Sigma Chemicals (St. Louis, MO). Gelatin and dialysis membrane, (molecular weight cut-off (MWCO) 12-14 kDa) were procured from Himedia (Mumbai, India). Glutaraldehyde and Sodium sulfate and Tween 20 were purchased from Spectrochem Pvt. Ltd. (Mumbai, India). All other reagents and solvents used were purchased from local suppliers unless mentioned. Fresh double-distilled water was used throughout the study.
Methods
Preparation of nanoparticles
GNPs were prepared by two step desolvation technique.[20] GNP batches, A1 to A7 were formulated with different AZT: Gelatin ratio (w/w) as presented in Table 1.
Table 1.
Composition of nanoparticle formulation
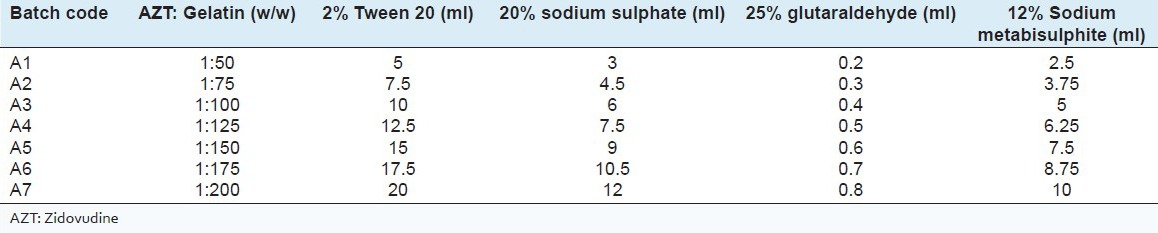
200 mg gelatin was dissolved in distilled water containing 2% w/w Tween 20. The resulting solution was heated at a constant temperature to 40°C and stirred at speed of 300 rpm. To this 2 ml of a 20% w/v aqueous solution of sodium sulphate was added slowly, followed by the addition of 1 ml distilled water containing 2 mg of AZT. 5 ml of sodium sulphate solution was added until the solution turned turbid, which indicated the formation of GNPs. 400 μl of 25% glutaraldehyde solution was added to cross-link the gelatin followed by the addition of 5 ml of 12% sodium metabisulfite solution to stop the cross-linking process. The turbid dispersion was finally purified on a Sephadex G-50 column. The nanoparticle containing fraction was lyophilized over a 48 h period.[21] Freezing of samples was carried out at 0.4°C/min. From 20°C to 54°C for 3 h under atmospheric pressure. Further vacuum was applied for 10 min. followed by primary drying with the pressure reduced to 0.062 mbar at 20°C. Final drying was carried out at a reduced pressure of 0.002 mbar. 2% mannitol was added as a cryoprotectant to improve stability and retain the size of the colloidal particles.
Mannosylation of GNPs
Coupling of mannose to GNPs was performed using the method described by with a few modifications.[22] the method involves ring opening of mannose followed by reaction of its aldehyde group with free amino groups present over the surface of GNPs. Briefly, mannose (8.0 μmol) was dissolved in 0.1 M sodium acetate buffer (0.1 M, pH 4.0) which was further added to GNPs (0.1 μmol), agitated and allowed to stand at ambient temperatures for 2 days. The resulting solution was concentrated under vacuum at 70°C. Mannosylated gelatin nanoparticles (M-GNPs) were purified by dialyzing against double-distilled water in a dialysis tube for 24 h to remove the unreacted mannose, salts, and partially M-GNPs followed by lyophilization. The M-GNPs were characterized by infrared (FTIR) spectroscopy.
CHARACTERISATION OF AZT LOADED NANOPARTICLES
Particle size analysis and polydispersity index (PI)
Mean particle size and PI of GNPs and M-GNPs were determined using the photon correlation spectroscopy (Beckman Coulter Delsa Nano instrument, USA). The analysis was performed at a scattering angle of 90° and a temperature of 25°C.
Scanning electron microscopy (SEM)
The GNPs and M-GNPs were characterised morphologically using SEM (JEOL JSM-6390 LV, USA). Samples were coated with Platinum using Auto Fine Coater for 75 sec at a 40 mA operating current. Thickness of the coating was less than 25 nm. Images were taken using JEOL JSM-6390 LV SEM attached with two turbo pumps, creating high vacuum inside the body of the instrument and a secondary electron detector.
EE%
EE of GNPs and M-GNPs were determined indirectly. Briefly, the AZT loaded nanoparticles were separated from the dispersion using Sephadex G50 column on centrifugation for 10 min at 2500 ± 100 rpm. The supernatant containing the unentrapped drug was diluted with distilled water appropriately and ultrasonicated. The amount of AZT entrapped in nanoparticles was determined spectrophotometrically at 266.8 nm by subtracting the quantity of drug in the supernatant from the total amount used for the preparation using the following equation.[11]
![]()
Zeta potential
Zeta potential of GNPs and M-GNPs were determined using Malvern Zeta-Sizer (Malvern instrument, UK). For the measurement, 100 μl of nanoparticle suspension was diluted to 4 ml with 10 mM NaCl solution, further adjusting the pH to 7.4 using 0.25 N NaOH. An electric field of 150 mV was applied to observe the electrophoretic velocity of the particles. All measurements were made at room temperature (25 ± 2°C) in triplicate and data were taken by setting refractive index to 1.8.
FTIR analysis
A Jasco FTIR spectrophotometer (Jasco FTIR 4100, UK) was used for infrared analysis of AZT, GNPs and M-GNPs. The technique used very small amount of each sample, which was directly loaded into the system. The spectra were obtained over a wave number range from 4000 cm−1-400 cm−1.
In-vitro release studies
In-vitro drug release studies were conducted for cross-linked GNPs and M-GNPs loaded with AZT. GNPs equivalent to 10 mg of AZT were dispersed in 100 ml phosphate buffer saline (release fluid) of pH 7.4 and incubated at 37 ± 0.5°C at a stirring rate of 50 rpm for 24 h. Serial samples of 1 ml were withdrawn at a predetermined time intervals and were used to measure the absorbance at peak-absorption wave length of 266.8 nm using UV-visible spectrophotometer (Jasco V-530 UV). The data obtained were put in Poona college of Pharmacy (PCP) Disso V 3.0 (Pune, India) software to type the drug release kinetics.
RESULTS AND DISCUSSION
It was observed that, GNPs prepared using a two-step desolvation technique showed less aggregation compared to single step desolvation. The obtained nanoparticles were found to be spherical and exhibited small particle size, low PI, and high EE.
Particle size analysis and PI
It was noted that the cross-linking process renders the system having a uniformly round morphology of the drug loaded particles by the self-aggregation of GNPs. SEM studies revealed the smooth surface and spherical shape of GNPs and M-GNPs [Figure 1]. The particle size of M-GNPs was found to be 797.2 ± 2.89 nm, which is much higher than the GNPs, which was found to be 394.5 ± 3.21 nm [Table 2]. This could be due to the anchoring of the mannose molecules at the surface of the nanoparticles. A very low PI of less than 0.1 was obtained for both formulations, indicating a narrow size distribution of the nanoparticles suspension and consequently a homogeneous distribution.
Figure 1.
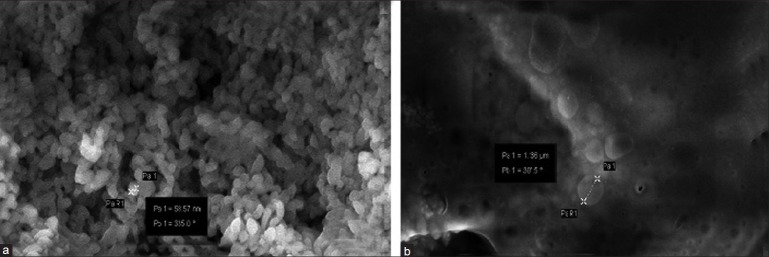
Scanning electron microscopy microphotographs of drug loaded (a) gelatin nanoparticles (magnification × 82.53 K) (b) mannosylated gelatin nanoparticles (magnification × 15.22 K)
Table 2.
Evaluation data for GNPs and M-GNPs*
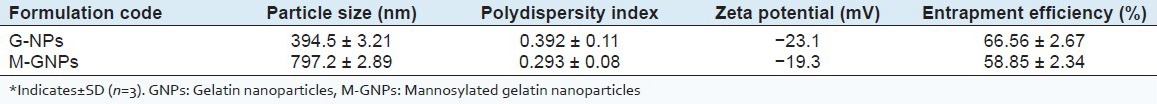
SEM
SEM studies revealed the smooth surface and spherical shape of GNPs and M-GNPs both [Figure 1].
EE%
The percent EE was determined and expressed as the percentage of drugs incorporated to the nanoparticles. Drug loading was determined spectrophotometrically at 266.8 nm. The AZT EE was found to be 66.56 ± 2.67% in GNPs while 58.85 ± 2.34% in M-GNPs [Table 2].
Zeta potential
A high zeta potential confers stability to nanoparticles, i.e., the solution or dispersion resists aggregation and when there is low zeta potential, attraction exceeds repulsion and the dispersion breaks and flocculates means the colloids with high zeta potential (negative or positive) are electrically stabilized while colloids with low zeta potentials tend to coagulate. Zeta potential of the M-GNPs was found to be -19.3 mV while that of the GNPs was found to be -23.1 mV [Table 2]. Thus, the GNPs are found to be more electrically stabilized compared to M-GNPs. the decrease in zeta potential on coupling with mannose may be due to a shielding effect of ions, over the charge present at the surface of the nanoparticles.
Fourier transform infrared analysis
The IR spectrum of AZT showed characteristic peak at the wave number 1109.37 cm−1 indicating the presence of C-N (amine) stretching, 3463.53 cm−1 for the presence of NH stretching, 1685.48 cm−1 for the presence of C=O stretching, 2086.78 cm−1 for the presence of azide group stretching, C-H aromatic at the wave number 2879.48 cm−1, 1469.78 cm−1 for C-H deformation (CH3) and at 1276 cm−1 for C-O stretch. The FTIR spectra of drug loaded GNPs showed weak N-H stretch at 3250-3450 cm−1 and strong N-H bending at 1663 cm−1 that reveal the presence of primary amine group, C = O stretching and azide group stretching at 1667 cm−1 and 2032 cm−1 respectively. The peaks of drug loaded GNPs were similar (but with lesser intensity) to the spectrum of AZT. The peaks of various functional groups as described in the IR spectrum of AZT were also present in the drug loaded GNPs without any shift or change. These observations revealed the intact nature of the AZT present in the nanoparticles. From these results, the absence of drug-polymer interaction and the stability of the loaded drug in the nanoparticles were confirmed. In the FTIR spectra of M-GNPs, weak N-H stretch at 3460-3580 cm−1 and strong N-H bending at 1690 cm−1 revealed the presence of primary amine groups in them. The N-H bending of secondary amines at 1575 cm−1 and C=N stretch at 1505-1465 cm−1 revealed the formation of Schiff's base (RCH=N-R bond), confirming the formation of a linkage between mannose ligand and amine terminal of the nanoparticles. Also, broad strong and intense O-H stretch of mannose at 3710-3580 cm−1 and a strong C-O stretch at 1075 cm−1 proved the presence of hydroxyl groups (of mannose) in large numbers in M-GNPs [Figure 2].
Figure 2.
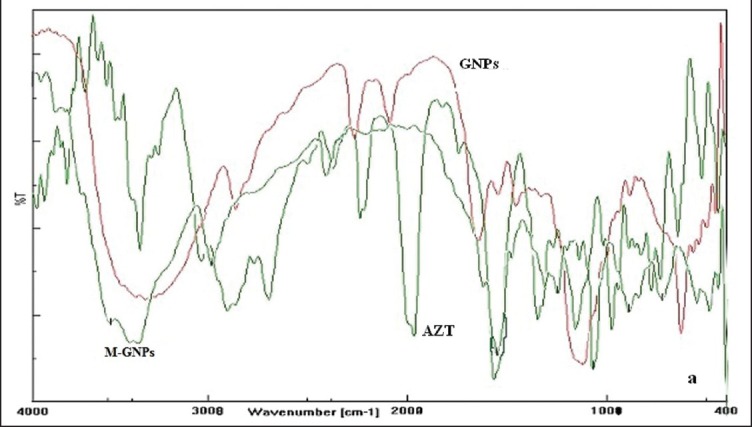
Fourier transform infrared spectroscopy spectrum of nanoparticles
In-vitro drug release studies
Both, AZT loaded GNPs and M-GNPs, extended the drug release up to almost 24 h [Figure 3]. The GNPs showed 80.56% ± 4.5% drug release, whereas the M-GNPs release 74.45% ± 4.8% at the end of 24 h and releases were significantly different (P < 0.05) with respect to time. From in-vitro release data, it can be concluded that nanoparticle formulations exhibited a biphasic pattern of drug release. An initial burst release, 15.39 ± 0.5% and 12.45 ± 1.2%, for GNPs and M-GNPs, respectively was observed until 3 h, due to immediate release of the surface associated drug and a prolonged release in the later stage due to the slow diffusion of drug from the matrix. Thus, both GNPs and M-GNPs followed Korsmeyer-Peppas release kinetics model (GNPs, k = 14.7225, r = 0.9760; M-GNPs, K = 19.6684, r = 0.9712).
Figure 3.
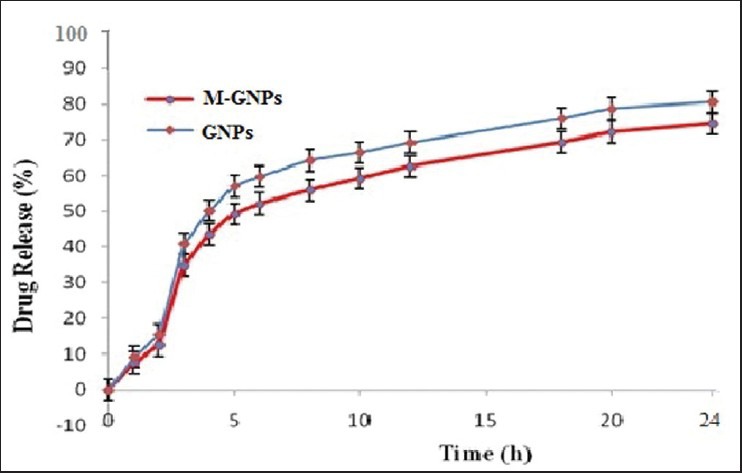
In-vitro drug release profile of gelatin nanoparticles and mannosylated gelatin nanoparticles
CONCLUSIONS
AZT loaded M-GNPs were successfully prepared from desolated GNPs, by coupling with mannose as ensured from the formation of Schiff's base between mannose ligand and amine terminal of the nanoparticles. The size of nanoparticles was found to be in the range of 200-800 nm; thus, can be easily uptaken by the RES, where HIV is prominent. Further mannosylation will help binding nanoparticles to mannose receptors present over the macrophages, achieving selectivity in targeting of AZT and improvement in therapeutic efficacy and reduction in associated side-effects.
ACKNOWLEDGMENT
The authors Ratan Tone and Sameer Nadaf are thankful to AICTE for providing scholarship in terms of JRF.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Sperling RS, Shapiro DE, Coombs RW, Todd JA, Herman SA, McSherry GD, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1996;335:1621–9. doi: 10.1056/NEJM199611283352201. [DOI] [PubMed] [Google Scholar]
- 2.Blum MR, Liao SH, Good SS, de Miranda P. Pharmacokinetics and bioavailability of zidovudine in humans. Am J Med. 1988;85:189–94. [PubMed] [Google Scholar]
- 3.Gill PS, Rarick M, Brynes RK, Causey D, Loureiro C, Levine AM. Azidothymidine associated with bone marrow failure in the acquired immunodeficiency syndrome (AIDS) Ann Intern Med. 1987;107:502–5. doi: 10.7326/0003-4819-107-4-502. [DOI] [PubMed] [Google Scholar]
- 4.Auger MJ, Ross JA. The biology of the macrophage. In: Lewis CE, Mcgee JOD, editors. the macrophage: The natural immune system. Oxford: Oxford University Press; 1992. pp. 1–74. [Google Scholar]
- 5.Levy JA. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss RA. How does HIV cause AIDS? Science. 1993;260:1273–9. doi: 10.1126/science.8493571. [DOI] [PubMed] [Google Scholar]
- 7.Largent BL, Walton KM, Hoppe CA, Lee YC, Schnaar RL. Carbohydrate-specific adhesion of alveolar macrophages to mannose-derivatized surfaces. J Biol Chem. 1984;259:1764–9. [PubMed] [Google Scholar]
- 8.Haltiwanger RS, Hill RL. The ligand binding specificity and tissue localization of a rat alveolar macrophage lectin. J Biol Chem. 1986;261:15696–702. [PubMed] [Google Scholar]
- 9.Singh S, Dobhal AK, Jain A, Pandit JK, Chakraborty S. Formulation and evaluation of solid lipid nanoparticles of a water soluble drug: Zidovudine. Chem Pharm Bull (Tokyo) 2010;58:650–5. doi: 10.1248/cpb.58.650. [DOI] [PubMed] [Google Scholar]
- 10.Nesalin AJ, Smith AA. Preparation and evaluation of chitosan nanoparticles containing zidovudine. Asian J Pharm Sci. 2012;7:80–4. [Google Scholar]
- 11.Mainardes RM, Khalil NM, Gremião MP. Intranasal delivery of zidovudine by PLA and PLA-PEG blend nanoparticles. Int Pharm. 2010;395:266–71. doi: 10.1016/j.ijpharm.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Sankar V, Nareshkumar PN, Ajitkumar GN, Penmetsa SD, Hariharan S. Comparative studies of lamivudine-zidovudine nanoparticles for the selective uptake by macrophages. Curr Drug Deliv. 2012;9:506–14. doi: 10.2174/156720112802650707. [DOI] [PubMed] [Google Scholar]
- 13.Margret C, Muruganantham SV, Debjit B, Kumudhavalli MV, Jayakar B. Formulation and evaluation of sustained release matrix tablets of zidovudine. Int J Curr Pharm. 2009;1:14–31. [Google Scholar]
- 14.Samal HB, Sreenivas V, Dey S, Sharma H. Formulation and evalution of sustained release zidovudine matrix tablets. Int J Pharm Sci. 2011;3:32–41. [Google Scholar]
- 15.Mitra S, Mitra AN. Nanoparticulate carriers in drug delivery targeting. Proc Indian Natn Sci Acad. 2002;68:349–60. [Google Scholar]
- 16.Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases. 2007;2:MR17–71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 17.Mladenovska K, Kumbaradzi E, Dodov G, Makraduli L, Goracinova K. Biodegradation and drug release studies of BSA loaded gelatin microspheres. Int J Pharm. 2002;242:247–9. doi: 10.1016/s0378-5173(02)00167-9. [DOI] [PubMed] [Google Scholar]
- 18.Zhao L, Yang B, Dai X, Wang X, Gao F, Zhang X, et al. Glutaraldehyde mediated conjugation of amino-coated magnetic nanoparticles with albumin protein for nanothermotherapy. J Nanosci Nanotechnol. 2010;10:7117–20. doi: 10.1166/jnn.2010.2820. [DOI] [PubMed] [Google Scholar]
- 19.Fang H, Huang J, Ding L, Li M, Chen Z. Preparation of magnetic chitosan nanoparticles and immobilization of laccase. J Wuhan Univ Technol-Mater Sci. 2009;24:42–7. [Google Scholar]
- 20.Oppenheim RC. Solid colloidal drug delivery system: Nanoparticles. Int J Pharm. 1981;8:217–34. [Google Scholar]
- 21.Lu Z, Yeh TK, Tsai M, Au JL, Wientjes MG. Paclitaxel-loaded gelatin nanoparticles for intravesical bladder cancer therapy. Clin Cancer Res. 2004;10:7677–84. doi: 10.1158/1078-0432.CCR-04-1443. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell JP, Roberts KD, Langley J, Koentgen F, Lambert JN. A direct method for the formation of peptide and carbohydrate dendrimers. Bioorg Med Chem Lett. 1999;9:2785–8. doi: 10.1016/s0960-894x(99)00480-1. [DOI] [PubMed] [Google Scholar]


