Abstract
Background:
Multiple-organ failure is the main cause of death in diabetes mellitus (DM). Hyperglycemia-induced oxidative stress is responsible for major diabetic complications, including multiple-organ failure. Medicinal plants possessing antioxidant activity may reduce oxidative stress and improve the functions of various organs affected by hyperglycemia.
Objectives:
This study was designed to evaluate the antioxidant effect of Aqueous Extract of Cassia sophera (AECS) in streptozotocin (STZ)-induced diabetic Wistar rats.
Materials and Methods:
AECS (200 mg/kg body weight (bw)) and the standard antidiabetic drug glibenclamide (10 mg/kgbw) were administered orally by gavaging for 28 days.
Results:
Oral administration of AECS inhibited STZ-induced increase in lipid peroxidation (LPO), aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), bilirubin, creatinine and urea in liver of diabetic rats. Significant increase in activity of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx), and a reduced level of glutathione (GSH), were observed in the liver, kidney, pancreas and testis on AECS treatment.
Conclusion:
The results demonstrate that AECS is not only useful in controlling blood glucose, but also has antioxidant potential to protect the liver, kidney, pancreas and testis against damage caused by hyperglycemia-induced oxidative stress.
Keywords: Antioxidants, Cassia sophera, streptozotocin
INTRODUCTION
Diabetes mellitus (DM) is a heterogeneous metabolic disorder with alterations in carbohydrate, lipid and protein metabolism.[1] It results from shortage or lack of insulin secretion or reduced sensitivity of tissue to insulin, leading to overt hyperglycemia. Hyperglycemia-induced oxidative stress is a crucial etiological factor implicated in DM.[2] Based on growing interest in free-radical biology and the lack of effective therapy, the usefulness of antioxidants in delaying DM-induced complications has been proposed. The antioxidants may mediate their effect by directly quenching ROS induced by streptozotocin (STZ) exposure or by chelating the catalytic metal ions responsible for initiating peroxidation reaction.[3] Several synthetic antioxidants, for example, butylated hydroxyl anisole and butylated hydroxyl toluene are available for commercial use, but safety is a major issue associated with these antioxidants.[4] Therefore, natural plant-based antioxidants hold the promise for protection against the toxic oxidizing agent.[5,6] A large number of aromatic, spice and medicinal plants with antioxidant properties have been reported. Several studies carried out on some of these plants have resulted in the development of natural antioxidant formulations for food, cosmetic and other applications.[7,8]
Cassia sophera (CS) is a medicinally important plant belonging to family Caesalpiniaceae. Known as ‘Kasondi’, in ‘Hindi,’ the plant is an important drug used in Ayurvedic and Unani medicine. The plant is used as blood purifier, carminative, purgative, digestive and diaphoretic, as well as for treatment of epilepsy, ascites, skin disorders, piles, jaundice, fever, articular pain and palpitation.[9,10] The plant is used as expectorant in asthma, inflammatory diseases, psoriasis, cough, arthritis, diabetes and convulsions of children, and as remedy for various skin aliments in folk medicine.[11] Recently the antidiabetic potential of the plant has also been reported.[12] Although the mechanism of antidiabetic action of CS or any of its phytochemical is not known, it may be partly due to an antioxidant mechanism and protection of pancreatic cells. Hence the present study was planned to evaluate the antioxidant potential of Aqueous Extract of CS (AECS) in the liver, kidney, pancreas and testis of diabetic Wistar rats. Liver and kidney function tests were also performed form serum to assess the possible protective role of AECS in STZ-induced pancreatic damage and toxicity caused by hyperglycemia.
MATERIALS AND METHODS
Plant material
Dried seeds of CS were obtained from local herbalists and authenticated with the help of experts in Department of Botany, Bundelkhand University, Jhansi. The seed sample was preserved in Department of Biomedical Sciences, Bundelkhand University, under accession number BU/BMS/VS/2010/04.
Animals
Wistar rats, weighing about 150–200 g, obtained from Indian Institute of Toxicology Research, Lucknow, were reared in the animal house of Bundelkhand University. The animals were kept for acclimatization for 15 days in the animal house at an ambient temperature of 25°C and 45–55% relative humidity, with 12-h dark and light cycles, and were fed pelleted diet and water ad libitum. The animal experimental protocols were in accordance with the recommendations of the institutional animal ethical committee (BU/Pharma/IAEC/10/029).
Chemicals
Alkaline phosphate (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea and creatinine were estimated by a Euro Diagnostics kit, Chennai, India and bilirubin by a Crest Biosystems kit, Chennai, India. All other biochemical estimations were performed using chemicals and reagents of high purity.
Preparation of seed extract of CS
The dried seeds of CS were pulverized in a grinder to obtain a coarse powder. A 100-g weight of the powder was soaked overnight in 400 ml distilled water at room temperature with constant stirring using a mechanical stirrer. The extract was filtered with Whatman filter paper no. 01 and centrifuged at 5000 rpm for 10 min to remove any residual material. The supernatant was lyophilized to obtain AECS.
Induction of diabetes
Diabetes was induced by a single intra-peritoneal injection of a freshly prepared solution of STZ (75 mg/kgbw, in 0.1 M citrate-buffered saline, pH 4.5). Fasting blood glucose was measured form the tail vein after 1 week for assessment of the diabetic state of the animals. Rats with a fasting blood glucose level of approximately 250 mg/dl were used in this study.
Treatment schedule
Twenty-four rats were divided into four groups of six each and treated with AECS (200 mg/kgbw) and the standard antidiabetic drug glibenclamide (10 mg/kgbw) for 28 days as per given treatment schedule [Table 1]. AECS and glibenclamide were dissolved in distilled water and administered orally by gavaging.
Table 1.
Treatment schedule

Collection and processing of blood and tissue samples
After the 28th day (at the end of the study) of the treatment, the overnight fasted rats were sacrificed under mild ether anesthesia and blood was collected by heart puncture in vials for serum isolation for performing various liver and kidney function tests. The liver; kidneys; pancreas and testis were removed, washed with ice-cold saline, homogenized and used for biochemical estimations.
Liver function tests
ALP, AST and ALT were estimated by a Euro Diagnostics kit. Bilirubin was estimated by a Crest Biosystems kit.
Kidney function tests
Urea and creatinine estimation were estimated by the Euro Diagnostics kit methods.
Preparation of tissue homogenate
Tissues were homogenized with 10 times (w/v) homogenizing buffer (0.1 M phosphate buffer, pH 7.4 + 150 mM KCl) and designated as 10% homogenate. Part of the 10% homogenate was used for lipid peroxidation (LPO) and reduced glutathione (GSH) estimation, and the remaining part was centrifuged at 9000 rpm for 20 min to get a supernatant (S). The ‘S’ was used for enzyme estimations.
Biochemical and enzyme estimation
LPO was estimated by the method of Ohkawa et al;[13] activity of superoxide dismutase (SOD) was assayed by the method of Kakkar et al;[14] catalase (CAT) was assayed by the method of Sinha;[15] activity of glutathione peroxidase (GPx) was estimated by the method of Rotruck et al;[16] glutathione-S-transferase (GST) was estimated by the method of Habig et al.;[17] glutathione reductase (GR) was estimated by the method of by Calberg and Mannervik;[18] and GSH was assayed by the method of Ellman.[19] Protein content in tissue homogenate was measured by the method of Lowry et al.[20]
Statistical analysis
Results were expressed as mean ± SD. Data were subjected to one-way analysis of variance. The treatment groups were compared with a control group using Dunnett's test. All statistics were carried out using GraphPad InStat (v.3.06; GraphPad Software Inc., San Diego, CA, USA).
RESULTS
Liver function tests
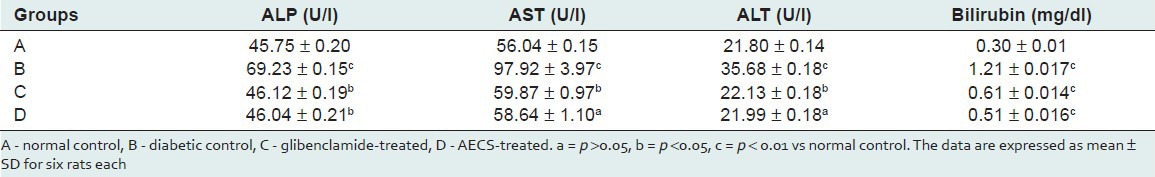
Significant (P < 0.01) increase in ALP (51.32%), AST (74.73%), ALT (63.66%) and bilirubin (299.33%) was observed in the serum of diabetic rats as compared with the control. Treatment of diabetic rats with AECS resulted in a significant (P < 0.05) decrease in the activity of ALP (33.49%) and bilirubin (57.85%), and a non-significant (P > 0.05) decrease in AST (40.11%) and ALT (38.36%) when compared with the diabetic control. Treatment of diabetic rats with glibenclamide caused a significant (P < 0.05) reduction in the activity of ALP, AST and ALT (33.38%, 38.85% and 37.97%, respectively), and a significant (P < 0.01) decrease in bilirubin (49.58%) as compared with the diabetic control [Table 2].
Table 2.
Effect of AECS and glibenclamide treatment on liver function markers
Kidney function test
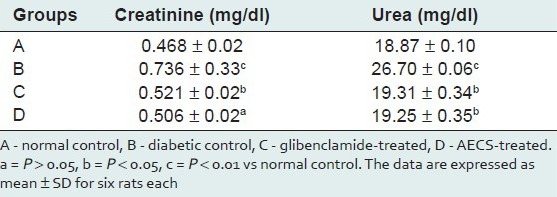
Diabetic rats showed a significant increase (P < 0.01) in creatinine and urea (57.26%, 41.49%, respectively) as compared with the control. AECS treatment showed a non-significant (P > 0.05) decrease in creatinine (31.25%) and a significant (P < 0.05) decrease in urea (27.90%), whereas glibenclamide treatment showed a significant decrease (P < 0.05) in the level of creatinine and urea (29.21%, 27.68% respectively) as compared with the untreated diabetic control [Table 3].
Table 3.
Effect of AECS and glibenclamide treatment on kidney function markers
Lipid peroxidation
Diabetic rats showed a significant increase (P < 0.01) in the concentration of LPO in different organs (liver 166.6%, kidney 84.5%, pancreas 271.2%, testis 188.4%) as compared with the control. Treatment of diabetic rats with AECS significantly (P < 0.05) decreased LPO level in liver (57.19%) and kidney (44.17%), highly significant decreased (P < 0.01) the same in pancreas (45.88%), and non-significantly decrease (P > 0.05) it in testis (127.27%) as compared with the untreated diabetic control. Glibenclamide-treated animals showed a significant decrease (P < 0.01) in liver, kidney and pancreas (52.2%, 36.41%, 35.66%, respectively), and a non-significant decrease (P > 0.05) in testis (81.81%) as compared with the untreated diabetic control [Figure 1].
Figure 1.
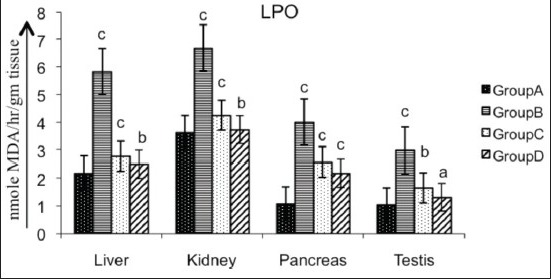
Effect of AECS on LPO in liver, kidney, pancreas and testis A - normal control, B - diabetic control, C - glibenclamide-treated, D - AECS-treated. a = P > 0.05, b = P < 0.05, c = P < 0.01 vs normal control. The data are expressed as mean ± SD for six rats each
Enzymatic antioxidants
Various organs of the diabetic rats showed a significant decrease (P < 0.01) in SOD (liver 32.19%, kidney 44.06%, pancreas 39.84%, testis 74.83%; Figure 2), CAT (liver 17.24%, kidney 28.24%, pancreas 28.34%, testis 18.58%; Figure 3), GPx (liver 42.60%, kidney 54.95%, pancreas 61.42%, testis 63.69%; Figure 4), GR (liver 90.55%, kidney 87.66%, pancreas 55.46%, testis 86.87%; Figure 5) and GST (liver 45.74%, kidney 68.29%, pancreas 75.80%, testis 50.94%; Figure 6) as compared with the control. Treatment of diabetic rats with AECS resulted in a non-significant increase (P > 0.05) in SOD in liver (40.32%), and a significant increase (P < 0.05) in kidney (72.33%), pancreas (58.76%) and testis (269.89%) [Figure 2]. CAT activity was non-significantly (P > 0.05) increased in liver and pancreas (19.71% and 36.53%), highly significantly (P < 0.01) increased in kidney (35.52%) and significantly (P < 0.05) increased in testis (19.02%) [Figure 3]. GPx activity was significantly increased (P < 0.05) in liver (71.64%) and kidney (107.39%), non-significantly (P > 0.05) increased in pancreas (155.40%), and highly significant increased (P < 0.01) in testis (158.19%) [Figure 4]. GR activity was significantly (P < 0.05) increased in liver (896.38%), non-significantly (P > 0.05) increased in kidney and testis (703.31%, 640.83%), and highly significantly increased (P < 0.01) in pancreas (110.34%) [Figure 5]. GST was significantly raised (P < 0.05) in liver and pancreas (64.70%, 293.33%), highly significantly increased (P < 0.01) in kidney (153.84%), and non-significantly increased (P > 0.05) in testis (96.15%) [Figure 6] as compared with the diabetic rats. Glibenclamide-treated animals showed a significant increase (P < 0.05) in SOD levels in liver (32.79%), and a highly significant increase (P < 0.01) in kidney, pancreas and testis (44.06%, 39.84%, 74.83%, respectively) [Figure 2]. CAT was significantly increased (P < 0.05) in liver and pancreas (19.18% and 32.69%), and highly significantly raised (P < 0.01) in kidney and testis (35.06% and 15.54%) [Figure 3]. GPx showed a highly significant increase (P < 0.01) in liver, kidney and testis (32.70%, 85.92%, 142.21%, respectively), and a significant increase (P < 0.05) in pancreas (148.47%) [Figure 4]. GR was highly significantly raised (P < 0.01) in liver, pancreas and testis (819.49%, 97.55%, 615%, respectively), and significantly increased (P < 0.05) in kidney (692.89%) [Figure 5]. GST was highly significantly increased (P < 0.01) in liver, kidney, pancreas (41.17%, 100%, 253.33%, respectively), and significantly raised (P < 0.05) in testis (80.76%) [Figure 6] as compared with the diabetic rats.
Figure 2.
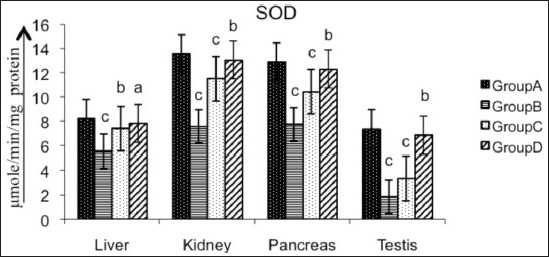
Effect of AECS on SOD in liver, kidney, pancreas and testis A - normal control, B - diabetic control, C - glibenclamide-treated, D - AECS-treated. a = P > 0.05, b = P < 0.05, c = P < 0.01 vs normal control. The data are expressed as mean ± SD for six rats each
Figure 3.
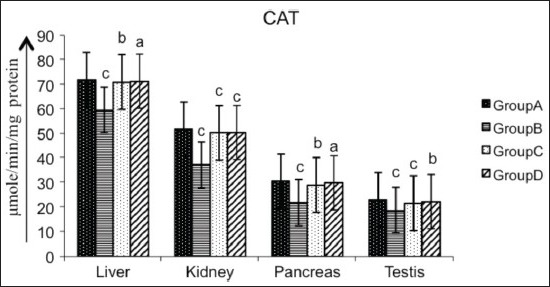
Effect of AECS on CAT in liver, kidney, pancreas and testis A - normal control, B - diabetic control, C - glibenclamide-treated, D - AECS-treated. a = P > 0.05, b = P < 0.05, c = P < 0.01 vs normal control. The data are expressed as mean ± SD for six rats each
Figure 4.
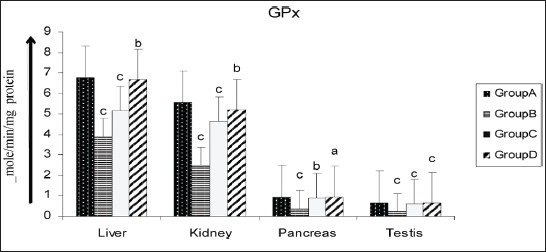
Effect of AECS on GPx in liver, kidney, pancreas and testis A - normal control, B - diabetic control, C - glibenclamide-treated, D - AECS-treated. a = P > 0.05, b = P < 0.05, c = P < 0.01 vs normal control. The data are expressed as mean ± SD for six rats each
Figure 5.
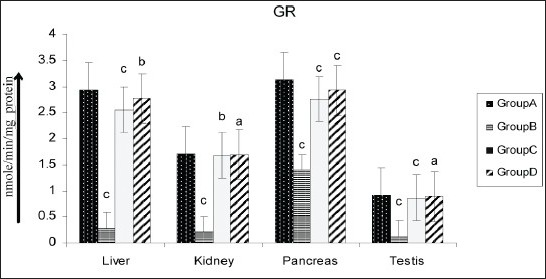
Effect of AECS on GR in liver, kidney, pancreas and testis A - normal control, B - diabetic control, C - glibenclamide-treated, D - AECS-treated. a = P > 0.05, b = P < 0.05, c = P < 0.01 vs normal control. The data are expressed as mean ± SD for six rats each
Figure 6.
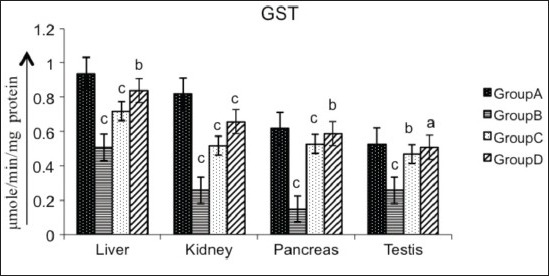
Effect of AECS on GST in liver, kidney, pancreas and testis A - normal control, B - diabetic control, C - glibenclamide-treated, D - AECS-treated. a = P > 0.05, b = P < 0.05, c = P < 0.01 vs normal control. The data are expressed as mean ± SD for six rats each
Non-enzymatic antioxidant
Reduced glutathione
Diabetic rats showed a significant reduction (P < 0.01) in GSH activity in liver (27.52%), kidney (37.29%), pancreas (64.82%) and testis (58.78%) as compared with the control. Treatment of diabetic rats with AECS resulted in a non-significant increase (P > 0.05) in GSH levels in liver (40%) and a significant increase (P < 0.05) in kidney (54.91%) and pancreas (175.89%), and a highly significant increase (P < 0.01) in testis (118.18%). Glibenclamide-treated animals showed a significant increase (P < 0.05) in GSH levels in liver (25.16%), and a significant increase (P < 0.01) in kidney (37.29%), pancreas (64.82%) and testis (58.78%) as compared with the diabetic control [Figure 7].
Figure 7.
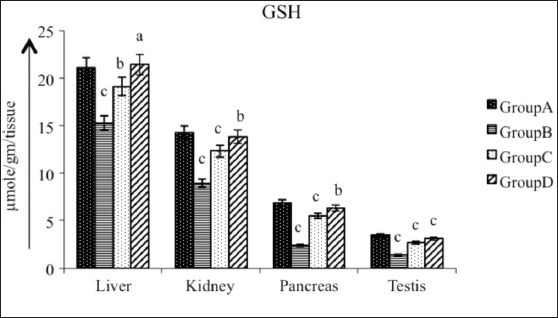
Effect of AECS on GSH in liver, kidney, pancreas and testis A - normal control, B - diabetic control, C - glibenclamide-treated, D - AECS-treated. a = P > 0.05, b = P < 0.05, c = P < 0.01 vs normal control. The data are expressed as mean ± SD for six rats each
Protein
Diabetic rats showed a significant decrease (P < 0.01) in the concentration of protein in liver (40.87%), kidney (45.62%), pancreas (70.85%) and testis (70.85%) as compared with the control. Oral administration of AECS to STZ-induced diabetic rats significantly increased protein level (P < 0.05) in liver (54.76%) and pancreas (223.95%), and showed a significant increase (P < 0.01) in kidney (65.09%), and a non-significant raise (P > 0.05) in testis (238.73%). Glibenclamide-treated animals showed a significant increase in protein level (P < 0.01) in liver, kidney and pancreas (29.58%, 50.98%, 204.79%, respectively), and significant increase (P < 0.05) in testis (232.43%) as compared to diabetic control [Figure 8].
Figure 8.
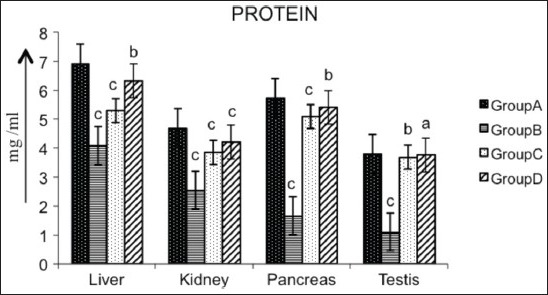
Effect of AECS on protein in liver, kidney, pancreas and testis A - normal control, B - diabetic control, C - glibenclamide-treated, D - AECS-treated. a = P > 0.05, b = P < 0.05, c = P < 0.01 vs normal control. The data are expressed as mean ± SD for six rats each
DISCUSSION
The present study showed the antioxidant effect of AECS in STZ-induced diabetic Wistar rats. STZ is a pancreatic toxin that induces diabetes by destroying pancreatic β-cells.[21] The cytotoxic effects of STZ are dependent on DNA alkylation by site-specific action with DNA bases and by free-radical generation.[22]
In the present study, a significant increase in serum ALP was observed after administration of STZ. The increased activity of this enzyme may be by either STZ- or hyperglycemia-induced tissue damage. Rai et al.,[23] reported an increase in ALP activity after induction of diabetes with STZ. In the present study, treatment with AECS and glibenclamide for 28 days showed a marked decrease in serum ALP activity. Decrease in ALP activity has been reported in Wattakaka volubilis-treated, alloxan-induced diabetic rats.[24] The activities of serum AST and ALT were increased in diabetic rats as compared with their normal control. The increase in the activities of AST and ALT in blood may be mainly due to leakage of these enzymes from the hepatocytes into the blood stream,[25] indicating a hepatotoxic effect of STZ. Treatment of diabetic rats with AECS and glibenclamide resulted in a reduction of AST and ALT compared with diabetic rats, suggesting a protective role of AECS in STZ-induced liver damage. Decrease in transaminases activity has been reported earlier on treatment with plant extracts.[26] Bilirubin is a breakdown product of heme. It is a reducing species and hence a potential antioxidants. It may provide physiological defence against oxidative injury.[27,28] Our study showed an increase in the level of serum bilirubin in diabetic rats as compared with the control. Increase in plasma bilirubin (hyper-bilirubenimia) may have resulted from decrease of liver uptake, conjugation or increased bilirubin production from hemolysis.[29] The AECS- and glibenclamide-treated rats showed a decrease in serum bilirubin compared with the diabetic control. The reduced level of bilirubin may be due to reduced oxidative stress after AECS treatment and hepatoprotection. Similar decrease in bilirubin was also reported in Citrullus colocynthis administration.[30]
Severe renal damage is observed in DM due to abnormal glucose regulation, including elevated glucose and glycosylated protein tissue levels, hemodynamic changes within the kidney tissue and increased oxidative stress.[31] In the present study, serum urea and creatinine levels were increased in untreated diabetic rats as compared with the diabetic control group. The level of creatinine had reduced after 28 days of treatment with AECS and glibenclamide. The level of urea was also reduced on treatment with AECS and glibenclamide, compared with the diabetic rats. Our findings are in agreement with the result from Sphagneticola trilobata,[32] and from leaf extracts of Ruellia tuberosa L. and Dipteracanthus patulus (Jacq).[33]
Chronic hyperglycemia lowers antioxidant status and enhances lipids, inducing oxidative stress. The increase in oxygen free radicals in diabetes could be primarily due to increase in blood glucose levels, which upon auto-oxidation generates free radicals.[34] An elevated level of lipid peroxides in the plasma of STZ-induced diabetic rats has also been reported by Karpen et al.,[35] Insulin secretion is also closely associated with lipoxygenase-derived peroxides.[36] In the current study, the level of LPO was increased in the liver, kidney, pancreas and testis of STZ-induced diabetic rats. Administration of AECS and glibenclamide to diabetic rats decreased the level of LPO in these tissues.
SOD and CAT are the two major radical scavenging enzymes. SOD is the main enzymatic defence against the superoxide anion. This enzyme detoxifies the superoxide anion, thus converting it into H2O2 and water. CAT is a heme protein that catalyses the reduction of hydrogen peroxides and protects tissues from hydroxyl radicals.[37] The activity of SOD was lowered in diabetic rats, probably due to glycation of the enzyme due to hyperglycemia. Kaleem et al.,[38] proposed that decrease in activities of SOD and CAT in both liver and kidney during a diabetic state may be due to over-production of reactive oxygen species in diabetic animals.[38] Administration of AECS and glibenclamide showed increase in SOD and CAT activity in liver, kidney, pancreas and testis. The phytochemicals present in AECS may either be scavenging the STZ metabolites or may be reducing oxidative stress by decreasing blood glucose. Increases in SOD activities in liver and kidney with Capparis aphylla,[39] and in pancreas by administration of ellagic acid,[40] have been reported earlier. GPx, an enzyme with selenium, works together with GST in the metabolism of H2O2 and organic hydroperoxides to non-toxic products at the expense of GSH.[41] Reduced activities of GPx may result from radical-induced inactivation and glycation of the enzyme.[42] Reduced activity of GPx in the liver, kidney, pancreas and testis of diabetic rats has been observed in this study. Administration of AECS and glibenclamide significantly increased GPx activity. Previously, oral administration of C. aphylla extracts and glibenclamide to STZ-treated rats showed increase in GPx level in liver and kidney.[39] GR is an enzyme that reduces glutathione disulfide (GSSG) to the sulfhydryl form GSH, which is an important cellular antioxidant. Administration of AECS and glibenclamide to diabetic rats significantly increased the level of GR in the liver, kidney, pancreas and testis of diabetic rats. SOD, CAT and GPx are involved in the elimination of H2O2. SOD converts the superoxide radical to H2O2, which is then acted upon by CAT and GPx. The functions of all three enzymes are interconnected and a lowering of their activities resulted in the accumulation of lipid peroxides and increased oxidative stress in diabetic rats.
GST plays an important role in the detoxification and metabolism of many xenobiotic and endobiotic compounds.[43] GST also has peroxidase and isomerase activity. It binds covalently with reactive metabolites formed from xenobiotics and non-covalently with lipophilic molecules, thereby offering protection against oxidative stress.[44] Induction of diabetes by STZ in this study caused reduction in GST activity. The decrease may be due to suppression of the mRNA of GST by excessive free radicals.[45] AECS and glibenclamide treatment showed a significant increase in GST in liver, kidney, pancreas and testis. Increase in SOD and GST activity has been reported earlier by Trigonella foenum-graecum seed powder (TSP) in diabetic liver.[46]
Decline in GSH content in the liver, kidney, pancreas and testis of the diabetic rats, and its normalization in AECS- and glibenclamide-treated animals, revealed the antioxidative potential of CS. The decrease in GSH in diabetic rats may probably be due to its increased utilization by hepatocytes in an attempt to counteract the increased formation of lipid peroxides on STZ exposure. GSH is a direct scavenger of free radicals as well as a co-substrate for peroxide detoxification by GPx and GST.[6] STZ-induced diabetic rats showed a decrease in GSH possibly due to the destruction of pancreatic β-cells by STZ, reinforcing the view that STZ induces diabetes through generation of oxygen free radicals. Oral administration of AECS and glibenclamide significantly increased the level of GSH in the tissues (liver, kidney, pancreas and testis) of diabetic rats. This indicates that the extract can either increase the biosynthesis of GSH or reduce oxidative stress, leading thereby to reduced conjugation of GSH, or have both effects. Increase in GSH levels on treatment with Sphagneticola trilobata,[32] Cinnamomum tamala[47] and C. aphylla39 have been reported earlier.
CONCLUSION
In the present study, AECS exhibited significant antioxidant activity in STZ-induced diabetic rats. The antidiabetic activity of AECS reported earlier may be partly due to its antioxidant and pancreatic β-cell-protecting activities.
ACKNOWLEDGEMENT
The authors are thankful to Bundelkhand University, Jhansi, India, for providing research facilities for undertaking this work.
Footnotes
Source of Support: Bundelkhand University, Jhansi, India
Conflict of Interest: No.
REFERENCES
- 1.Mutalik S, Sulochana B, Chetana M, Udupa N, Uma Devi P. Preliminary studies on acute and subacute toxicity of an antidiabetic herbal preparation, Dianex. Indian J Exp Biol. 2003;41:316–20. [PubMed] [Google Scholar]
- 2.Pong K. Therapeutic Pharmacognosy. New Delhi: Vallabh Prakashan; 2003. Oxidative stress in neurodegenerative diseases; pp. 107–13. [Google Scholar]
- 3.Robak J, Marcinkiewicz E. Scavenging of reactive oxygen species as the mechanism of drug action. Pol J Pharmacol. 1995;47:89–98. [PubMed] [Google Scholar]
- 4.Madhavi DL, Salunkhe DK. Toxicological aspects of food antioxidants. In: Madavi DL, Deshpande SS, Salunkhe DK, editors. Food Antioxidants. New York: Marcel Dekker Inc; 1995. p. 267. [Google Scholar]
- 5.Tepe B, Sokmen M, Akpulat HA, Sokmen A. Screening of the antioxidant potentials of six Salvia species from Turkey. Food Chem. 2006;95:200–4. [Google Scholar]
- 6.Winterbourn CC. Concerted antioxidant activity of glutathione and superoxide dismutase. In: Packer L, Fuchs J, editors. Biothiols in Health and Disease. New York: Marcel Dekker Inc; 1995. pp. 117–34. [Google Scholar]
- 7.Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–60. [Google Scholar]
- 8.Miliauskas G, Venskutonis PR, Van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–7. [Google Scholar]
- 9.Bilal A, Khan NA, Inamuddin GA. Pharmacological investigation of Cassia sophera Linn. Var. purpurea, Roxb. Med J Islam World Acad Sci. 2005;15:105–9. [Google Scholar]
- 10.Chopra RN, Nayar SL, Chopra IC. New Delhi: CSIR; 1956. Glossary of Indian Medicinal Plant; p. 25. [Google Scholar]
- 11.Shastri AD. Varanasi: Chaukhamba Sanskrit Sansthan; 1981. Bhaisajyaratnawali; p. 619. (623-5). [Google Scholar]
- 12.Ashoke D, Sanjib D, Amol K. Antihyperglycemic effect of Cassia sophera Linn. Asian J Tradit Med. 2012;7:8–13. [Google Scholar]
- 13.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 14.Kakkar P, Dos B, Viswnathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem. 1984;21:130–2. [PubMed] [Google Scholar]
- 15.Sinha KA. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 16.Rotruck JT, Pope AL, Ganther HE, Swanson AB. Selenium: Biochemical roles as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 17.Habig WJ, Pabst M, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 18.Carlberg I, Mannervik B. Purification and characterization of glutathione reductase from calf liver. An improved procedure for affinity chromatography on 2’,5’-ADP–Sepharose 4B. Anal Biochem. 1981;116:531–6. doi: 10.1016/0003-2697(81)90398-5. [DOI] [PubMed] [Google Scholar]
- 19.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 20.Lowry OH, Rosebrough NJ, Farr Al, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 21.Fu Z, Zhang W, Zhen W, Lum H, Nadler J, Bassaganya-Riera J, et al. Genistein induces pancreatic β-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology. 2010;151:3026–37. doi: 10.1210/en.2009-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolzan AD, Bianchi MS. Genotoxicity of streptozotocin. Mutat Res. 2002;512:121–34. doi: 10.1016/s1383-5742(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 23.Rai PK, Jaiswal D, Mehta S, Rai DK, Sharma B, Watal G. Effect of Curcuma longa freeze dried rhizome powder with milk in STZ-induced diabetic rats. Ind J Clin Biochem. 2010;2:175–81. doi: 10.1007/s12291-010-0032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maruthupandian A, Mohan VR, Sampathraj R. Antidiabetic, antihyperlipidaemic and antioxidant activity of Wattakaka volubilis (L.F) Stapf leaves in alloxan induced diabetic rats. Int J Pharma Sci Res. 2010;1:83–90. [Google Scholar]
- 25.Navarro C, Montilla P, Martin A, Jimenez J, Utrilla P. Free radicals scavenger and antihepatotoxic activity of Rosmarinus. Planta Med. 1993;59:312–4. doi: 10.1055/s-2006-959688. [DOI] [PubMed] [Google Scholar]
- 26.Pactrick EE, Item JA, Eyong UE, Godwin EE. The antidiabetic efficacy of combined extracts from two continental plants: Azadirachta indica and Vernonia amygdalina (Africa bitter leaf) Am J Biochem Biotechnol. 2008;4:239–44. [Google Scholar]
- 27.Elbirt KK, Bonkovsky HL. Heme oxygenase: Recent advances in understanding its regulation and role. Proc Assoc Am Physicians. 1999;111:438–47. [PubMed] [Google Scholar]
- 28.Hayashi S, Takamiya R, Yamaguchi T. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: Role of bilirubin generated by the enzyme. Circ Res. 1999;85:663–71. doi: 10.1161/01.res.85.8.663. [DOI] [PubMed] [Google Scholar]
- 29.Rana SV, Singh R, Verma S. Protective effects of few antioxidants on liver function in rats treated with cadmium and mercury. Indian J Exp Biol. 1996;34:177–9. [PubMed] [Google Scholar]
- 30.Abd El-Baky A, Abdulla A, Abd El-Mawgoud H, Abd El-Hay E. Hypoglycemic and hypolipidaemic action of bitter melon on normoglycemic and hyperglycemic diabetic rats. Res J Med Med Sci. 2009;4:519–25. [Google Scholar]
- 31.Aurell M, Bjorck S. Determination of progressive renal disease in diabetes mellitus. Kidney Int. 1992;4:38–42. [PubMed] [Google Scholar]
- 32.Kade IJ, Barbosa NB, Ibukun EO, Igbakin AP, Nogueira CW, Rocha JB. Aqueous extracts of Sphagneticola trilobata attenuates streptozoticin-induced hyperglycaemia in rat models by modulating oxidative stress parameters. Biol Med. 2010;2:1–13. [Google Scholar]
- 33.Manikandan A, Doss DV. Effect of 50% hydroethanolic leaf extract of Ruellia tuberosa L. and Dipteracanthus patulus (Jacq.) on non-enzymatic antioxodants and other biochemical parameters in liver, kidney, serum of alloxan induced diabeteic Swiss albino rats. J Biomed Sci Res. 2010;2:190–201. [Google Scholar]
- 34.Ivorra MD, Paya M, Villar A. A review of natural products and plants as potential antidiabetic drugs. J Ethnopharmacol. 1989;27:243–75. doi: 10.1016/0378-8741(89)90001-9. [DOI] [PubMed] [Google Scholar]
- 35.Karpen CW, Pritchaed KA, Jr, Merola AJ, Panganamala RV. Alterations of the prostaglandin thromboxane ratio in streptozotocin induced diabetic rats. Prostaglandins Leukot Med. 1982;8:93. doi: 10.1016/s0262-1746(82)80001-2. [DOI] [PubMed] [Google Scholar]
- 36.Walsh MF, Pek SB. Possible role of endogenous arachidonic acid metabolites in stimulated release of insulin and glucagons from the isolated, perfused rat pancreas. Diabetes. 1984;33:929–36. doi: 10.2337/diab.33.10.929. [DOI] [PubMed] [Google Scholar]
- 37.Searle AJ, Wilson RL. Glutathione peroxidase: Effect of superoxide, hydroxyl and bromine free radicals on enzyme activity. Int J Radiat Biol Relat Stud Phys Chem Med. 1980;37:213. doi: 10.1080/09553008014550261. [DOI] [PubMed] [Google Scholar]
- 38.Kaleem M, Asif M, Ahmed QU, Bano B. Antidiabetic and antioxidant activity of Annona squamosa extract in streptozotocin-induced diabetic rats. Singapore Med J. 2006;47:670–5. [PubMed] [Google Scholar]
- 39.Dangi KS, Mishra SN. Antioxidative and a cell regeneration effect of Capparis aphylla stem extract in streptozotocin induced diabetic rat. Biol Med. 2011;3:82–91. [Google Scholar]
- 40.Palanisamy M, Ganesan K, Murugan R. Antiperoxidative and antioxidant effect of ellagic acid on normal and streptozoticin induced diabetes in albino Wistar rats. RJPBCS. 2011;2:24. [Google Scholar]
- 41.Freeman BA, Crapo JD. Biology of disease: Free radicals and tissue injury. Lab Invest. 1982;47:412. [PubMed] [Google Scholar]
- 42.Hodgson EK, Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: Inactivation of the enzyme. Biochemistry. 1975;24:5294. doi: 10.1021/bi00695a010. [DOI] [PubMed] [Google Scholar]
- 43.Ji X, Zhang P, Armstrong RN, Gilliland GL. The three-dimensional structure of a glutathione S-transferase from the mu gene class. Structural analysis of the binary complex of isoenzyme 3-3 and glutathione at 2.2-A resolution. Biochemistry. 1992;1:10169–84. doi: 10.1021/bi00157a004. [DOI] [PubMed] [Google Scholar]
- 44.Semiz A, Sen A. Antioxidant and chemoprotective properties of Momordica charantia L. (bitter melon) fruit extract. Afr J Biotechnol. 2007;6:273–7. [Google Scholar]
- 45.Andallu B, Varadacharyulu NC. Antioxidant role of mulberry leaves in streptozotocin-diabetic rats. Clin Chim Acta. 2003;338:3. doi: 10.1016/s0009-8981(03)00322-x. [DOI] [PubMed] [Google Scholar]
- 46.Kumar P, Kale RK, Mukherjee S, Prakash K, McLean P, Baquer NZ. Antidiabetic effects of Trigonella foenum-graecum seed powder in a rat model. Toxicol Environ Chem. 2011;93:2085–97. [Google Scholar]
- 47.Chakraborty U, Das H. Antidiabetic and antioxidant of Cinnamomum tamala leaf extracts STZ-treated diabetic rats. GJBBR. 2010;5:12–8. [Google Scholar]


