Abstract
Background:
The present study was designed to investigate the hepato protective effect of methanolic extract of Ficus religisoa L., Moraceae, on isoniazid-rifampicin and paracetamol induced hepatotoxicity in rats.
Materials and Methods:
Male Wistar albino rats were divided into six groups; group 1 served as a control received vehicle (Distilled water), group 2 served as a toxic control, received isoniazid-rifampicin (100 mg/ kg, i.p.) or paracetamol 200mg/kg, p.o in sterile water, groups 3, 4 and 5 received 100, 200 and 300mg/kg bw, p.o. methanolic extract of F. religisoa along with INH+RIF or paracetamol and group 6 received Liv 52 as reference standard. All the treatment protocols followed 21 days for INH+RIF model and seven days for paracetamol model, after treatment rats were sacrificed and blood was used for biochemical and liver was used for histological studies.
Results:
Administration of INH+RIF and paracetamol caused a significant elevation in the levels of liver marker enzymes (P < 0.05 and P < 0.01) and thiobarbituric acid reactive substances (P < 0.001) in experimental rats. Administration of methanolic extracts of F. religisoa significantly prevented isoniazid-rifampicin and paracetamol induced elevation in the levels of serum diagnostic liver marker enzymes and TBARS level in experimental groups of rats. Moreover, total protein and reduced glutathione levels were significantly (P < 0.001) increased in treatment group. The effect of extract was compared with a standard drug, Liv 52. The changes in biochemical parameters were supported by histological profile.
Conclusion:
The methanolic extract of F. religisoa protects against isoniazid- rifampicin and paracetamol induced oxidative liver injury in rats.
Keywords: Drug induced liver injury, Flavonoids, hepatotoxin, oxidative stress
INTRODUCTION
Liver is a key organ that regulates metabolism, secretion, storage, and detoxifying functions in the body, and hepatic damage is often associated with distortion of these functions. Drug-induced liver toxicity is a common cause of liver injury. It accounts for approximately one-half of the cases of acute liver failure and mimics all forms of acute and chronic liver disease.[1] Different types of drugs such as acetaminophen, chloroquine and isoniazid are inducers of hepatotoxicity in world. Most of the hepatotoxic chemicals damage liver cells mainly by inducing lipid peroxidation and other oxidative damages. Liver possesses a unique metabolism and plays a pivotal role in the removal of substances from the portal circulation due to which it is susceptible to toxicity of drugs, xenobiotics, and oxidative stress. The current treatment for hepatotoxicity includes drugs which influence the p-450 enzyme mechanism either by inhibiting (amiodarone, cimetidine, ciprofloxacin, etc.) or inducing (rifampicin, carbamazepine, phenobarbital, phenytoin) the metabolic activity of enzymes.[2] Herbal medicines have put forward a number of formulations for liver disorders. In this modern age it is very important to provide scientific proof to justify the various medicinal uses of herbs.[3]
Ficus religiosa (Moraceae) commonly known as Bodhi tree is regarded as a sacred tree to both Hindus as well as Buddhists; it is used for medicinal as well as religious purposes in India.[4] In Ayurveda it is claimed that Ficus religiosa possesses anticonvulsant activity.[5] Many such reports have been validated pharmacologically for its actions on CNS viz.: different parts of Ficus religiosa showed acetyl cholinesterase inhibitory activity[5] and antianxiety activity.[7] Fruits of this plant contain numerous amino acids like asparagine and tyrosine in fruit edible part, alanine, threonine, tyrosine, and valine in seeds, alanine and valine in proteins.[8] Apart from amino acids fruits of this plant has been reported to contain highest amount of serotonin (5-HT) as compare to fruits of other Ficus species.[9,10] Singh et al,[11] reported the anticonvulsant activity of methanolic extract of Ficus religiosa. The aqueous extract of leaves was reported for Antidiabetic activity by Srinivasan BP et al.[12] The hepatoprotective activity is not yet evaluated in this plant but most of the ficus species were reported for hepato protective activity[13]. Hence the present study was undertaken to investigate hepatoproptective role of methanolic extract of Ficus religiosa leaves by isoniazid, rifampicin (INH+RIF) and paracetamol (PML) induced hepatotoxic model.
MATERIALS AND METHODS
Chemicals
Bilirubin, total protein, alkaline phosphatase (ALP), alanine transaminases (ALT), and aspartate transaminases (AST) were assayed by using kits from Ranbaxy Diagnostic, New Delhi. All the drugs, chemicals and reagents used for biochemical estimation were purchased from Sigma-Aldrich, USA.
Animals
Male Wistar albino rats, weighing about 150-200g and Swiss albino mice weighing about 25-30g were obtained from Institute Animal Center were used in the experiments. The protocol was approved by the Institute's Animal Ethical Committee (1220/a/08/CPCSEA/ANCP/04). Animals were kept in the animal house at an ambient temperature of 25°C and 45-55% relative humidity, with 12 hrs each of dark and light cycles. Animals were fed pellet diet and water ad-libitum.
Plant material and preparation of extract
The leaves of Ficus religiosa Linn were freshly collected in the month of August-September 2009 in and around of Rajampet, Andhra Pradesh, India. The plant materials were identified and authenticated by Dr. K. Madhava Chetty, Department of botany, Sri Venkateswara University, Tirupathi. The vide voucher specimen (ANCP-Medicinal Plants-019-2011) has been deposited in the department. Authenticated leaves were washed with water, shade-dried, ground to a moderately coarse powder. The powdered leaves were subjected to extraction by refluxing with methanol in a Soxhlet extractor for 72 hrs. The resultant extract was evaporated to dryness using rotavapor (Evator rotary vaccum evaporator EV111) and stored at 4 ◦C (yield: 5.3%, w/w).
Phytochemical screening
The obtained extracts were subjected to preliminary phytochemical screening and thin layer chromatography to identify the chemical constituents. TLC was performed by using mobile phase Benzene: Chloroform in the ratio of 7:3 and the compound were detected under UV chamber at 365 nm. The methods of analysis employed were those described in standard procedures.[14,15]
Hepatotoxicity induced by isoniazid and rifampicin
Isoniazid and rifampicin solution were prepared separately in sterile distilled water. Rats were treated with isoniazid (100 mg/kg, i.p.) and co-administered with rifampicin (100 mg/kg, i.p.), for 21 days.[16,17] In order to study the effect of MEFR in rat, 100, 200 and 300 mg/kg bw, p.o. were used respectively. Liv 52 (10 mg/kg bw, p.o.) was used as a standard drug in this study. Rats were divided into Six different groups (n = 6), group 1 was served as a control, group 2 was toxic control receive isoniazid+rifampicin (100 mg/kg bw i.p.), group 3 served as standard group received Liv 52 and group 4, 5 and 6 were served as extract treatment groups received 100, 200 and 300 mg/kg bw, p.o MEFR. Rats were treated as per the treatment protocol. Body weights of these rats were monitored sequentially in control and experimental animals for a period of 21 days.
Hepatotoxicity induced by paracetamol
Rats were divided into Six different groups (n = 6), group 1 was served as a control, group 2 was toxic control receive paracetamol (200 mg/kg bw p.o), group 3 served as standard group received Liv 52. and group 4,5 and 6 were served as extract treatment groups received 100, 200 and 300 mg/kg bw, p.o MEFR. Rats were treated as per the treatment protocol. Body weights of these rats were monitored sequentially in control and experimental animals for a period of 7 days. They were sacrificed 1hr after administration on seventh day and the blood was collected by retro orbital artery bleeding. Blood samples were kept for 30 minutes without any disturbance in dry test tubes. Then the supernatant layer was centrifuged for 10 minutes at 3000 rpm to separate the serum.
Biochemical estimation
Rats were sacrificed 1hr after administration on day 21 for isoniazid-rifampicin model and seventh day for paracetamol model. The blood was collected by retro-orbital artery bleeding. Blood samples were centrifuged for 10 min at 3000 rpm to separate the serum. ALP, ALT, AST, total protein and bilirubin levels were estimated from the serum by using standard kits. Liver was excised immediately, quickly cooled and perfused with cold normal saline. Ten percent homogenate was prepared by homogenizing the liver tissue by using 0.3 m phosphate buffer. TBARS[18] and GSH[19] levels were estimated from the liver homogenate by using spectrophotometric determination.
Histopathological studies
The tissues of liver were fixed in 10% formalin and embedded in paraffin wax. Sections of 4-5 microns thickness were made using rotary microtome and stained with haematoxylin-eosin and histological observations were made under light microscope.
Statistics
All values were expressed as means ± SEM (n = 6 in each group). One way ANOVA was applied to test for significance of biochemical data of the different groups. Significance is set at P ≤ 0.05.
RESULTS
Phytochemical screening
Phytochemical screening of MEFR revealed the presence of triterpenoids, alkaloids, flavonoids and phenolic compound. TLC of plant extract produced pink fluorescent compounds at 365nm and Rf value was 0.2, 0.36 and 0.58 [Figure 1].
Figure 1.

TLC of methanolic extract of Ficus relegeosa
Acute toxicity activity
Acute oral toxicity studies, the extracts treated animals were observed for mortality up to 48 hrs. There was no mortality or any signs of behavioral changes observed after oral administration of methanol extract up to 5000 mg/kg body weight.
Isoniazid – rifampicin and paracetamol induced hepatotoxicity
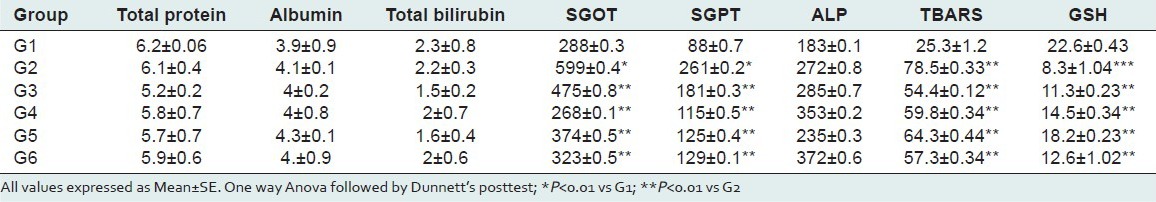
The results of biochemical parameters revealed the elevation of biochemical markers like SGPT and SGOT in toxicant treated group indicating that INH+RIF and PM induces damage to the liver. Pretreatment with methanolic extract of Ficus religeosa at three different doses significantly reduced (P < 0.01) the elevated levels of SGPT and SGOT. There is no significant different in toxicant group as well as extract treatment group for the level of Total protein and albumin and alkaline phosphatase. The extract of Ficus religiosa treated rats when compared with standard Liv 52 group and extract control group there was no significant different in biochemical parameters. The results were present in Table 1 and 2.
Table 1.
Results of biochemical parameters of INH+RIF intoxicated group
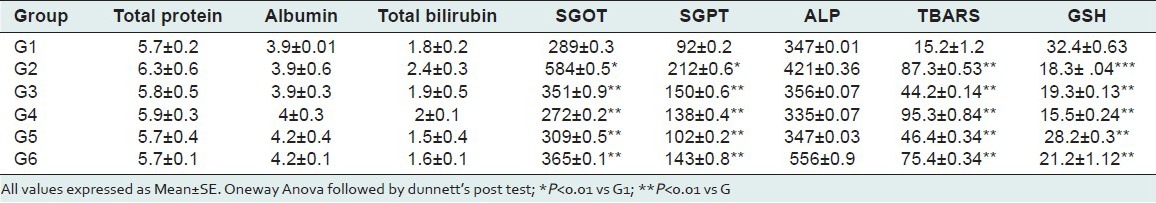
Table 2.
Results of biochemical parameters of paracetamol intoxicated group
Histopathological studies
Histopathological examination of the liver section of the rats treated with INH+RIF and PM showed an intense centrilobular necrosis and vacuolization. The rats treated with Liv 52 and methanolic extract of Ficus religiosa showed a good sign of protection against the toxicant to considerable extent as it was evident from the formation of normal hepatic cords and absence of necrosis and vacuoles [Figures 2 and 3].
Figure 2.
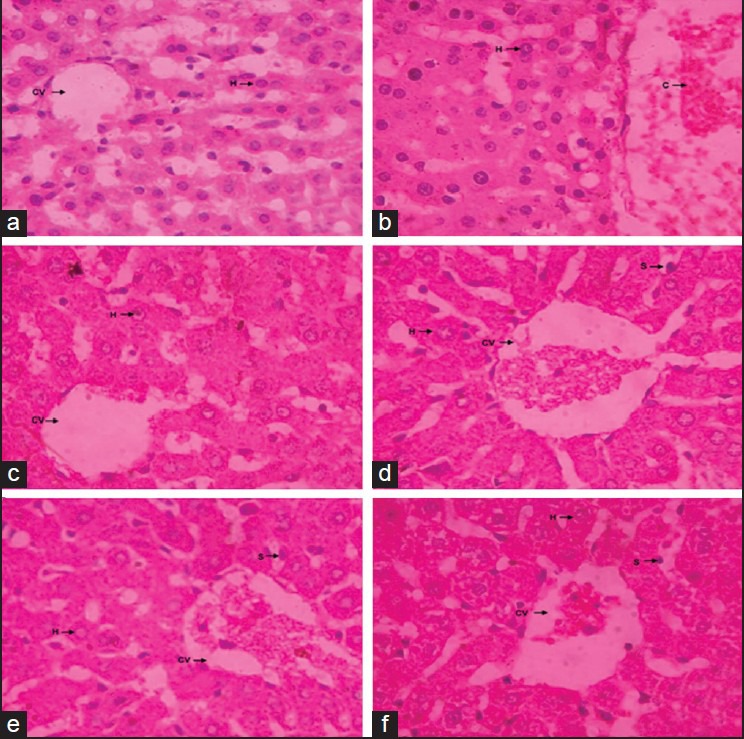
Histopathology report. (a) Hepatocytes of the normal control group showed a normal lobular architecture of the liver; (b) Hepatocytes of toxic control group showed hepatocytic necrosis and inflammation and neutrophil infiltration also observed in the centrilobular region with portal triaditis; (c) Liv 52 pretreated group showed minimal inflammation and hepatic congestion with moderate portal triditis and their lobular architecture was normal; (d-f) MEFB pretreated group at all dose of 100, 200 and 300 mg/kg showed minimal inflammation with moderate portal triditis and their lobular architecture was normal
Figure 3.
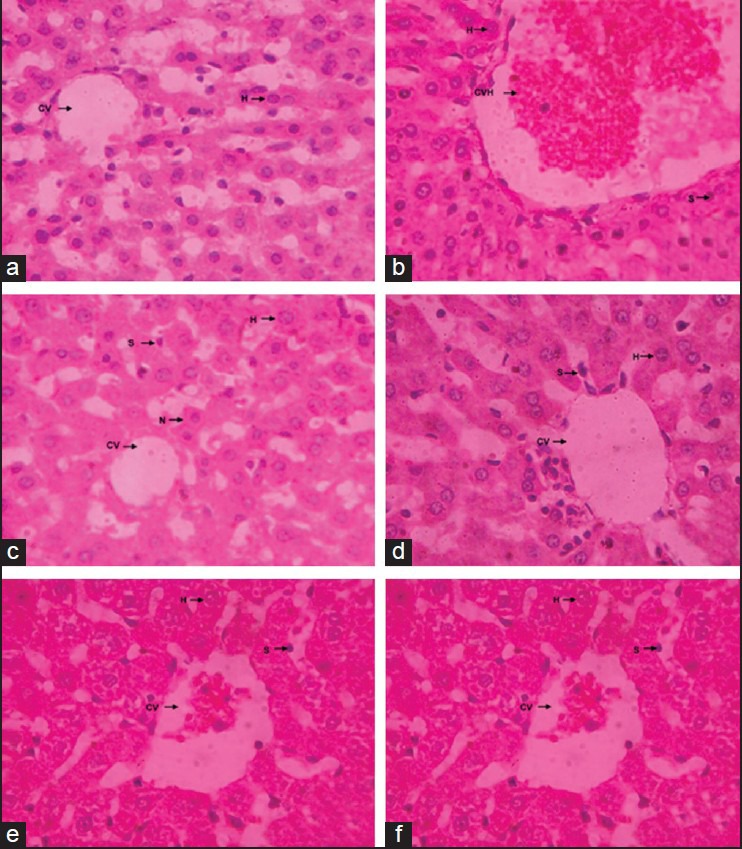
Histopathology report. (a) Hepatocytes of the normal control group showed a normal lobular architecture of the liver; (b) Hepatocytes of toxic control group showed hepatocytic necrosis and inflammation and neutrophil infiltration also observed in the centrilobular region with portal triaditis; (c) Liv 52 pretreated group showed minimal inflammation and hepatic congestion with moderate portal triditis and their lobular architecture was normal; (d-f) MEFB pretreated group at all dose of 100, 200 and 300 mg/kg showed minimal inflammation with moderate portal triditis and their lobular architecture was normal
DISCUSSION
From the phytochemical investigation the methanolic extract contains flavonoid was proved by chemical test and TLC. In UV chamber the compound shows pink fluorescent indicating the presence of flavonoids rich in the extract. During the metabolism of INH, hydrazine is produced directly (from INH) or indirectly (from acetyl hydrazine). From earlier study[20] it is evident that hydrazine play a role in INH induced liver damage in rats, which is consistent with the report by Sarich et al.[21] The combination of INH and RIF was reported to result in higher rate of inhibition of biliary secretion and an increase in liver cell lipid peroxidation, and cytochrome P450 was thought to be involved the synergistic effect of RIF on INH.[22] However, its role in INH induced hepatotoxicity is not clear, because, INH itself is an inducer of CYP2E1.[23] According to previous report it is cleared that INH itself does not produce complete damage to the liver.[24,25] INH is metabolized in the liver primarily by acetylation and hydrolysis, and these acetylated metabolites are thought to be hepatotoxins.[26,27] Previous report in rats suggest that the hydrazine metabolite of INH and is subsequent effect on CYP2E1 induction is involved in the development of INH-induced hepatotoxicity.[16] And also oxidative stress as one of the mechanism for INH+RIF induced hepatic injury.[27]
Acetaminophen was metabolically activated by cytochrome P450 enzymes to a reactive metabolite that depleted glutathione (GSH) and covalently bound to protein. It was shown that repletion of GSH prevented the toxicity. The reactive metabolite was found to be N-acetyl-p-benzoquinone imine (NAPQI), which is formed by a direct two-electron oxidation.[28] More recently, the cytochromes 2E1, 1A2, 3A4, and 2A6[29,30,31] have been reported to oxidize acetaminophen to the reactive metabolite. Acetaminophen toxicity increased formation of superoxide would lead to hydrogen peroxide and peroxidation reactions by Fenton-type mechanisms.
In this study the results suggest that the statistically significant different in biochemical parameters in toxic control group G2, indicate that hepatic damage has been induced by INH+RIF and paracetamol. Following treatment with Liv 52 and MEFR (100, 200 and 300 mg/kg), all the parameters were reduced and total protein restored to normal value. Thakare et al.[32] also reported that administration of methanolic extract of F. Religiosa revert the elevated level of serum liver marker enzymes and malondialdehyde (MDA) (an index of lipid per oxidation) formation in edematous tissue at a dose of 200 and 400 mg/kg.
Histopathology also revealed that the significant protection from the hepatic damage by the treatment of MEFR. Metabolism of chemicals takes place largely in the liver, which accounts for the organ's susceptibility to metabolism-dependent, drug induced injury. The drug metabolites can be electrophilic chemicals or free radicals that undergo or promote a variety of chemical reactions, such as depletion of reduced glutathione; covalently binding to proteins, lipids, or nucleic acids; or inducing lipid peroxidation.[1]
In present study in toxic control group increased level of TBARS (a marker for oxidative stress), reduction in the GSH concentration is indication for increased oxidative stress in INH+RIF and paracetamol treatment group. Elevation of TBARS were significantly reduced by co-administration of MEFR and Liv 52 and elevation of GSH level after MEFR and Liv 52 treatments indicate that the extracts is useful for the treatment of drug injury caused by INH+RIF and paracetamol. Recently we have reported the similar type of protective role of ficus benghalensis in INH+RIF induced hepatotoxicity model.[33]
The correlation between antioxidant activities and quantity of the flavonoids is still under discussion, a good linear relationship was observed in some published works.[34] Number of medicinal plants has been reported with good hepatoprotective activity with potential antioxidative mechanism due to presence of flavonoids and phenolic compounds.[35,36,37] The protective role of this plant against liver toxicity might be through antioxidative effect of flavonoids rich in the plant.
CONCLUSION
The present study showed that Methanolic extract of Ficus religiosa linn produce protective action against the hepatotoxicity induced by isoniazid+rifampicin and paracetamol. The hepatoprotective role of MEFR might be due to its chemical constituent like flavonoids and phenolic compound. Traditionally flavonoids produce antioxidant activity so this mechanism suggesting that the extract of plant may be useful to prevent the oxidative stress induced damage in liver. Hence MEFR may be act as prophylactic as well as curative drug in treating hepato toxic conditions. Further studies needs to isolate the active constituents and also to evaluate the exact mechanism of action.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kaplowitz N. Drug induced liver injury. Clin Infect Dis. 2004;38(Suppl 2):S44–8. doi: 10.1086/381446. [DOI] [PubMed] [Google Scholar]
- 2.Hiraganahalli DB, Chandrasekaran CV, Dethe S, Mundkinajeddu D, Pandre MK, Balachandran J, et al. Hepatoprotective and antioxidant activity of standardized herbal extracts. Phcog Mag. 2012;8:116–23. doi: 10.4103/0973-1296.96553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valiathan MS. Healing plants. Curr Sci. 1998;75:1122–7. [Google Scholar]
- 4.Kala CP, Dhyani PP, Sajwan BS. Developing the medicinal plants sector in northern India: Challenges and opportunities. J Ethnobiology Ethnomedicine. 2006;2:32–46. [Google Scholar]
- 5.Vyawahare NS, Khandelwal AR, Batra VR, Nikam AP. Herbal anticonvulsants. J Herbal Med Toxicol. 2007;1:9–14. [Google Scholar]
- 6.Vinutha B, Prashanth D, Salma K, Sreeja SL, Pratiti D, Padmaja R, et al. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J Ethnopharmacol. 2007;109:359–63. doi: 10.1016/j.jep.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Ratnasooriya WD, Jayakody JR, Dharmasiri MG. An aqueous extract of trunk bark of Ficus religiosa has anxiolytic activity. Med Sci Res. 1998;26:817–9. [Google Scholar]
- 8.Ali M, Qadry JS. Amino acid composition of fruits and seeds of medicinal plants. J Indian Chem Soc. 1987;64:230–31. [Google Scholar]
- 9.Bliebtrau JN. New York: Macmillan Company; 1968. The Parable of the Beast; p. 74. [Google Scholar]
- 10.Bagdy G, Kecskemeti V, Riba P, Jakus R. Serotonin and epilepsy. J Neurochem. 2007;100:857–73. doi: 10.1111/j.1471-4159.2006.04277.x. [DOI] [PubMed] [Google Scholar]
- 11.Singh D, Rajesh KG. Anticonvulsant effect of Ficus religiosa: Role of serotonergic pathways. J Ethnopharmacol. 2009;123:330–4. doi: 10.1016/j.jep.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 12.Kirana H, Agrawall SS, Srinivasan BP. Aqueous extract of Ficus religiosa linn. Reduces oxidative stress in experimentally induced type 2 diabetic rats. Indian J Exp Biol. 2009;47:822–6. [PubMed] [Google Scholar]
- 13.Krishna Mohan G, Pallavi E, Ravi Kumar B, Ramesh M, Venkatesh S. Hepatoprotective activity of Ficus carica Linn. leaf extract against carbon tetrachloride-induced hepatotoxicity in rats. DARU J Pharm Sci. 2007;15:162–6. [Google Scholar]
- 14.Harbone JB, Baxter HH. Washington: Taylor and Francis; 1993. Phytochemical Dictionary: A hand Book of Bioactive Compound from plants; p. 237. [Google Scholar]
- 15.Trease GE, Evans MC. London: Bailiere Tindall; 1989. Text book of Pharmacognosy; pp. 200–1. [Google Scholar]
- 16.Yue J, Peng RX, Yang J, Kong R, Liu J. CYP2E1 mediated isoniazid-induced hepatotoxicity in rats. Acta Pharmacol Sin. 2004;25:699–704. [PubMed] [Google Scholar]
- 17.Saleem TS, Christina AJ, Chidambaranathan N, Ravi V, Gauthaman K. Hepatoprotective activity of Annona squamosa Linn. on experimental animal model. Int J Appl Res Nat Prod. 2008;1:1–7. [Google Scholar]
- 18.Okhawa H, Qohishi N, Yagi K. Assay of lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 19.Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 20.Garner P, Holmes A, Ziganahina L. Tuberculosis. Clin Evid. 2004;11:1081–93. [PubMed] [Google Scholar]
- 21.Sarich TC, Youssefi M, Zhou T, Adams SP, Wall RA, Wright JM. The role of hydrazine in the mechanism of isoniazid hepatotoxicity in rabbits. Arch Toxicol. 1996;70:835–40. doi: 10.1007/s002040050347. [DOI] [PubMed] [Google Scholar]
- 22.Skakun NP, Shmanko VV. Synergistic effect of Rifampicin on hepatotoxicity of isoniazid. Antibiot Med Biotek. 1985;30:185–9. [PubMed] [Google Scholar]
- 23.Ramaiah SK, Apte U, Mehendale HI. Cytochrome P4502E1 induction increases thioacetamide liver injury in diet-restricted rats. Drug Metab Dispos. 2001;29:1088–95. [PubMed] [Google Scholar]
- 24.Yasuda K, Sato A, Chida K. Pulmonary tuberculosis with chemotherapy related liver dysfunction. Kekkadu. 1990;65:407–13. [PubMed] [Google Scholar]
- 25.Wu JC, Lee SD, Yeh PF, Chan CY, Wang YJ, Huang YS, et al. Isoniazid-Rifampicin induced hepatitis in hepatitis B carriers. Gastroentrology. 1990;98:502–4. doi: 10.1016/0016-5085(90)90846-s. [DOI] [PubMed] [Google Scholar]
- 26.Steele MA, Burk RF, Des Prez RM. Toxic hepatitis with Isoniazid and rifampicin: A meta-analysis. Chest. 1991;99:465–71. doi: 10.1378/chest.99.2.465. [DOI] [PubMed] [Google Scholar]
- 27.Peretti E, Karlaganis G, Lauterburg BH. Acetylating of acetylhydrazine, the toxic metabolite of Isoniazid, in humans: Inhibition by concomitant administration of Isoniazid. J Pharmacol Exp Ther. 1987;243:686–9. [PubMed] [Google Scholar]
- 28.Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-Acetyl-p-benzoquinone imine: A cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci USA. 1984;81:1327–31. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patten CJ, Thomas PE, Guy RL, Lee M, Gonzalez FJ, Guengerich FP, et al. Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol. 1993;6:511–8. doi: 10.1021/tx00034a019. [DOI] [PubMed] [Google Scholar]
- 30.Thummel KE, Lee CA, Kunze KL, Nelson SD, Slattery JT. Oxidation of acetaminophen to N-acetyl-p-aminobenzoquinone imine by human CYP3A4. Biochem Pharmacol. 1993;45:1563–9. doi: 10.1016/0006-2952(93)90295-8. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Koenigs LL, Thompson SJ, Peter RM, Rettie AE, Trager WF, et al. Oxidation of acetaminophen to its toxic quinone imine and nontoxic catechol metabolites by baculovirus-expressed and purified human cytochromes P450 2E1 and 2A6. Chem Res Toxicol. 1998;11:295–301. doi: 10.1021/tx9701687. [DOI] [PubMed] [Google Scholar]
- 32.Thakare VN, Suralkar AA, Deshpande AD, Naik SR. Stem bark extraction of Ficus benghalensis Linn for anti-inflammatory and analgesic activity in animal models. Indian J Exp Biol. 2010;48:39–45. [PubMed] [Google Scholar]
- 33.Angala Parameswari S, Mohamed Saleem TS, Chandrasekar KB, Madhusudhana Chetty C. Protective role of Ficus benghalensis against isoniazid-rifampicin induced oxidative liver injury in rat. Revista Brasileira de Farmacognosia Brazilian Journal of Pharmacognosy. 2012;22(3):604–10. [Google Scholar]
- 34.Gutierrez RP, Navarro YG. Antioxidant and hepatoprotective effects of the methanol extract of the leaves of Satureja macrostema. Phcog Mag. 2010;6:125–31. doi: 10.4103/0973-1296.62901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma SK, Arogya SM, Bhaskarmurthy DH, Agarwal A, Velusami CC. Hepatoprotective activity of the Phyllanthus species on tert-butyl hydroperoxide (t-BH)-induced cytotoxicity in HepG2 cells. Phcog Mag. 2011;7:229–33. doi: 10.4103/0973-1296.84237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahon K, Das S. Hepatoprotective activity of Ocimum sanctum alcoholic leaf extract against paracetamol-induced liver damage in Albino rats. Phcog Res. 2011;3:13–8. doi: 10.4103/0974-8490.79110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lima de Medeiros BJ, Costa Kd, Ribeiro JF, Silva JC, Jr, Barbosa WR, Carvalho JT. Liver protective activity of a hydroethanolic extract of Arrabidaea chica (Humb. and Bonpl.) B. Verl. (pariri) Phcog Res. 2011;3:79–84. doi: 10.4103/0974-8490.81954. [DOI] [PMC free article] [PubMed] [Google Scholar]


