Abstract
Background:
Gomphostemma parviflorum (Lamiaceae) is a medicinal plant of Bangladesh which has been used traditionally in the treatment of painful and inflammatory conditions such as asthma, headache, fever, etc.
Objective:
To investigate the antinociceptive, anti-inflammatory, central nervous system (CNS) depressant and antimicrobial activities of ethanolic extracts of leaves (GPLE) and roots (GPRE) of the plant.
Materials and Methods:
The antinociceptive potentials of the extracts were studied using acetic acid-induced writhing test in mice, anti-inflammatory activity was investigated using carrageenan-induced paw edema in rats, CNS depressant activities were evaluated using pentobarbitone-induced sleeping time, Hole cross and Open field tests in mice while the anti-microbial activity was studied by in vitro disc diffusion method.
Results:
The extracts GPLE and GPRE significantly (P < 0.001) and dose dependently inhibited the acetic acid-induced writhing in mice with 73.15% and 53.69% inhibition, respectively at the dose of 200 mg/kg. At the same dose GPLE and GPRE significantly inhibited carrageenan-induced rats paw edema at the end of 4 hour with 35.54% and 28.17% inhibition, respectively. The extracts significantly prolonged the pentobarbitone-induced sleeping time and decreased the locomotory activities in open field and Hole cross tests in mice. The GPLE showed strong antimicrobial activity against Gram-positive and Gram-negative bacteria with zones of inhibition ranging from 8 to 20 mm at a concentration of 400 μg/disc.
Conclusion:
The findings of the study indicate that the leaves and roots of G. parviflorum possess antinociceptive, anti-inflammatory and CNS depressant activity and revealed the antimicrobial activities of leaves extract of the plant. The results justify the traditional use of the plant in the treatment of painful and inflammatory disorders.
Keywords: Anti-inflammatory, antimicrobial activities, antinociceptive, central nervous system depressant, Gomphostemma parviflorum, writhing
INTRODUCTION
Medicinal plants have been used since the beginning of human civilization to treat different diseases and thus represent a rich source of new molecules with pharmacological properties, which are lead compounds for the development of new drugs. Gomphostemma parviflorum var. parviflorum Wall. (Family: Lamiaceae) commonly known in Bangladesh as Jateri bormala and Kudugo-jhunjhuni, is an important medicinal plant and usually found in the shade of tropical green-forest. The plant is a tall robust perennial herb and distributed throughout India, Myanmar, Thailand, Southeast China, Malaysia, and Bangladesh.[1] The species grows in hilly forest areas of the central and eastern parts of Bangladesh. In Bangladesh, the plant is used traditionally in the treatment of asthma and hepatomegaly. The leaf-paste of the plant is applied on the forehead of the patient suffering from giddiness, headache, and fever. Roots are used for irregular menstruation.[1] The leaves of the plant contain two nortriterpenoids, Gomphoparvins A, and Gomphoparvins B, asperphenamate,[2] ergosta-7,22-diene-3,5,6,9-tetrol,[3] ergosta-7,22-dien-3-ol,[4] β-sitosterol,[5] and daucosterol.[6]
Though the plant has traditionally been used in the treatment of various types of pain and inflammatory disorders in Bangladesh, to date, no scientific reports exist about the possible antinociceptive, anti-inflammatory, and other biological activities of Gomphostemma parviflorum in experimental animals. Therefore, the present study was designed to evaluate the antinociceptive, anti-inflammatory and central nervous system (CNS) depressant activity of the ethanolic extracts of leaves and roots of the plant in experimental animals. The in vitro antimicrobial activity of the plant extracts was also investigated.
MATERIAL AND METHODS
Chemicals and reagents
The chemicals used were: ethanol and acetic acid (Merck, Germany), carrageenan and pentobarbitone (Sigma Chemicals, USA), diclofenac sodium, and diazepam (Square Pharmaceuticals Ltd; Dhaka, Bangladesh). Normal saline solution (0.9% NaCl) was collected from Orion Infusion Ltd, Bangladesh. Dimethylsulfoxide and Tween-80 were from Sigma –Aldrich and rests of the chemicals used were of BDH and E-Merck analytical grade.
Plant material
The leaves and roots of Gomphostemma parviflorum were collected from Dinajpur, Bangladesh in October 2009 at the mature stage. The plant sample was identified and a voucher specimen (accession No: DACB 35246) was deposited in Bangladesh National Herbarium for future reference. The roots and leaves part of the plant, after cutting into small pieces were dried in shade at temperature between 21 and 30°C for 7 days. The plant materials were then oven dried for 24 hours at considerably low temperature for better grinding. The cutting pieces were pulverized by a mechanical grinder and passed through a 60-mesh sieve to obtain fine powder and stored into an air-tight container.
Extraction and sample preparation
Pulverized powdered leaves and roots (600 g each) of the plant were taken in separate clean, round bottomed flask (5 l) and soaked in 4 l of ethanol at room temperature for 15 days with occasional shaking and stirring. The whole mixture was then filtered through cotton plug followed by Whatman No.1 filter paper and the filtrate thus obtained was evaporated to dryness (45°C) under reduced pressure by Heidolph rotary evaporator. The concentrated extract was then air dried to solid residue. The weight of the crude ethanolic extracts obtained from the powdered leaves and roots were 6.5 and 8 g, respectively. The extracts and standard drug diclofenac sodium were suspended in normal saline using 0.1% Tween-80.
Phytochemical screening
The freshly prepared crude ethanolic extract of leaves and roots were qualitatively tested for the presence of alkaloids, tannins, reducing sugar, flavonoids, steroids, terpenoids, and anthraquinone by standard phytochemical methods.[7,9]
Experimental animals
Swiss albino mice (weighing 20-25 g, aged 4-5 weeks) and Long Evans rats (weighing 100-150 g) of either sex were collected from the animal resource branch of the International Centre for Diarrheal Diseases and Research, Bangladesh (ICDDR,B). The animals were kept in polyvinyl cages in groups of five animals each under controlled room temperature (25 ± 2°C) in the laboratory environment (12h dark/12 h light cycle) for 7 days for acclimatization. They were given standard feed developed by ICDDR,B and water ad libitum. The animals were fasted overnight and weighed before the experiment. The design and performance of research study involving mice and rats have been approved by the Ethical Review Committee, Faculty of Biological Science, University of Dhaka through the submission of a research protocol before the study.
Acute toxicity study
The intraperitoneal acute toxicity and lethality of ethanolic extract of leaves and roots were determined in mice using the method described by Lorke[10] with slight modification. Eighteen experimental animals were randomly selected and divided into six groups consisting 3 mice in each group. The test was divided into two stages. In stage one, nine randomly selected mice of both sexes were divided into 3 groups (n = 3) and received 10, 100, and 1000 mg/kg of ethanolic extract, respectively and observed for number of deaths in 24 h. After 24 h, based on a record of zero death in the stage one test, a fresh batch of animals were divided into 3 groups (n = 3) and received 2000, 3000, and 5000 mg/kg of ethanolic extract. The number of deaths in each group within 24 h was recorded and the final LD50 values were calculated as the geometric mean of the highest nonlethal dose (with no deaths) and the lowest lethal dose (where deaths occurred).
Acetic acid-induced writhing response in mice
The response to an intraperitoneal injection of acetic acid solution (i.e., the contractions of the abdominal muscles and stretching of hind limbs) was studied according to procedures described by Koster et al.[11] Initially, the mice were divided into five groups (n = 5). Subsequently, GPLE (200, and 400 mg/kg), GPRE (200, and 400 mg/kg), vehicle (saline/Tween 80 0.1%, 10 mL/kg, as control group), and diclofenac sodium (50 mg/kg) were administered p.o. 40 min before an injection of acetic acid (0.6%, 10 mL/kg). The forty minutes interval between the oral administration of test materials and intraperitoneal administration of acetic acid was given to assure proper absorption of the administered samples. Five minutes after the administration of acetic acid, each animal was isolated in an individual observation chamber and the cumulative number of writhing responses was recorded for 15 min. Percentage inhibition of writhing in comparison to control group was taken as an index of analgesia and was calculated using the following formula:
Inhibition (%) = [(Wc -Wt)/Wc ] × 100
where Wc is the average number of writhing reflex in the control group and Wt is the average number of writhing in the test groups.
Carrageenan-induced rat's paw edema test
The ethanolic extracts of leaves and roots of the plant were subjected to screening for anti-inflammatory activity by the carrageenan-induced rat paw edema test.[12,13] Thirty experimental animals were randomly selected and divided into six groups consisting of 5 rats in each. Prior to any treatment, each rat was weighed properly and the doses of the test samples and control materials were adjusted accordingly. Group I was kept as control giving 0.1% tween-80 in normal saline water and Group II received the standard anti-inflammatory agent diclofenac sodium (10 mg/kg p.o.). Group III and IV were given ethanolic extracts of leaves of the plant at a dose of 200, and 400 mg/kg body weight p.o., respectively, while Group V and VI were administered ethanolic extracts of roots of the plant (200, and 400 mg/kg body weight p.o.). One hour after administration of the various agents, acute hind paw edema was produced by injecting 0.1 mL of carrageenan (1%, prepared as a suspension in distilled water plus Tween 80 at 0.1%) locally into the subplantar tissue of the right hind paw of each rat of each group. The paw volumes were measured by plethysmometer (Model 7140, Ugo Basile, Italy) prior to and at 1, 3 and 4 h after the carrageenan injection. Thus edema volumes in control and in groups treated with test materials were calculated and the percentage inhibition of paw edema was determined according to the following formula:
% Inhibition = [(Vc - Vt)/Vc ] × 100
where Vc and Vt represent average paw volume of control and treated animals respectively.
CNS depressant activities
The ethanolic extract of the leaves and roots of G. parviflorum were assessed for effect on the CNS using a number of neuropharmacological experimental models in mice.
Pentobarbitone-induced sleeping time test
Thirty minutes after the oral administration of GPLE (200, and 400 mg/kg), GPRE (200, and 400 mg/kg), vehicle control (1% Tween-80 solution in saline, 10 mL/kg) and intraperitoneal injection of diazepam (1 mg/kg), all mice were injected with sodium pentobarbital (50 mg/kg, i.p.). The animals were observed for the latent period (time between pentobarbitone administration to loss of righting reflex) and duration of sleep (time between the loss and recovery of righting reflex.[14,15]
Open field test
The effect of the ethanolic extracts of leaves and roots of the plant on the spontaneous locomotor activity of the experimental animals was evaluated by the method described by Gupta et al.[16] Thirty mice were divided into six groups (n = 5). GPLE, (200 and 400 mg/kg; p.o.), GPRE (200, and 400 mg/kg; p.o.), vehicle control (1% Tween-80 solution in saline, 10 mL/kg; p.o.) and diazepam (1 mg/kg, i.p.) were administered to different groups. The floor of an open field of half square meter was divided into a series of squares each alternatively colored black and white. The apparatus had 40-cm height a wall. The number of squares visited by the animals was counted for 3 min, on 0, 30, 60, and 90 min during the study period.
Hole cross test
The method described by Takagi et al.[17] was adopted for this study. A steel partition was fixed in the middle of a cage having a size of 30 × 20 × 14 cm. A hole of 3 cm diameter was made at a height of 7.5 cm in the center of the cage. Thirty mice were divided into six groups (n = 5) and the respective groups were administered with vehicle (1% Tween-80 solution in saline, 10 mL/kg p.o.), GPLE (200, and 400 mg/kg p.o.), GPRE (200, and 400 mg/kg p.o.) and diazepam (1 mg/kg i.p.). After the treatment, the number of passages of a mouse through the hole from one chamber to other was counted for a period of 3 min on 0, 30, 60, and 90 min during the study period.
Antimicrobial screening
The disc diffusion method (Bauer et al., 1966; Rios et al., 1988; Barray, 1980)[18,19,20] was used to evaluate the antimicrobial activities of ethanolic crude extracts of leaves and roots of the plant. The bacterial and fungal strains used for the experiment were collected as pure culture from the Institute of Nutrition and Food Sciences (INFS), University of Dhaka. The ethanolic extracts of leaves and roots were dissolved in calculated volumes of solvent (Chloroform) to obtain the desired concentrations and applied to sterile discs (6 mm diameter) at a concentration of 400 μg/disc and carefully dried to evaporate the residual solvents in an aseptic hood. Discs containing the test samples were placed on nutrient agar medium uniformly seeded with the test microorganisms. Standard antibiotic Kanamycin (30 μg/disc) discs and blank discs (impregnated with solvents) were used as positive and negative control, respectively. These plates were kept at 4°C for 24 hours to allow maximum diffusion of the test materials to the surrounding media. The plates were then inverted and incubated at 37°C for 24 hours for optimum growth of the organisms. The antimicrobial activity of the test agents was then determined by measuring the diameter of zone of inhibition expressed in millimeter. The experiment was carried out in duplicate.
Statistical analysis
All data are presented as mean ± standard error of the mean (SEM) and the differences between control and treated groups were evaluated by one-way analysis of variance (ANOVA) followed by Dunnett's t test. In all cases differences were considered significant if P < 0.05. The statistical analysis was carried out using the SPSS program (version 17.0).
RESULTS
Phytochemical screening
Preliminary qualitative analysis revealed that the root part of G. parviflorum is reach in tannin, alkaloid, flavonoid, carbohydrate, terpenoid, and steroid whereas the leaf part is reach in alkaloid, flavonoid, carbohydrate, terpenoid, and steroid [Table 1].
Table 1.
Result of chemical group test of the ethanol extracts of leaves (GPLE) and roots (GPRE) of Glomphostemma parviflorum

Acute toxicity study
The LD50 of ethanolic extract of the leaves of G. parviflorum was found to be 15, 00 mg/kg. Absence of death at all doses up to 5000 mg extract/kg showed that the LD50 of the ethanolic extract of roots of the plant is greater than 5000 mg/kg body weight.
Acetic acid–induced abdominal writhing
At the dose of 200, and 400 mg/kg body weight p.o. the ethanolic extract of leaves (GPLE) dose dependently produced a significant (P < 0.001) reduction in the number of writhes with 73.15% and 91.28% of inhibition, respectively when compared to the control group which were even higher than that of the standard drug diclofenac sodium (67.79% inhibition; P < 0.001) [Table 2]. The ethanolic extract of roots of G. parviflorum (GPRE) also significantly and dose dependently produced 53.69 and 73.83% of writhing inhibition respectively at the doses of 200, and 400 mg/kg p.o. [Table 2].
Table 2.
Effect of ethanolic leaf (GPLE) and root extract (GPRE) of G. parviflorum against acetic acid-induced writhing in mice
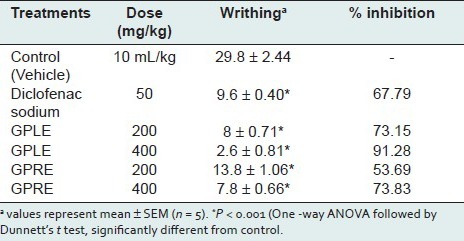
Carrageenan-induced hind paw edema
In the carrageenan-induced rat's paw edema test, ethanolic extract of leaves (GPLE) significantly (P < 0.001) and dose dependently reduced edema in rats at the 4th hour after carrageenan injection with 19.85 and 35.54% inhibition of paw edema at a dose of 200, and 400 mg/kg body weight p.o., respectively [Table 3]. The rate of anti-inflammatory activity of GPLE (400 mg/kg) was increased with time and reached the peak level at the fourth hour of the study which was comparable to that of standard drug diclofenac sodium (40.26% of inhibition; P < 0.01). The ethanolic extract of roots (GPRE) also significantly (P <0.001) protected the rats against carrageenan-induced edema with 23.06 and 28.17% inhibition of edema after 4th hour of carrageenan injection at a dose of 200, and 400 mg/kg body weight p.o., respectively [Table 3]. However, the effect was not dose dependent.
Table 3.
Effect of ethanolic extracts of leaves (GPLE) and roots of G. parviflorum (GPRE) on carrageenan-induced rat paw edema
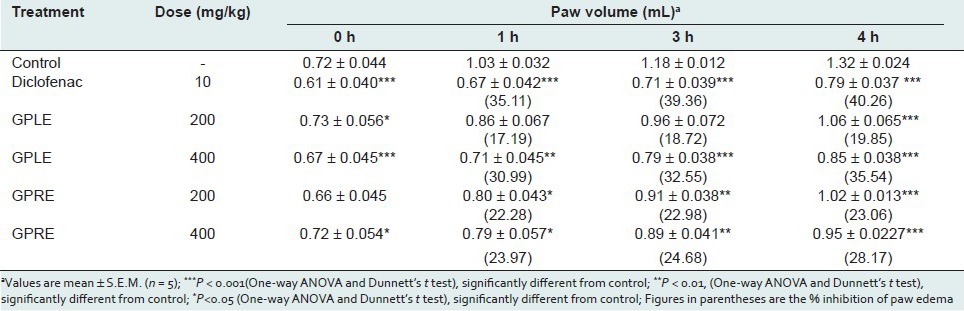
Pentobarbitone-induced sleeping time
In the pentobarbitone-induced hypnosis test, the ethanolic extract of leaves and roots of G. parviflorum at the doses of 200, and 400 mg/kg significantly induced the sleep at an earlier stage and also prolonged the duration of sleeping time in test animals as compared to control [Table 4]. Administration of GPLE at doses of 200, and 400 mg/kg significantly and dose dependently (P <0.01) increased the recovery time from the pentobarbitone-induced sleep by 82.23%, and 123.02%, respectively, compared to the control group. The effect was comparable to that of standard drug diazepam (130.26 % prolongation). GPRE also prolonged the duration of pentobarbitone-induced sleeping time by 15.78%, and 30.26% compared to the control group at the doses of 200, and 400 mg/kg p.o. but the effect was not found to be statistically significant.
Table 4.
Effect of ethanolic extract of leaves (GPLE) and roots of G. parviflorum (GPRE) on phenobarbitone-induced sleeping time in mice
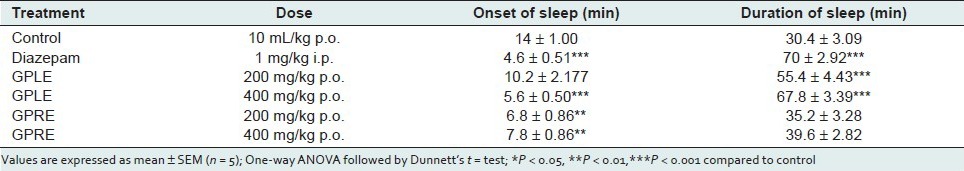
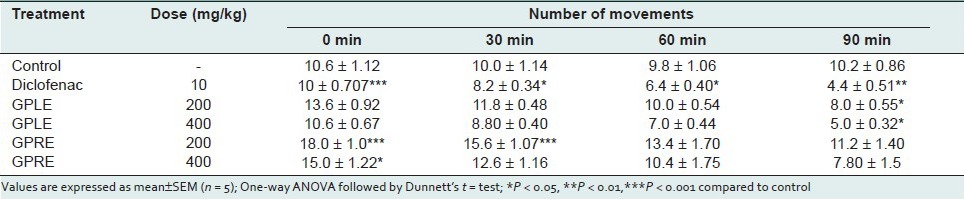
Open field and hole cross test in mice
In the open field test, the ethanolic extract of leaves (GPLE) and roots (GPRE) of G. parviflorum showed a dose dependent and significant (P < 0.001) decrease in locomotion in the test animals during the observation period at the dose levels of 200, and 400 mg/kg body weight [Table 5]. The depressant actions were comparable to that of standard drug diazepam (P < 0.001). In the hole cross test, the extracts also showed a dose-dependent decrease in locomotion in the test animals from the second observation period at both dose levels (200 and 400 mg/kg body weight) [Table 6].
Table 5.
Effect of ethanolic extract of leaves (GPLE) and roots of G. parviflorum (GPRE) on locomotor activity of mice in Open field test
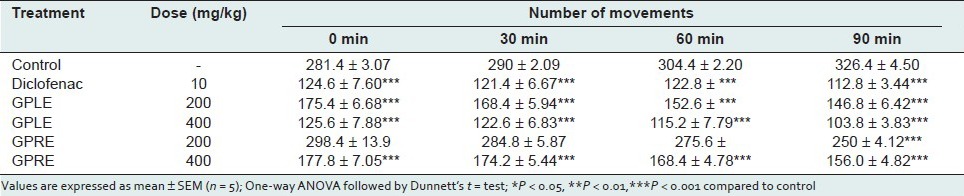
Table 6.
Effect of ethanolic extract of leaves (GPLE) and roots of G. parviflorum (GPRE) on locomotor activity of Mice in Hole cross test
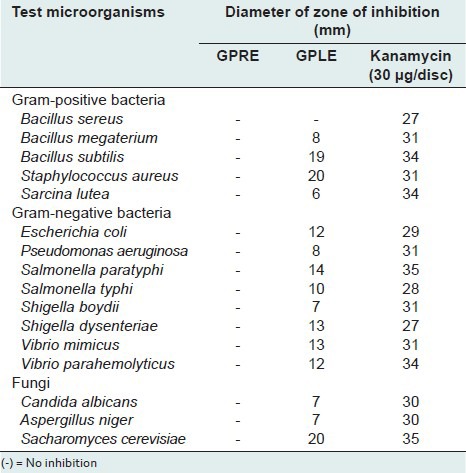
In vitro antimicrobial screening
The zones of inhibition produced by the ethanolic extract of leaves of Gomphostemma parviflorum (GPLE) were ranged from 8 to 20 mm at a concentration of 400-μg/disc. The GPLE showed strong antimicrobial activity against two Gram-positive bacteria Staphylococcus aureus and Bacillus subtilis and a fungi Saccharromyces cerevisiae (zone of inhibition 20, 19, and 20 mm, respectively) [Table 7]. GPLE also showed moderate antimicrobial activity against some Gram-negative bacteria such as E. coli, Shigella dysenteriae, Salmonella paratyphi, Salmonella typhi, Vibrio mimicus, Vibrio parahemolyticus, etc. where zones of inhibition produced were ranged from 10 to 14 mm. But the ethanolic extract of roots (GPRE) showed no antimicrobial activity.
Table 7.
Antimicrobial activity ethanolic leaf (GPLE) and root extract (GPRE) of G. parviflorum
DISCUSSION
In this study we evaluated the antinociceptive, anti-inflammatory, antimicrobial and CNS depressant activities of ethanolic extracts obtained from the leaves and roots of G. parviflorum using acetic acid-induced writhing in mice, carrageenan-induced edema tests in rodents, in vitro disc diffusion method and a number of neuropharmacological tests in mice, respectively. The LD50 of ethanolic extract of the leaves (15, 00 mg/kg) and roots ( > 5000 mg/kg) of G. parviflorum, found in the acute toxicity study indicated that the GPLE and GPRE extracts were safe at the given doses to the experimental animals.
The acetic acid-induced writhing test has been used widely for the evaluation of peripheral antinociceptive activity.[21]
This method is not only simple and reliable, but also affords rapid evaluation of antinociceptive action.[22,23] The nociceptive mechanism of abdominal writhing induced by acetic acid involves the process of release of arachidonic acid metabolites prostaglandins and leukotrienes via COX (cyclooxygenase) and lipo-oxygenase pathway[24] and other endogenous pain mediators, such as histamine, serotonin (5-HT), bradykinin, and substance P that sensitize pain nerve endings.[22,25,26] In the first 30 min after acetic acid injection, the levels of prostaglandins PGE2 and PGF2α was found to be increased during writhing test.[27] The ability of the extracts (GPLE and GPRE) to attenuate the acetic acid-induced writhing in mice suggests that they possess peripherally mediated antinociceptive activity[28] which might be due to its possible interference in the biosynthesis, release and/or action of some chemical agents such as prostaglandins and leukotrienes from cyclo-oxygenase and lipo-oxygenase pathway.
The carrageenan-induced rat paw edema assay has frequently been used to evaluate the anti-inflammatory effect of natural products. The induction of edema by using carrageenan is believed to be biphasic in nature.[29] The initial phase (1–2 h after carrageenan injection) of carrageenan paw edema is mediated by histamine and serotonin released from mast cells in the surroundings of the damaged tissues. The second phase of inflammatory reaction is attributed to the production and release of bradykinin, protease, arachidonate metabolites (prostaglandins and leukotrienes) and lysosome.[29,30,31,32] In our experiments, since GPLE and GPRE extracts showed peak inhibition of paw edema in rats in the second phase of edema (at 3-4 h after carrageenan injection), the observed anti-inflammatory activity may be due to inhibition of cyclooxygenase and/or 5-lipoxygenase, both enzymes involved in the formation of prostaglandins and leukotrienes. This edematous response was also significantly reduced in rats pretreated with diclofenac sodium, a nonsteroidal anti-inflammatory agent, known to be a cyclooxygenase inhibitor.
The phytochemical study revealed that the ethanolic extract of leaves and roots of the plant contain sterols, alkaloids, terpenoids and flavonoids. The leaves of the plant was reported to contain two nortriterpenoids, Gomphoparvins A and Gomphoparvins B and some sterols such as ergosta-7,22-diene-3,5,6,9-tetrol, ergosta-7,22-dien-3-ol, β-sitosterol and daucosterol. The antinociceptive and anti-inflammatory activities showed by GPLE and GPRE extracts may be attributed to the presence of one or a combination of steroids, alkaloids, flavonoids, and terpenoids as previously it has been reported that alkaloids,[33,34] flavonoids,[33,35,37] terpenoids,[36] and steroidal compounds[37,38] possess antinociceptive and/or anti-inflammatory activities.
This study has established the central nervous system depressant properties of ethanolic extracts of leaves and roots of the plant. The potentiation of action of pentobarbitone (prolongation of sleeping time) and the significant decrease in the spontaneous motor activity in Open field and Hole cross tests indicated the presence of compounds with a CNS depressant action in leaves and roots of G. parviflorum. Pentobarbitone when given at appropriate dose induces sedation or hypnosis in animals by potentiating the GABA mediated postsynaptic inhibition through an allosteric modification of GABA receptors.[22] Substances that have CNS depressant activity either decrease the time for onset of sleep or prolong the duration of sleep or both. Since GABA is the major inhibitory neurotransmitter in the CNS, it is possible that GPLE, and GPRE act by potentiating GABAergic inhibition in the CNS via membrane hyperpolarization which leads to a reduction in the firing rate of critical neurons in the brain. This inhibition may be due to direct activation of GABA receptor by GPLE, and GPRE. It may also be due to enhanced affinity for GABA[39] or an increase in the duration of the GABA-gated channel opening.[40]
The phytochemical research based on ethnopharmacological information is generally considered an effective approach in the discovery of new anti-infective agents from higher plants. The data from the in vitro antimicrobial screening test showed that the ethanolic extract of leaves of G. parviflorum (GPLE) showed moderately potent and broad spectrum antimicrobial activities. It has been reported that alkaloids and flavanoids are known to have curative activity against several pathogenic bacteria and fungus.[41] The broad antibacterial activities of the extract GPLE might be as a result of these compounds (alkaloids and flavanoids). Thus, the GPLE extract may be a potential source of new anti-infective agents and it may be used in the treatment of infection.
CONCLUSIONS
The findings of the study indicate that the leaves and roots of G. parviflorum possess antinociceptive, anti-inflammatory and CNS depressant activity and revealed the antimicrobial activities of leaves of the plant. These results validated the traditional use of the plant parts in the treatment of painful and inflammatory disorders like headache, fever, asthma, etc. Further studies are required to establish the potential mechanism of action of these activities and elucidate structure of the active phytoconstituents responsible for these bioactivities.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Uddin SN. 1st ed. Dhaka, Bangladesh: National Herbarium; 2006. Traditional uses of Ethnomedicinal plants of the Chittagong Hill Tracts. [Google Scholar]
- 2.Wang Q, Luo SD, Ju P, Wang YF. Two Novel Nortriterpenoids from Gomphostemma parviflorum. Helv Chim Acta. 2007;90:1360–5. [Google Scholar]
- 3.Yue JM, Chen SN, Lin ZW, Sun HD. Sterols from the fungus Lactarium volemus. Phytochemistry. 2001;56:801–6. doi: 10.1016/s0031-9422(00)00490-8. [DOI] [PubMed] [Google Scholar]
- 4.Keller AC, Maillard MP, Hostettmann K. Antimicrobial steroids from the fungus Fomitopsis pinicola. Phytochemistry. 1996;41:1041–6. doi: 10.1016/0031-9422(95)00762-8. [DOI] [PubMed] [Google Scholar]
- 5.Kojima H, Sato N, Hatano A, Ogura H. Sterol glucosides from Prunella vulgaris. Phytochem. 1990;29:2351–5. [Google Scholar]
- 6.Calis I, Lahloub MF, Rogenmoser E, Sticher O. Isomartynoside, a phenylpropanoid glycoside from Galeopsis pubescens. Phytochem. 1984;23:2313–5. [Google Scholar]
- 7.Trease GE, Evans WC. 11th ed. London: Brailliar Tindall Ltd; 1989. A text book of Pharmacognsy. [Google Scholar]
- 8.Sofowora A. Ibadan, Nigeria: Spectrum Books Ltd; 1993. Medicinal Plants and Traditional Medicine in Africa. [Google Scholar]
- 9.Ghani A. 2nd ed. Dhaka: Asiatic society of Bangladesh; 2003. Medicinal plants of Bangladesh with chemical constituents and uses. [Google Scholar]
- 10.Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54:275–87. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 11.Koster R, Anderson M, De Beer EJ. Acetic acid for analgesic screening. Fed Proc. 1959;18:412–6. [Google Scholar]
- 12.Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 13.Roy A, Roy SM, Gupta JK, Lahiri SC. A simple device for rapid measurement of rat paw oedema for evaluation of anti-inflammatory activity. Indian J Physiol Pharmacol. 1980;24:369–72. [PubMed] [Google Scholar]
- 14.Ferrini R, Miragoli G, Taccardi B. Neuro-pharmacological studies on SB 5833, a new psychotherapeutic agent of the benzodiazepine class. Arzneimittelforschung. 1974;24:2029–32. [PubMed] [Google Scholar]
- 15.Williamson EM, Okpako DT, Evans FJ. Arzneimittelforschung. 1st ed. Vol. 1. New York: Wiley and Sons; 1996. Selection, preparation and pharmacological evaluation of plant material. [Google Scholar]
- 16.Gupta BD, Dandiya PC, Gupta ML. A psycho-pharmacological analysis of behaviour in rats. Jpn J Pharmacol. 1971;21:293–8. doi: 10.1254/jjp.21.293. [DOI] [PubMed] [Google Scholar]
- 17.Takagi K, Watanabe M, Saito H. Studies of the spontaneous movement of animals by the hole cross test; effect of 2-dimethyl-aminoethanol and its acyl esters on the central nervous system. Jpn J Pharmacol. 1971;21:797–810. doi: 10.1254/jjp.21.797. [DOI] [PubMed] [Google Scholar]
- 18.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by standardized single disc method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 19.Rios JL, Recio MC, Villar A. Screening methods for natural products with antimicrobial activity: A review of the literature. J Ethnopharmacol. 1988;23:127–49. doi: 10.1016/0378-8741(88)90001-3. [DOI] [PubMed] [Google Scholar]
- 20.Rios JL, Recio MC, Villar A. Screening methods for natural products with antimicrobial activity: A review of the literature. In: Lorian V, editor. Antibiotic in laboratory medicine. Baltimore, USA: Williams and Wilkin's Co; 1980. pp. 1–23. [DOI] [PubMed] [Google Scholar]
- 21.Trongsakul S, Panthong A, Kanjanapothi D, Taesotikul T. The analgesic, antipyretic and anti-inflammatory activity of Diospyros variegata Kruz. J Ethnopharmacol. 2003;85:221–5. doi: 10.1016/s0378-8741(03)00020-5. [DOI] [PubMed] [Google Scholar]
- 22.Collier HO, Dinneen LC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh S, Majumdar DK. Analgesic activity of Ocimum sanctum and its possible mechanism of action. Int J Pharmacog. 1995;33:188–92. [Google Scholar]
- 24.Basbaum AI, Julius D. Molecular mechanisms of nociception. Nature. 2001;413:203–10. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 25.Raj PP. Pain mechanisms. In: St. Louis, editor. Pain Medicine, A Comprehensive Review. USA: Mosby-Year Book; 1996. [Google Scholar]
- 26.Ribeiro RA, Vale ML, Thomazzi SM, Paschoalato AB, Poole S, Ferreira SH, et al. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol. 2000;387:111–8. doi: 10.1016/s0014-2999(99)00790-6. [DOI] [PubMed] [Google Scholar]
- 27.Deraedt R, Jougney S, Delevalcee F, Falhout M. Release of prostaglandin E and F in an algogenic reaction and its inhibition. Eur J Pharmacol. 1980;51:17–24. doi: 10.1016/0014-2999(80)90377-5. [DOI] [PubMed] [Google Scholar]
- 28.Melo MG, Araújo AA, Rocha CP, Almeida EM, Siqueira RS, Bonjardim LR, et al. Purification, physicochemical properties, thermal analysis and antinociceptive effect of atranorin extracted from Cladina kalbii. Biol Pharm Bull. 2008;31:1977–80. doi: 10.1248/bpb.31.1977. [DOI] [PubMed] [Google Scholar]
- 29.Vinegar R, Truax JF, Selph JL, Johnston PR, Venable AL, McKenzie KK. Pathway to carrageenan-induced inflammation in the hind limb of the rat. Fed Proc. 1987;46:118–26. [PubMed] [Google Scholar]
- 30.Crunkhorn P, Meacock SC. Mediators of the inflammation induced in the rat paw by carrageenin. Br J Pharmacol. 1971;42:392–402. doi: 10.1111/j.1476-5381.1971.tb07124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Rosa M, Giroud JP, Willoughby DA. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971;104:15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- 32.Hwang SB, Lam MH, Li CL, Shen TY. Release of platelet activating factor and its involvement in the first phase of carrageenin-induced rat foot edema. Eur J Pharmacol. 1986;120:33–41. doi: 10.1016/0014-2999(86)90636-9. [DOI] [PubMed] [Google Scholar]
- 33.Larkins N, Wynn S. Pharmacognosy: phytomedicines and their mechanisms. Vet Clin North Am Small Anim Pract. 2004;34:291–327. doi: 10.1016/j.cvsm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Raaman N. Phytochemical Techniques. Pitam Pura, New Delhi: New India Publishing Agency; 2006. 2006. Categories of phytochemicals. [Google Scholar]
- 35.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernandez-Perez M, Rabanal RM. Evaluation of the antinflammatory and analgesic activity of Sideritis canariensis var. pannosa in mice. J Ethnopharmacol. 2002;81:43–7. doi: 10.1016/s0378-8741(02)00033-8. [DOI] [PubMed] [Google Scholar]
- 37.Ramaswamy S, Pillai NP, Gopalakrishnan V, Parmar NS, Ghosh MN. Analgesic effect of O-(beta-hydroxy ethyl)rutoside in mice. Indian J Exp Biol. 1985;23:219–20. [PubMed] [Google Scholar]
- 38.Ramadan A, Harraz FM, EI-Mougy SA. Anti-inflammatory, analgesic and antipyretic effects of the fruit pulp of Adansonia digitata. Fitoterapia. 1994;65:418–22. [Google Scholar]
- 39.Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem. 1997;272:32727–30. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- 40.Stanley KJ. Ionic mechanisms of neuronal excitation by in hibitory GABAa receptors. Science. 1995;269:977–81. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- 41.Hassan MM, Oyewala AO, Amupitan JO, Abdullahi MS, Okonkwo EM. Preliminary phytochemical and antibacterial investigation of crude extracts of the root bark of Datarium microcarpum. J Chem Soc Nigeria. 2004;29:26–9. [Google Scholar]


