Abstract
Introduction:
Incorporation of pH modifier has been the usual strategy employed to enhance the dissolution of weakly basic drug from floating microspheres. Microspheres prepared using a combination of both ethyl cellulose (EC) and hydroxypropyl methylcellulose (HPMC) which shows highest release were utilize to investigate the effect of fumaric acid (FA), citric acid (CA), ascorbic acid (AA) and tartaric acid (TA) (all 5-20% w/w) incorporation on metoprolol succinate (MS) release.
Materials and Methods:
EC, HPMC alone or in combination were used to prepare microspheres that floated in simulated gastric fluid and evaluated for a percent yield, drug entrapment, percent buoyancy and drug release. The higher drug release in combination (MS:HPMC:EC, 1:1:2) was selected for the evaluation of influence of pH modifiers on MS release. CA (5-20% w/w), AA (5-20% w/w), FA (5-20% w/w) and TA (5-20% w/w) were added and evaluated for drug release. Present investigation is directed to develop floating drug delivery system of MS by solvent evaporation technique.
Results:
The microspheres of MS:HPMC:EC (1:1:2) exhibited the highest entrapment (74.36 ± 2.18). The best percentage yield was obtained at MS:HPMC (1:1) (83.96 ± 1.50) and combination of MS:HPMC:EC (1:1:2) (79.23 ± 1.63).
Conclusion:
MS release from the prepared microspheres was influenced by changing MS-polymer, MS-polymer-polymer ratio and pH modifier. Although significant increment in MS release was observed with CA (20% w/w), TA (20% w/w) and AA (20% w/w), addition of 20% w/w FA demonstrated more pronounced and significant increase in drug entrapment as well as release from MS:HPMC:EC (1:1:2) buoyant microspheres.
Keywords: Ethyl cellulose, floating microspheres, hydroxypropyl methyl cellulose, metoprolol succinate, microenvironmental pH, pH modifiers
INTRODUCTION
Metoprolol succinate (MS) is a β1-selective (cardioselective) adrenoceptor blocking agent[1] used extensively in the treatment of hypertension, angina pectoris and coronary heart diseases has oral bioavailability of <50% perhaps because of its rapid first pass metabolism and degradation in colon.[2,3] The maintenance of constant plasma level of cardiovascular drug is important in ensuring the desired therapeutic response which is not achieved with conventional tablets. Since the t1/2 of MS is 3-5 h, multiple doses are needed to be administered to maintain constant plasma concentration for therapeutic response with improved patient compliance. It has also been reported that MS absorption in the duodenum and jejunum is directly proportional to the dose availability.[4] Various attempts have been made to prolong the retention time of the dosage form in the stomach for the drug which primarily absorbs from upper gastro intestinal tract. One such approach is the preparation of a device that remains buoyant in the stomach contents due to its lower density than that of the gastric fluids.[5,6,7,8,9] A floating drug delivery system can overcome at least some of these problems and is particularly useful for drugs that are principally absorbed in the duodenum and upper jejunum segments. A floating system made up of multiple unit forms has relative merits compared to a single unit preparation[10] and is able to prolong the retention time of dosage form in stomach, increase in the rate of absorption improving the oral bioavailability of drug. Hence it was thought worthwhile to prepare the floating microspheres of MS, which can reside in the stomach for a longer period and provide extended drug release.
Polymeric microspheres and microcapsules have received much attention as drug delivery systems in recent years to modify and retard drug release. Hydroxypropyl methylcellulose (HPMC) and ethyl cellulose (EC) have been investigated for their utility in formulating buoyant microparticles of cimetidine with prolonged release.[11] Variability and bioavailability for weakly basic drug substances can be overcome by releasing the drug at a controlled rate. Incorporation of pH modifiers into controlled release matrix system is used to alter microenvironmental pH (pHM) within solid dosage form have been the usual strategy to obtain the desired release profile.[12] An optimized pH can be used to modulate the release rate of drug compounds exhibiting pH-dependent solubility[13] and to overcome stability issues of pH-sensitive drug compounds.[14,15] Several researchers have successfully enhanced the release of weakly basic drug compounds from swellable tablets using hydrophilic polymers by incorporating pH modifiers, such as succinic, fumaric, or adipic acid.[16,17,18,19] They act principally by reducing the pHM, and thereby enhancing the drug solubility and dissolution. The ability of pH modifier to alter the pH is dependent on diffusivity and solubility of pH modifier. The maintenance of a low pHM depends on the physicochemical properties of the incorporated pH modifiers and was favored by high acidic strength and low aqueous solubility.[12] The majority of frequently used pH modifiers is more soluble at higher pH and diffuses out more rapidly as compared to the drugs which are weak bases or salts thereof showing distinct solubility in higher pH environments. As the pH increases along the gastrointestinal tract, the solubility of weakly basic drugs decreases as the fraction of unionized form is enhanced.
While much has been studied on the effect of pH modifier in the matrix type of the doses form the effect of these modifiers in the floating microspheres remained unexplored. In the present study, we prepared the floating microparticle of MS using EC and HPMC alone or in combination. Further the influence of different pH modifiers on the performance of buoyant microspheres prepared using a combination of EC and HPMC was also investigated.
EXPERIMENTAL
Materials
MS (Aurobindo Pharma Ltd., Hyderabad, India), EC (Glenmark pharmaceuticals, Nashik, India) and hydroxy propyl methyl cellulose K100M CR (HPMC) (Colorcon Asia Ltd., Goa, India), Tween 80 (Loba Chemi Ltd., Mumbai, India), methanol and dichloromethane (DCM) (Qualigens Fine Ltd., Mumbai, India), fumaric acid (FA), citric acid (CA), tartaric acid (TA) and ascorbic acid (AA) (Loba Chemi Ltd., Mumbai, India) were used. All chemicals were of analytical grade.
METHODS
Preparation of floating microparticles
The solvent evaporation technique was used to produce MS microspheres.[20] MS, EC and/or HPMC were mixed in DCM at various ratios using methanol as blending solvent (DCM:Methanol 1:1). Solutions of MS and polymer/s were mixed with added Tween 80 (0.2% v/w) as a stabilizer in the slurry. Prepared solution was introduced into 200 ml of liquid paraffin while being stirred at 2000 rpm by mechanical stirrer (REMI-RQT-124A) for 2 h at 35 ± 2°C to allow the solvent to evaporate and microspheres were collected by filtration. The microspheres were washed repeatedly with petroleum ether until freed from oil, dried for 24 h at room temperature and subsequently stored in desiccators over fused calcium.[20] Yield (%) of microspheres was calculated by dividing the total weight of microspheres by the total mass of nonvolatile compounds used.
Evaluation of floating microspheres
Morphology
The shape and surface morphology of MS floating microspheres with polymer EC and/or HPMC were investigated using scanning electron microscopy (SEM) (Joel, JSM-6380, USA). The samples for SEM study were prepared by sprinkling the formulation on a double-adhesive tape stuck to an aluminum stub. The stuck were then coated with gold to a thickness of ~300 A° under an argon atmosphere using a gold sputter module in a high-vacuum evaporator. The coated samples were then randomly scanned and photomicrographs were taken with SEM.
Particle size determination
Size distribution analysis of microspheres was done by optical microscopy using motic microscope.[21] A small quantity of microspheres was dispersed on the slide with the help of capillary tube and diameters were sized using a suitable objective (×10 and ×40). An average of 50 particles was calculated for each variable studied.
Measurement of flow properties
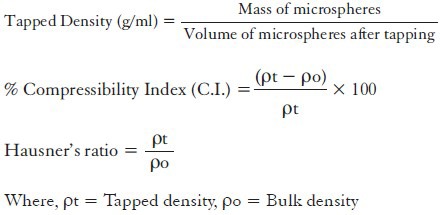
The bulk and tapped density were determined by digital automatic tap/bulk density test apparatus (Veego Instruments: VTAP/MATIC-II). Accurately weighed microspheres (100 ± 0.1 g) placed in the graduated cylinder and unsettled volume was noted. The cylinder was then tapped 100 times to determine the tapped volume. The bulk density and tapped density was determined (n = 3) as per formulae.[22]
Angle of repose
Angle of repose of prepared microspheres (n = 3) was determined by fixed funnel standing method.[23] The granules were allowed to flow through funnel orifice on a plane paper kept on the horizontal surface to form a pile of granules. The angle of repose was calculated by substituting the values of radius ‘r’ and height ‘h’ in the following equation.
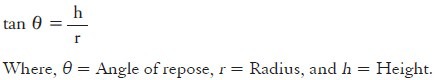
Determination of drug entrapment
Accurately weighed 100 mg ± 0.1% of microspheres was triturated with 50 ml 0.1 N HCl, sonicated for 2 h and filtered to remove the debris. Volume was made to 100 ml with 0.1 N HCl and diluted suitably before the recording of absorbance at 221 nm using UV-spectrophotometer (UV-1700, Shimadzu, Japan). No interference at 221 nm was found due to the other floating microparticle components.
![]()
In vitro evaluation of floating ability
Buoyancy studies were carried out to ascertain the floating behavior of microspheres prepared with EC or HPMC or their combinations. Microspheres (300 mg ± 0.1%) were spread over the surface of 900 ml of 0.1 N HCl containing 0.2% w/v tween 80 in United States Pharmacopeia (USP) dissolution apparatus type II. The medium was agitated with a paddle rotating at 100 rpm for 10 h. The floated and settled portions of microspheres were separated, dried in a desiccator to a constant weight to determine % buoyancy.[11]
![]()
Drug release rate determinations from floating microparticles
Floating microparticles corresponding to a weight of 100 mg drug was filled into a non-reacting mesh that had a smaller mesh size than the microspheres and placed in the basket containing 900 ml of 0.1 N HCl maintained at 37 ± 0.5°C stirred at 100 rpm (USP dissolution test apparatus type I). Samples were withdrawn at a suitable time intervals and diluted suitably before being assayed spectrophotometrically at 221 nm (UV-1700, Shimadzu, Japan).
Effect of pH modifiers
Following the above studies, formulation showing higher drug release in combination (MS:HPMC:EC, 1:1:2) was selected for the evaluation of influence of pH modifiers on MS release. Aqueous solubility and pKa were the parameters observed before the selection of pH modifier. CA (5-20% w/w), AA (5-20% w/w), FA (5-20% w/w) and TA (5-20% w/w) were added to the drug: polymer: polymer solutions and the prepared microspheres were evaluated for drug release as mentioned earlier.
RESULTS AND DISCUSSION
Physicochemical properties of the prepared floating microparticles
Morphology
The floating microspheres of MS prepared by solvent evaporation were found to be almost spherical, free-flowing, white or almost white in color. SEM was performed to study the surface and morphological characteristics are shown in Figure 1. SEM indicated that the prepared microspheres were spherical, rough surface with distinct pores evident on the surface which may contribute for drug release. The microphotographs also show the presence of loose crystals of drug on the surface of few microspheres.
Figure 1.
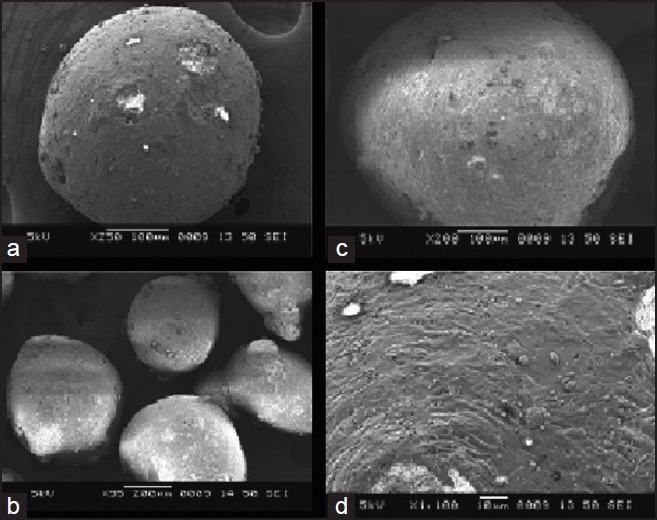
Scanning electron microphotographs of floating microspheres (a) microspheres with ethyl cellulose (EC), (b) microspheres with hydroxypropyl methyl cellulose (HPMC), (c) microspheres with HPMC:EC combination, (d) surface morphology of microspheres with HPMC: EC combination
Particle size
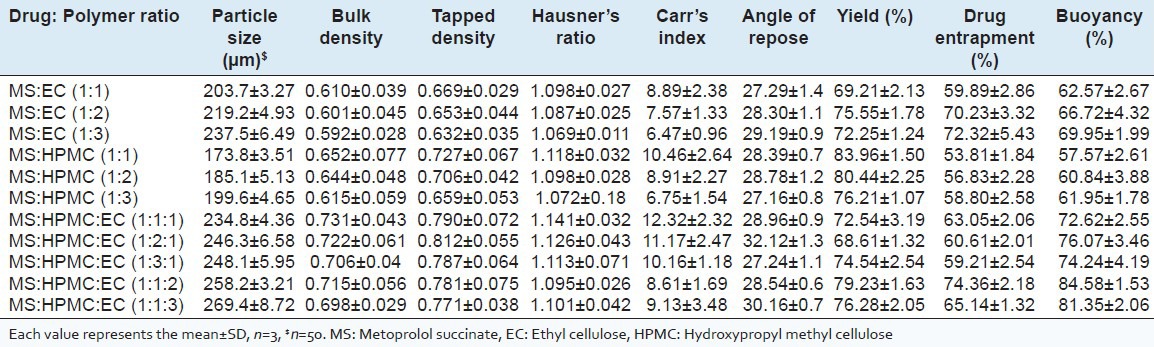
Particle size increased with increasing the polymer contribution in the drug: Polymer solution. Increasing polymer and keeping drug constant, particle size was recorded increased with elevated levels of EC (203.7-237.5 μm) and HPMC (173.8-199.6 μm) [Table 1] while in the microspheres prepared with EC-HPMC combination, the size increased and ranged from 234.8 to 269.4 μm. As the polymer concentration increases, viscosity increments influenced the interaction between disperse phase and dispersion medium that affected the size distribution of particle. Increased EC or HPMC in a fixed volume of solvent increases the viscosity of the medium which might have diminished the shearing efficiency leading to increased droplet size and hence microsphere size.[24,25]
Table 1.
Characterization of buoyant microspheres prepared with MS:EC, MS:HPMC, and MS:HPMC:EC
Micromeritic properties of floating microspheres of metoprolol
Bulk and tapped density determinations demonstrated good compaction property and good packability of the floating microspheres [Table 1]. All formulations showed excellent flowability as represented in terms of angle of repose (<40°) and percentage compressibility values (Carr's Index) <20 (Lin and Kao, 1989). Hausner's ratio decreased with increasing HPMC and EC concentration ranging from 1.069 ± 0.18 to 1.141 ± 0.032 which suggesting good flow properties of prepared microspheres.
Drug entrapment and percentage yield of floating microparticles
The yield of floating microparticles determined by weighing after dryness to constant weight was observed to be in the range of 69.2 ± 2.13-75.55 ± 1.78% for EC and decreased for microspheres containing increased HPMC (83.96 ± 1.50-76.21 ± 1.07%). The yield of floating microspheres containing HPMC:EC in combination was 68.61 ± 1.32-79.23 ± 1.63%. The best yield was obtained at drug:HPMC (1:1) (w/w) and combination of MS:HPMC:EC (1:1:2) (w/w/w) which indicates that the optimum diffusion rate of solvents was obtained at these polymer ratio [Table 2]. The decreased microsphere yield with increased concentration of HPMC may be the result of flocculation and aggregation due to increased viscosity.[26]
Table 2.
Characteristics of floating microspheres prepared with MS:HPMC:EC (1:1:2) containing pH modifiers viz. FA, CA, AA, TA (all 5-20% w/w)
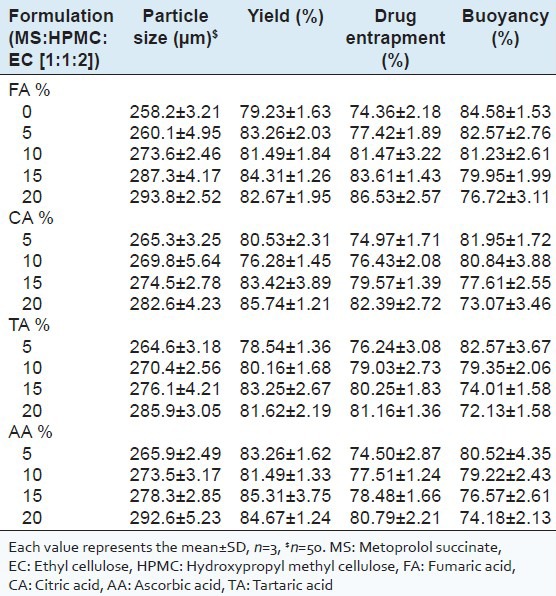
Drug entrapped in the floating microspheres containing HPMC and/or EC was found to be in the range of 53.81 ± 1.84-74.36 ± 2.18% w/w. The drug entrapment was found to be dependent on the nature of polymer used in the formulation[25] and increased EC or HPMC showed increment in drug entrapment. However, highest MS entrapment was seen in the microspheres prepared with MS:HPMC:EC (1:1:2) (74.36 ± 2.18). Increase polymer in a fixed volume of organic solvent has been demonstrated to increase drug retention in floating microspheres.[27] However, no such polymer dependant increase but drug entrapments of EC was observed to be higher than HPMC microparticles [Table 2]. This can be attributed to the structural differences between types and solubility of polymer used in the formulation. Notably, the drug entrapment in the microspheres prepared with EC/HPMC combination was little higher than those prepared using EC or HPMC individually.
Floating ability
The floating pattern differed according to the formulation tested and polymer used wherein good in-vitro percentage buoyancy (>57%) was observed for all the microspheres [Table 2]. Buoyancy of microspheres depends upon porosity and apparent density[28] and the nature of the polymer influences the floating behavior of the microspheres.[29] With increasing HPMC and EC the buoyancy increased and the microspheres prepared with EC were more buoyant than HPMC. The difference in the percentage buoyancy of microspheres containing EC and HPMC was significant, EC being insoluble and unswellable remains floated whereas HPMC swells and erodes with time hence EC predominately increases the buoyancy than HPMC. Average buoyancy of the microspheres was in the range of 62.57 ± 2.67-84.58 ± 1.53% at the end of 10 h. The microspheres prepared from a combination of both hydrophilic (HPMC) and hydrophobic (EC) polymer in different ratio showed greater buoyancy than those microspheres prepared with EC or HPMC alone because of their low density and internal voids. The percentage buoyancy of prepared floating microspheres containing MS:HPMC:EC in the ratio 1:1:2 was found to be the highest (84.58 ± 1.53) [Table 1].
Dissolution rate study
Drug release from floating EC and HPMC microspheres evaluated at pH 1.2 HCl influenced by polymer concentration and found decreased with an increasing amount of polymer. No significant difference in release rate was observed between microspheres containing either ratios 1:1 or 1:2 of EC or HPMC. However, drug release decreased significantly with further increase in the MS: Polymer ratio to 1:3 of either EC (F [2, 66] = 70.60, P < 0.001) or HPMC (F [2, 66] = 69.53, P < 0.001) (Two way analysis of variance [ANOVA]) [Figures 2a and b].
Figure 2.
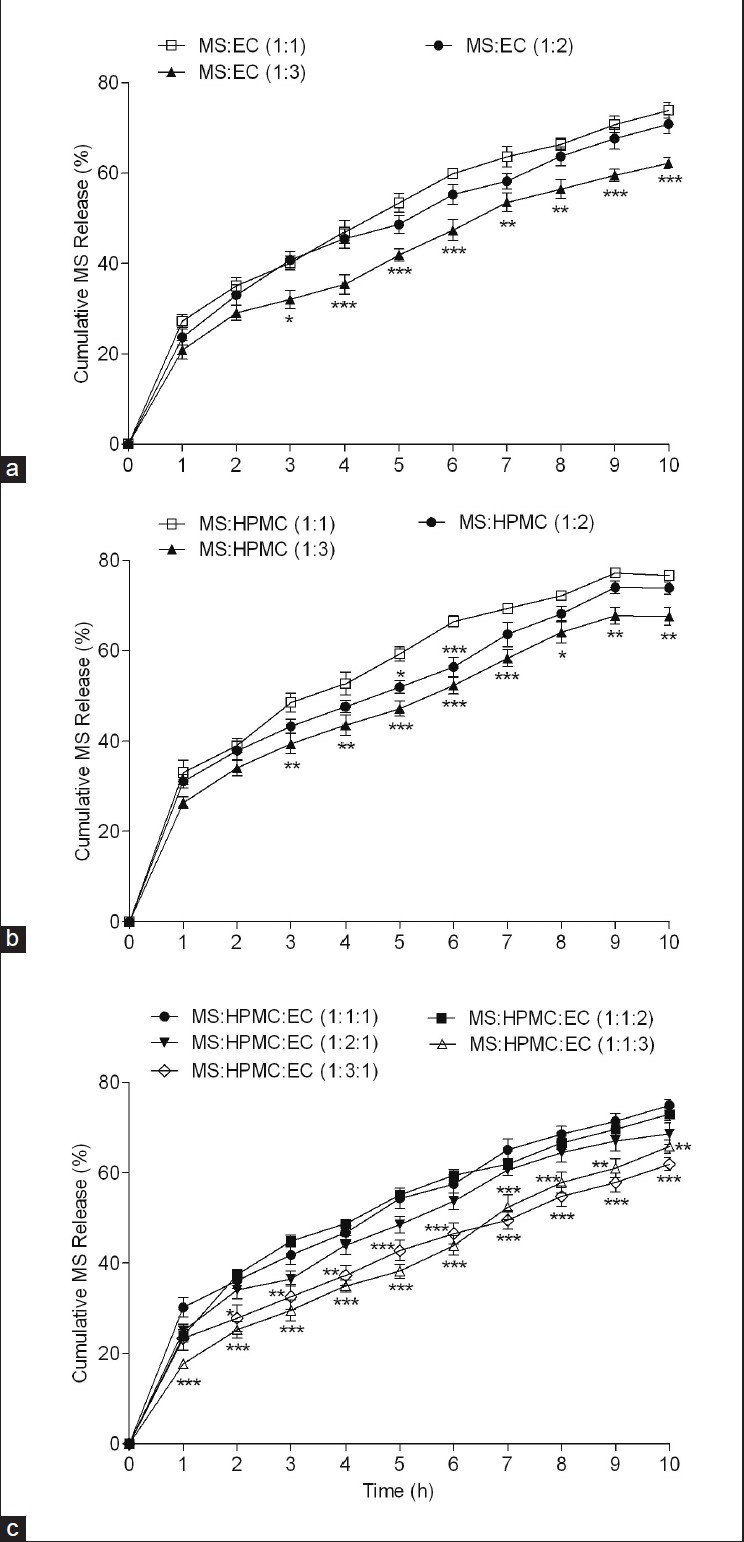
Effect ethyl cellulose (EC) (a) or hydroxypropyl methyl cellulose (HPMC) 100 alone (b) or in combination (c) on metoprolol succinate (MS) release from microspheres prepared by solvent evaporation; microspheres containing MS and EC and/or HPMC in different drug polymer ratio were prepared and evaluated for drug release in dissolution medium containing 0.1 N HCl pH 1.2. *P < 0.05, **P < 0.01, ***P < 0.001 when compared against respective control formulation (MS:Polymer/s, 1:1) (Two way analysis of variance post hoc Bonferroni mean comparisons)
Release rate usually depends upon the presence of drug closer to the surface which decreases with increasing polymer concentration and decreases the amount of uncoated drug.[25] The increased density of the polymer matrix at higher polymer concentrations increases diffusion path length which decreases the overall drug release from the polymer matrix. The release rate from HPMC microspheres was a little higher due to the high permeability and hydrophilic nature of HPMC which increases the porosity of matrix and accelerates the release.
The microspheres prepared with varying formulation containing different drug-polymer-polymer ratio (MS:HPMC:EC; 1:1:1, 1:2:1, 1:3:1, 1:1:2, 1:1:3) were evaluated for effect of polymer on drug release. The MS release was significantly reduced from 75.01 ± 1.18 to 65.83 ± 1.49 (F [2, 66] = 151.83, P < 0.001) with an increase in EC whereas HPMC increase the release decreased from 75.01 ± 1.18 to 61.95 ± 1.43 (F [2, 66] =75.08, P < 0.001) (Two way ANOVA) [Figure 2c]. The formulated microspheres containing MS:HPMC:EC in the ratio 1:1:2 satisfactorily released 72.99 ± 1.45% of total MS hence this combination prepared microparticles were further used for the study.
Effect of pH modifiers
As a result of lowered pHM, organic acids addition have been reported to delay or sustain drug release in formulations containing enteric polymers as matrix or film forming agents.[30] In order to achieve the desired release through pH modulation by FA, CA, TA and AA, MS:HPMC:EC (1:1:2) microspheres were prepared with added pH modifiers in 5-20% w/w by solvent evaporation method and evaluated for particle size, yield, drug entrapment and buoyancy. The results are depicted in Table 2.
The particle size of the microspheres was in the range of 260.1 ± 4.95 μm to 292.6 ± 5.23 μm, which increased with the increasing levels of all pH modifiers. As the concentration of pH modifier increases the boiling point of solvent increases which decreases the rate of evaporation which resulted in larger particle size of the microspheres. Drug entrapment also found increased as the concentration of pH modifiers increased. 20% w/w FA showed highest drug entrapment (86.53 ± 2.57) and the influence on MS entrapment was in the order FA > CA > TA> AA. The percentage buoyancy decreased with increased pH modifiers concentration may be due to increased water uptake caused by ionization of pH modifiers.
As shown in Figure 3, incorporation of FA in MS:HPMC:EC (1:1:2) microspheres significantly influenced the MS release (F [4, 110] = 69.67, P < 0.001) (Two way ANOVA). FA (20 but not 5-15% w/w) significantly (P < 0.001) enhanced the drug release as compared to MS:HPMC:EC (1:1:2) microspheres without pH modifier. However, statistically insignificant enhancement in MS release was also observed with increasing levels of FA (5-15% w/w). The addition of lower levels of FA (5-15% w/w) might be insufficient to achieve and maintain a favorable acidic microenvironment. The addition of higher FA amounts releases drug and pH modifier equivalently to achieve constant and low pHM over the entire dissolution period which resulted in increased drug release by decreasing the pHM within the microspheres thus increasing the solubility and in turn release of the weakly basic drug at higher pH.[12]
Figure 3.
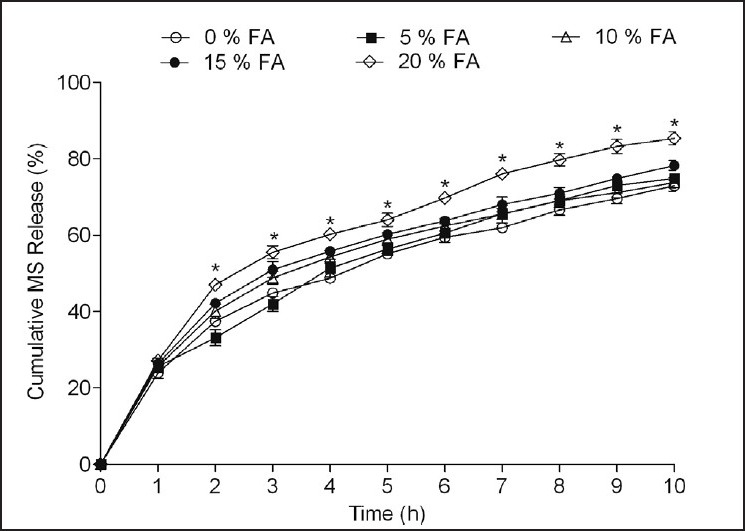
Effect of fumaric acid (FA) in different levels (5-20% w/w) on metoprolol succinate (MS) release from the microspheres prepared with MS: Hydroxypropyl methyl cellulose (HPMC): Ethyl cellulose (EC) (1:1:2) by solvent evaporation method. Drug release evaluated in dissolution medium containing 0.1 N HCl having pH 1.2. *P < 0.001 when compared against control formulation MS:HPMC:EC (1:1:2) with 0% FA (Two way analysis of variance post hoc Bonferroni mean comparisons)
Figure 4 demonstrates the effect of CA (5-20% w/w) on MS release from MS:HPMC:EC (1:1:2) microspheres. Two way ANOVA indicated the significant effect of CA addition on the MS release from prepared microparticles (F [4, 110] = 60.33, P < 0.001). CA (20% w/w) significantly (P < 0.001) stimulated the release of MS from the prepared microspheres.
Figure 4.
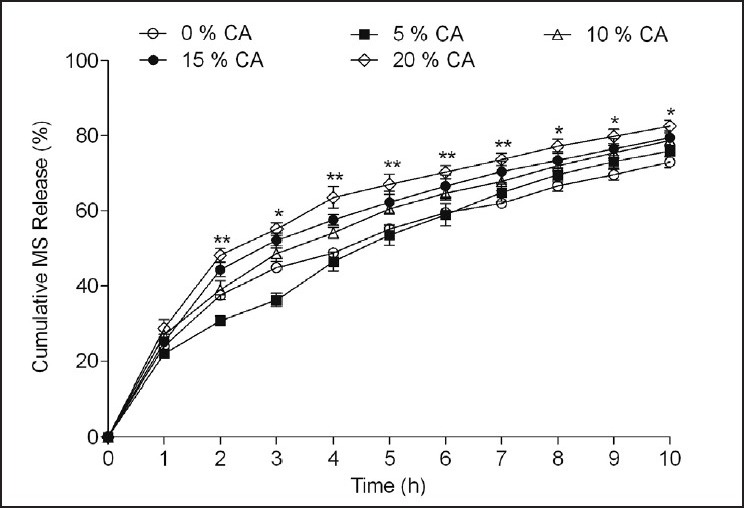
Effect of citric acid (CA) in different levels (5-20% w/w) on metoprolol succinate (MS) release from the microspheres prepared with MS: Hydroxypropyl methyl cellulose (HPMC): Ethyl cellulose (EC) (1:1:2) by solvent evaporation method. Drug release evaluated in dissolution medium containing 0.1 N HCl having pH 1.2. *P < 0.01, **P < 0.001 when compared against control formulation MS:HPMC:EC (1:1:2) with 0% CA (Two way analysis of variance post hoc Bonferroni mean comparisons)
Addition of TA (5-20% w/w) significantly affected the release of MS with increasing TA concentration (F [4, 110] = 45.51, P < 0.001) [Figure 5]. Drug release was significantly (P < 0.001, post hoc Bonferroni mean comparisons) enhanced from the microspheres containing 20% w/w TA than the microparticles prepared without pH modifiers.
Figure 5.
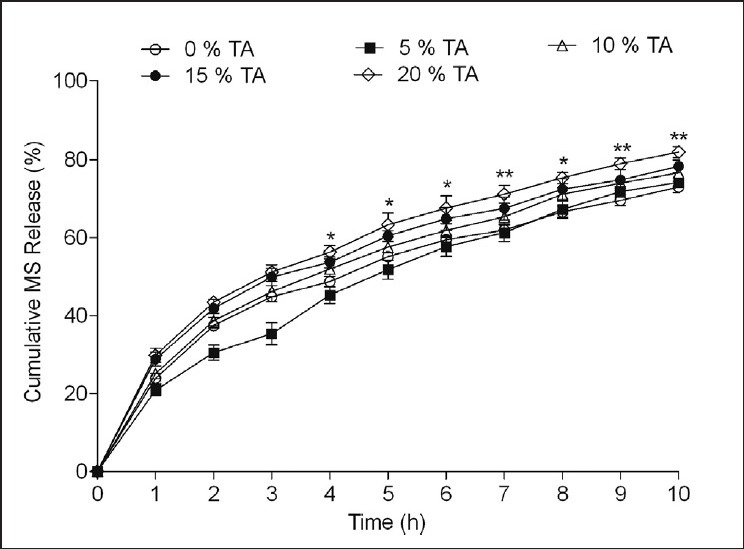
Effect of tartaric acid (TA) in different levels (5-20% w/w) on metoprolol succinate (MS) release from the microspheres prepared with MS: Hydroxypropyl methyl cellulose (HPMC): Ethyl cellulose (EC) (1:1:2) by solvent evaporation method. Drug release evaluated in dissolution medium containing 0.1 N HCl having pH 1.2. *P < 0.05, **P < 0.01 when compared against control formulation MS:HPMC:EC (1:1:2) with 0% TA (Two way analysis of variance post hoc Bonferroni mean comparisons)
Two way ANOVA demonstrated that the addition of AA (5-20% w/w) significantly influenced drug release (F [4, 110] = 14.78, P < 0.001) [Figure 6]. Post hoc Bonferroni mean comparisons showed the insignificant effect on MS release by AA in concentrations 5-15% w/w however, microspheres with 20% w/w AA showed significant effect on MS release at 4th h (P < 0.01) and 7th h (P < 0.05) of 10 h dissolution studies.
Figure 6.
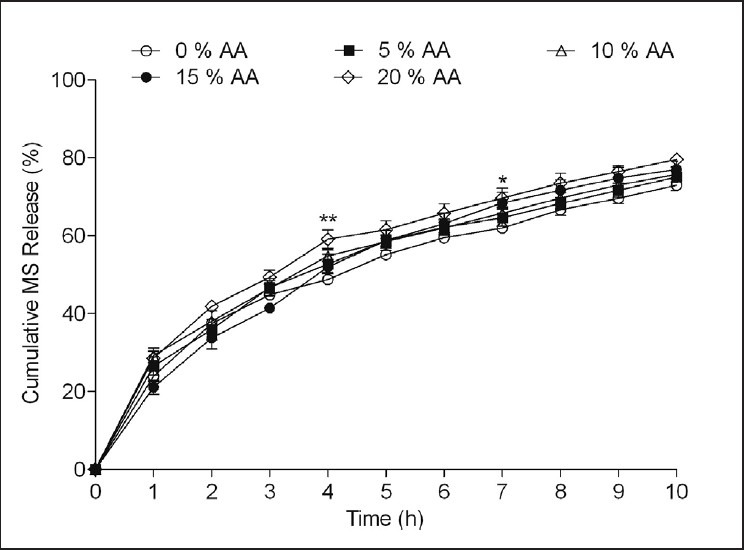
Effect of ascorbic acid (AA) in different levels (5-20% w/w) on metoprolol succinate (MS) release from the microspheres prepared with MS: Hydroxypropyl methyl cellulose (HPMC): Ethyl cellulose (EC) (1:1:2) by solvent evaporation method. Drug release evaluated in dissolution medium containing 0.1 N HCl having pH 1.2. *P < 0.05, **P < 0.01 when compared against control formulation MS:HPMC:EC (1:1:2) with 0% AA (Two way analysis of variance post hoc Bonferroni mean comparisons)
In the presence of either CA or FA an acidic and favorable environment was created initially, thus resulting in rapid and similar drug release profiles with both pH modifiers. However, as CA diffused out rapidly, increased pHM slow down drug release during the latter periods of dissolution. In contrast, fair amounts of FA remained within the microspheres owing to its lower solubility and consequently prolonged acidification led to further enhancement of drug release. Moreover, FA was released over the entire dissolution period, whereas CA release was completed much faster than the drug release of FA.[12]
The difference in the extent and duration of pH modulation depended on the physicochemical properties of the included pH modifiers, i.e., the acidic strength and the aqueous solubility. The enhanced release of weakly basic drugs by incorporated pH modifiers occurs mainly through modulation of the pHM.[12] The low pKa values and poor water solubility of FA led to a significant and extended effect on pHM modification. Despite of high pKa (4.1) and lower aqueous solubility (1-3.5 parts) AA affect release was less prominent as compared to other pH modifiers. The incorporation of acidic pH modifiers significantly enhanced the drug release by creating a more acidic microenvironment, thereby enhancing solubility and consequently, increased dissolution.
The result demonstrated that the incorporation of FA shows extended drug release, followed by CA, TA and AA. As compared to the soluble pH modifiers, FA containing MS microspheres showed a markedly improved drug release throughout the dissolution period at all concentrations.
In all, this study suggests that EC in combination with HPMC can be useful in floating microspheres, which can be proved beneficial to enhance the bioavailability of MS through incorporation of pH modifiers and therefore, can be a useful tool to improve the bioavailability of the drug like MS which degrades in the lower intestine. Since bioavailability of MS following oral administration is <50% because of its rapid first pass metabolism and degradation in a colon[2,3] it can be proposed that floating microspheres with added pH modifiers may enhance the absorption and bioavailability which however needs to be confirmed.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Thomas C, Westfall DP. Adernergic agonists and antagonists. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. NY: McGraw-Hill; 2006. pp. 278–84. [Google Scholar]
- 2.Kendall MJ, Maxwell SR, Sandberg A, Westergren G. Controlled release metoprolol. Clinical pharmacokinetic and therapeutic implications. Clin Pharmacokinet. 1991;21:319–30. doi: 10.2165/00003088-199121050-00001. [DOI] [PubMed] [Google Scholar]
- 3.Hwang SJ, Park H, Park K. Gastric retentive drug-delivery systems. Crit Rev Ther Drug Carrier Syst. 1998;15:243–84. [PubMed] [Google Scholar]
- 4.Jobin G, Cortot A, Godbillon J, Duval M, Schoeller JP, Hirtz J, et al. Investigation of drug absorption from the gastrointestinal tract of man. I. Metoprolol in the stomach, duodenum and jejunum. Br J Clin Pharmacol. 1985;19(Suppl 2):97S–105. doi: 10.1111/j.1365-2125.1985.tb02749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai S, Bolton S. A floating controlled-release drug delivery system: In vitro-in vivo evaluation. Pharm Res. 1993;10:1321–5. doi: 10.1023/a:1018921830385. [DOI] [PubMed] [Google Scholar]
- 6.Deshpande AA, Rhodes CT, Shah NH, Malick AW. Controlled-release drug delivery systems for prolonged gastric residence”: An overview. Drug Dev Ind Pharm. 1996;22:531–9. [Google Scholar]
- 7.Kawashima Y, Niwa T, Takeuchi H, Hino T, Ito Y. Preparation of multiple unit hollow microspheres (microballoons) with acrylic resins containing tranilast and their drug release characteristics (in vivo) J Control Release. 1991;16:279–90. [Google Scholar]
- 8.Oth M, Franz M, Timmermans J, Möes A. The bilayer floating capsule: A stomach-directed drug delivery system for misoprostol. Pharm Res. 1992;9:298–302. doi: 10.1023/a:1015870314340. [DOI] [PubMed] [Google Scholar]
- 9.Whitehead L, Fell JT, Collett JH, Sharma HL, Smith A. Floating dosage forms: An in vivo study demonstrating prolonged gastric retention. J Control Release. 1998;55:3–12. doi: 10.1016/s0168-3659(97)00266-6. [DOI] [PubMed] [Google Scholar]
- 10.Iannuccelli V, Coppi G, Bernabei MT, Cameroni R. Air compartment multiple unit system for prolonged gastric residence. Part I. Formulation study. Int J Pharm. 1998;174:47–54. [Google Scholar]
- 11.Srivastava AK, Ridhurkar DN, Wadhwa S. Floating microspheres of cimetidine: Formulation, characterization and in vitro evaluation. Acta Pharm. 2005;55:277–85. [PubMed] [Google Scholar]
- 12.Siepe S, Herrmann W, Borchert HH, Lueckel B, Kramer A, Ries A, et al. Microenvironmental pH and microviscosity inside pH-controlled matrix tablets: An EPR imaging study. J Control Release. 2006;112:72–8. doi: 10.1016/j.jconrel.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Rao VM, Engh K, Qiu Y. Design of pH-independent controlled release matrix tablets for acidic drugs. Int J Pharm. 2003;252:81–6. doi: 10.1016/s0378-5173(02)00622-1. [DOI] [PubMed] [Google Scholar]
- 14.Kabadi MB, Vivilecchia RV. Stabilized pharmaceutical compositions comprising an HMG-COA reductase inhibitor compound. 1994 U.S. Patent 5356896. [Google Scholar]
- 15.Nikfar F, Serajuddin AT, Jerzewiski N, Jain N. Pharmaceutical compositions having good dissolution properties. 1996;506:248. U.S. Patent 5. [Google Scholar]
- 16.Streubel A, Siepmann J, Dashevsky A, Bodmeier R. pH-independent release of a weakly basic drug from water-insoluble and -soluble matrix tablets. J Control Release. 2000;67:101–10. doi: 10.1016/s0168-3659(00)00200-5. [DOI] [PubMed] [Google Scholar]
- 17.Nie S, Pan W, Li X, Wu X. The effect of citric acid added to hydroxypropyl methylcellulose (HPMC) matrix tablets on the release profile of vinpocetine. Drug Dev Ind Pharm. 2004;30:627–35. doi: 10.1081/ddc-120037664. [DOI] [PubMed] [Google Scholar]
- 18.Burnside BA, Chang RK, Guo X. Sustained release pharmaceutical dosage forms with minimized pH dependent dissolution profiles. 2001;287:599. U.S. Patent 6. [Google Scholar]
- 19.Gabr KE. Effect of organic acids on the release patterns of weakly basic drugs from inert sustained release matrix tablets. Eur J Pharm Biopharm. 1992;38:199–202. [Google Scholar]
- 20.Streubel A, Siepmann J, Bodmeier R. Multiple unit gastroretentive drug delivery systems: A new preparation method for low density microparticles. J Microencapsul. 2003;20:329–47. doi: 10.1080/0265204021000058384. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh A, Nayak V, Roy P. Development, evaluation and method selection for the preparation of lamivudine microspheres. Pharma Times. 2006;38:12–6. [Google Scholar]
- 22.Lin S, Kao Y. Solid particulates of drug-cyclodextrin inclusion complexes directly prepared by a spray-drying technique. Int J Pharm. 1989;56:249–59. [Google Scholar]
- 23.Rahman Z, Kohli K, Khar RK, Ali M, Charoo NA, Shamsher AA. Characterization of 5-fluorouracil microspheres for colonic delivery. AAPS PharmSciTech. 2006;7:E47. doi: 10.1208/pt070362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Park TG, Choi H. Effect of formulation and processing variables on the characteristics of microspheres for water-soluble drugs prepared by w/o/o double emulsion solvent diffusion method. Int J Pharm. 2000;196:75–83. doi: 10.1016/s0378-5173(99)00440-8. [DOI] [PubMed] [Google Scholar]
- 25.Behera BC, Sahoo SK, Dhal S, Barik BB, Gupta BK. Characterization of glipizide-loaded polymethacrylate microspheres prepared by an emultion solvent evaporation method. Trop J Pharm Res. 2008;7:879–85. [Google Scholar]
- 26.Wu PC, Huang YB, Chang JS, Tsai MJ, Tsai YH. Design and evaluation of sustained release microspheres of potassium chloride prepared by Eudragit. Eur J Pharm Sci. 2003;19:115–22. doi: 10.1016/s0928-0987(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 27.Youan BB, Jackson TL, Dickens L, Hernandez C, Owusu-Ababio G. Protein release profiles and morphology of biodegradable microcapsules containing an oily core. J Control Release. 2001;76:313–26. doi: 10.1016/s0168-3659(01)00445-x. [DOI] [PubMed] [Google Scholar]
- 28.Yuasa H, Takashima Y, Kanaya Y. Studies on the development of intragastric floating and sustained release preparation, application of calcium silicate as a floating carrier. Chem Pharm Bull. 1996;44:1361–6. [Google Scholar]
- 29.Tanwar YS, Naruka PS, Ojha GR. Development and evaluation of microspheres of verapmil hydrochloride. Braz J Pharm Sci. 2007;43:529–34. [Google Scholar]
- 30.Nykänen P, Lempää S, Aaltonen ML, Jürjenson H, Veski P, Marvola M. Citric acid as excipient in multiple-unit enteric-coated tablets for targeting drugs on the colon. Int J Pharm. 2001;229:155–62. doi: 10.1016/s0378-5173(01)00839-0. [DOI] [PubMed] [Google Scholar]


