Abstract
Jasminum grandiflorum belongs to the family Oleaceae and is known to have anti-inflammatory, antimicrobial, antioxidant, and antiulcer activities. The present study was undertaken to study its analgesic and anticonvulsant effects in rats and mice. The antinociceptive activity of the hydroalcoholic extract of J. grandiflorum leaves (HEJGL) was studied using tail flick and acetic acid – induced writhing method. Similarly, its anticonvulsant activity was observed by maximal electroshock (MES) method and pentylenetetrazol (PTZ) method. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Dunnett's test. At doses of 50, 100, and 200 mg/kg, HEJGL showed significant analgesic and anticonvulsant effects in experimental animals. In view of its analgesic and anticonvulsant activity, the JGL extract can be used in painful conditions as well as in seizure disorders.
Keywords: Acetic acid, Jasminum grandiflorum, maximal electro shock, pentylenetetrazole, tail flick
INTRODUCTION
Jasmine (Jasminum grandiflorum) is an ornamental plant of the family Oleaceae. J. grandiflorum (chameli/yasmine) is native to tropical and warm temperate regions and cultivated in France, Italy, China, Japan, India, Morocco, and Egypt. It is a semievergreen-to-deciduous shrub reaching a length of eight meters, often with pendulous branches. The leaves are odd-pinnate shaped with seven to nine leaflets and have various uses in medicine.[1]
The plant is documented to possess beneficial effects as odontalgic, thermogenic, aphrodisiac, antiseptic, emollient, anthelmintic, deobstruent, suppurative, tonic, and useful in fixing loose teeth, ulcerative stomatitis, leprosy, skin diseases, ottorrhea, otalgia, wounds, corns, and aromatherapy. Pharmacological activities of the plant reported so far are spasmolytic, anti-inflammatory, antimicrobial, antioxidant, antiulcer, cytoprotective, chemoprotective, wound healing, and antiacne activities.[2]
On perusal of the literature, it appears that there are only a few studies on the analgesic effect of J. grandiflorum. However, its anticonvulsant activity has not been investigated as there is no report of such activity. Hence, it was of interest to study the analgesic and anticonvulsant activities of the hydroalcoholic extract of J. grandiflorum leaves (HEJGL).
Ethical clearance
The institutional animal ethics committee approved the protocol of the study (Ethics Committee, Ref. No. MC/IAEC/2/2011).
MATERIALS AND METHODS
Collection of plant
Fresh plant leaves were collected from Lloyds Nagar, Bhugaon, Wardha, Maharashtra, and were authenticated by a botanist.
Preparation of plant extract
Fresh leaves of J. grandiflorum were shade-dried and powdered. The powder was extracted with 70% ethanol and 30% distilled water (i.e., hydroalcoholic extract) using a Soxhlet extractor at 50-55°C for three days. After extraction, the extract was concentrated in an electronic hot water bath at 55°C; 50 g powder yielded 7 g (14%) of extract after drying and concentrating.
Acute oral toxicity study
An acute oral toxicity study of HEJGL was performed as described by Ghosh et al.[3] by administering the different dose levels of the extract to Wistar rats for determining the lethal dose (LD50). HEJGL was found to be nontoxic up to the dose of 2.0 g/kg body weight.
Animals
Wistar rats weighing 150-220 g (8 to 12 weeks old) and Swiss albino mice weighing 22-25 g of either sex were used for the study. The animals were procured from the Agnihotri College of Pharmacy, Wardha and housed in the animal house of the Mahatma Gandhi Institute Of Medical Sciences, Sevagram at least two weeks prior to the study, so that they could adapt to the new environment. The animal house was well maintained under standard hygienic conditions, at 22 ± 2°C, humidity (60 ± 10%) with 12-hour day and night cycle, and food and water ad libitum. For each study, the animals were divided into five groups of six animals each. Wistar rats were used for the tail flick method and maximal electroshock — induced (MES) convulsion method, whereas mice were used for acetic acid — induced writhing and pentylenetetrazol (PTZ)-induced seizures.
Group I: Control (distilled water, 10 mL/kg, p.o.)
Group II: Standard drug (1 mL/kg)
Group III: HEJGL (50 mg/kg, p.o.)
Group IV: HEJGL (100 mg/kg, p.o.)
Group V: HEJGL (200 mg/kg, p.o.)
Chemicals and drugs
Chemicals: Pentylenetetrazol, acetic acid, ethanol, and distilled water.
Drugs: Phenytoin, diazepam, aspirin, and buprenorphine. The drugs were dissolved in distilled water for injection and all the drugs were administered intraperitoneally.
Tail flick method[4]
The central analgesic activity of the plant material was studied by measuring drug-induced changes in the sensitivity of pre-screened (reaction: 2-4 sec) animals to heat stress applied to their tails by using an analgesiometer. Briefly, intensity of the current passing through a naked nichrome wire was maintained at 5 A (A: SI unit for ampere). Basal reaction time of animals to reactant heat was recorded by placing the tip of the tail on the radiant heat source. Withdrawal of the tail from the heat (flicking response) was taken as the end point. The animals which showed flicking response within 3-5 seconds were selected for the study. A cut-off period of 15 seconds was observed to avoid damage to the tail. The measurement of withdrawal time using the tail flick apparatus was conducted at 30, 60, and 120 minutes after administration of HEJGL. Pain inhibition percentage was calculated.
PIP = (T1-T0/T0) ×100 where T1 is the post-drug latency and T0 is the pre-drug latency.
Acetic acid – induced writhing[5]
The analgesic activity of HEJGL was also evaluated using acetic acid — induced writhing in mice. In this method, acetic acid is administered intraperitoneally to the experimental animals to create pain sensations as a positive control. Any standard nonsteroidal anti-inflammatory drug (NSAID) can be used; in this study, aspirin was used to serve the purpose. HEJGL was administered orally in three different doses (50, 100, and 200 mg/kg) to the Swiss albino mice after an overnight fast. HEJGL and distilled water were also administered orally 30 minutes prior to the intraperitoneal administration of 0.7% v/v acetic acid solution (0.1 mL/10 g), but aspirin was administered 15 minutes prior to the acetic acid injection. Then, the animals were placed on an observation table. Each mouse in all the groups was observed individually for counting the number of writhings it made in 30 minutes commencing just five minutes after the intraperitoneal administration of acetic acid solution. The writhing consisted of a wave of contraction and relaxation passing caudally along the abdominal wall, sometimes accompanied with extension of hind limbs. The number of writhes in each treated group was compared to that of a control group, whereas aspirin (50 mg/kg) was used as a reference substance (positive control). Writhing was induced 30 minutes later by intraperitoneal injection of 10 mL/kg of 0.1% acetic acid in distilled water. The number of writhes was counted for 30 minutes immediately after the acetic acid injection. The percentage protection was calculated.
Maximal electroshock seizure (MES) model[6]
Maximal electroshock seizure model was used to evaluate the anticonvulsant activity of HEJGL. Seizures were induced in the mice by delivering electroshock of 50 mA for 0.2 seconds by means of an electroconvulsiometer through a pair of corneal electrodes. The test animals (n = 6) received 50, 100, and 200 mg/kg of HEJGL orally and the standard group received phenytoin (25 mg/kg) injected intraperitoneally and tested after 30 minutes for MES-induced seizure response. All the experimental groups were compared with the control treated with vehicle.
PTZ-induced seizures[7]
PTZ at a dose of 80 mg/kg (minimal dose needed to induce convulsions) was injected intraperitoneally to induce clonic-tonic convulsions in mice. The test animals (n = 6) received 50, 100, and 200 mg/kg of HEJGL orally and the standard group received phenytoin (25 mg/kg) injected intraperitoneally; PTZ was injected intraperitoneally 60 minutes after the administration of the drug. Occurrence of hind limb tonic extension (HLTE) and duration of seizures were noted. If no HLTE occurred during the time limit, the animals were considered protected.
Statistical analysis
Results were expressed as mean ± standard error of measurement (SEM). Statistical analysis was performed using ANOVA, followed by Dunnett's test; P < 0.05 was considered statistically significant.
OBSERVATION AND RESULTS
Acute oral toxicity study
The acute oral toxicity study observed that HEJGL was safe and nontoxic up to 2 g/kg orally in Wistar rats. Hence, the LD50 value of HEJGL was determined as >2 g/kg of body weight.
DISCUSSION
In the present study, the effect of HEJGL was observed on analgesic activity in experimental models using the tail flick technique in rats and acetic acid — induced writhing in mice. Anticonvulsant activity of HEJGL extract was also studied in two experimental models, that is, MES- and PTZ-induced convulsions in rats and mice, respectively.
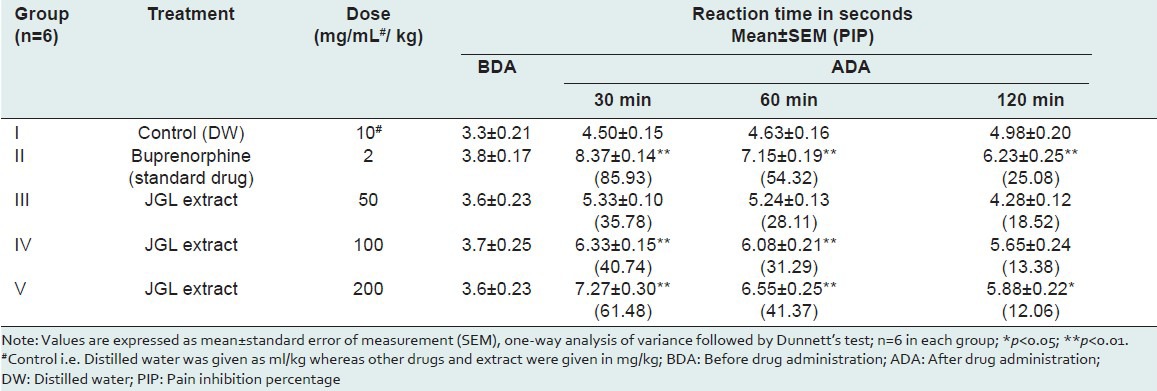
The tail flick method is considered to be selective for the drugs acting centrally. The tail flick test measures the complex response to a noninflammatory, acute nociceptive input and is one of the models normally used for studying central nociceptive activity. It is an established fact that any agent that causes a prolongation of the tail flick latency using this test must be acting centrally.[8] Therefore, HEJGL must have a central activity. In the tail flick model, the test drug in different doses increased the pain threshold significantly during the period of observation of 120 minutes, and this indicates the involvement of a higher center [Table 1].
Table 1.
Effect of Jasminum grandiflorum L (JGL) on reaction time in tail flick method in wistar rats
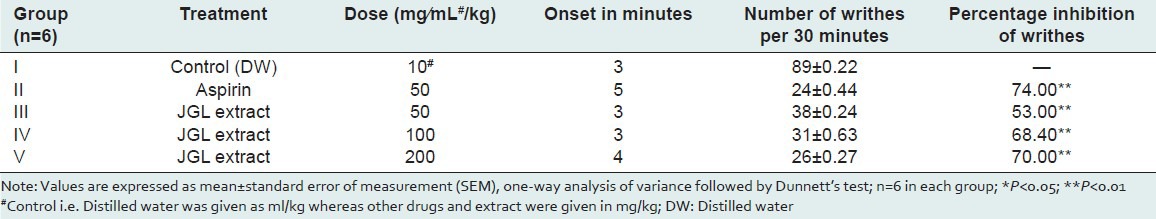
In the present study, the number of writhing movements during a 30-minute observation in the control group was 89 + 0.22, which was inhibited by JGL extract at doses of 50, 100, 200 mg/kg p.o. significantly to 53, 68, and 70%, respectively, as compared to the standard dose of aspirin which inhibited writhings up to 74% [Table 2]. The abdominal constriction response induced by acetic acid is a sensitive procedure to establish peripherally acting analgesics. This response is thought to involve local peritoneal receptors. This suggests that JGL extract possesses analgesia for pain produced by peripheral action.
Table 2.
Effects of Jasminum grandiflorum L (JGL) extract on acetic acid — induced writhing response in swiss albino mice
Again, narcotic analgesics inhibit both peripheral and central mechanisms of pain, whereas NSAIDs inhibit the pain produced by peripheral mechanisms only. The plant extracts of J. grandiflorum in our study inhibited both types of pain. The analgesic effect of the plant extract in both the models suggests that it has been acting through central as well as peripheral mechanisms.
In other studies, extracts of the plants which belong to the same family (Oleaceae) have shown antinociceptive action which supports our study.[9] However, this species (J. grandiflorum) has not been studied for analgesic action. In the absence of literature, it will be the first report on the analgesic action of JGL extract.
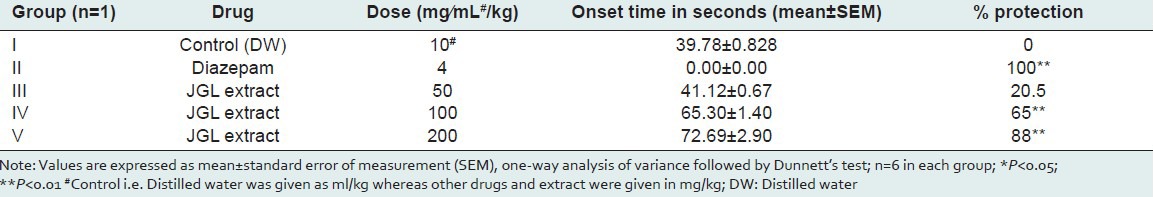
Epilepsy is the second most common neurological disorder in the world.[10,11] Despite many advances in research on epilepsy, the pharmacotherapy of epilepsy remains largely empirical, owing to the lack of understanding of the underlying pathology. Moreover, approximately 30% of the people with epilepsy have seizures that do not respond satisfactorily to the conventional antiepileptic drugs (CAEDs).[12] These limitations with the CAEDs alone highlighted the need for exploring the drugs that could potentiate the action of CAEDs so as to make the treatment of epilepsy more effective. The results of the present study indicate that the JGL extract at 50, 100, 200 mg/kg dose levels significantly decreased convulsions by the electroshock method to 56, 62, and 66% as compared to the standard drug phenytoin which showed protection against MES seizures to the extent of 72% [Table 3]. Similarly, in PTZ-induced convulsions, HEJGL significantly decreased seizures in the present study [Table 4]. PTZ is the most frequently used substance as well as an acute experimental model in the preliminary screening to test potential anticonvulsant drugs. Several biochemical hypotheses have been advanced involving the inhibitory GABAergic (GABA: Gamma-aminobutyric acid) system and the system of the excitatory amino acid glutamate and aspartate.[13] The mechanism by which PTZ is believed to exert its action is by acting as an antagonist at the GABAA receptor complex.[14] Drugs protecting against tonic-clonic seizures induced by PTZ are considered to be useful to control myoclonic and absence seizures in humans.[15] The present study reveals that the JGL extract exhibits a significant -dose-dependent protection against electrical- and chemical-induced seizures. No study on this aspect of HEJGL has been reported previously.
Table 3.
Effect of Jasminum grandiflorum L (JGL) extracts on MES-induced convulsions
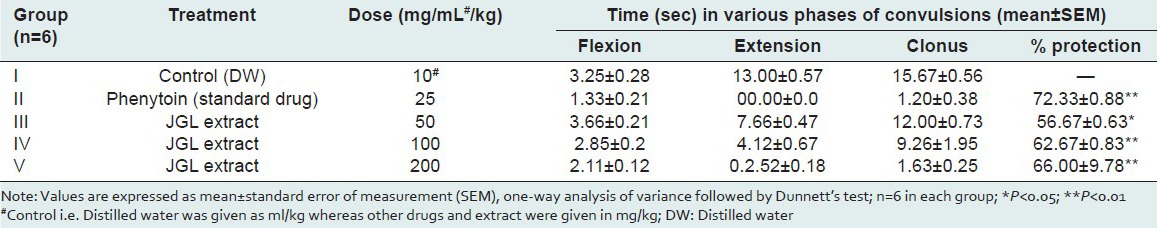
Table 4.
Effect of leaves of Jasminum grandiflorum L (JGL) on PTZ-induced convulsions
CONCLUSION
J. grandiflorum L possesses analgesic activity against pain induced by the tail flick method in rats and acetic acid — induced writhing method in mice. In the present study, JGL extract produced significant anticonvulsant activity in both experimental models of convulsions, that is, MES- and PTZ-induced seizures, respectively.
In view of its analgesic and anticonvulsant activity, JGL extract can be used in painful conditions as well as in seizure disorders. The study on the combination effect of JGL extract with a standard drug in all the experimental models of analgesic and anticonvulsant activities is in progress.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Eid RA, Lobna, Taha Soad, Ibrahim MM. Physiological properties studies on essential oil of Jasminum grandiflorum L. as affected by some vitamins. Ozean J Appl Sci. 2010;3:87–96. [Google Scholar]
- 2.Sandeep, Padmaa Paarakh. Jasminum grandiflorum Linn (Chameli): Ethnobotany, phytochemistry and pharmacology — A review. Pharmacol Online Newslett. 2009;2:586–95. [Google Scholar]
- 3.Ghosh MN. Fundamental of Experimental Pharmacology. Vol. 4. Kolkata, India: Bose Printing House; 2008. Toxicity studies; pp. 176–83. [Google Scholar]
- 4.Kamath JV, Rana AC. Pharmacological activities of ethanolic extract of Calotropis procera roots. Indian Drugs. 2003;40:292–5. [Google Scholar]
- 5.Collier HOJ, Dineen LC, Johnson CA, Schneirder C. Nociceptive response to prostaglandins and analgesic actions of aspirin and morphine. Br J Pharmacol. 1968;32:295–310. [Google Scholar]
- 6.Toman JE, Loewe S, Goodman LS. Studies on the physiology and therapy of convulsive disorders. Arch Neurol Psychiatry. 1947;58:312–24. doi: 10.1001/archneurpsyc.1947.02300320063003. [DOI] [PubMed] [Google Scholar]
- 7.Bastian JW, Krause WE, Ridlon SA, Ercoli N. CNS drug specificity as determined by the mouse intravenous pentylenetetrazole technique. J Pharmacol Exp Ther. 1959;127:75–80. [PubMed] [Google Scholar]
- 8.Saha A, Ahmed M. The analgesic and anti-inflammatory activities of the extract of Albizia lebbeck in animal model. Pak J Pharm Sci. 2009;22:74–7. [PubMed] [Google Scholar]
- 9.Jia Q, Su W, Peng W, Li P, Wang Y. Anti-diarrhoea and analgesic activities of the methanol extract and its fractions of Jasminum amplexicaule Buch.-Ham. (Oleaceae) J Ethnopharmacol. 2008;119:299–304. doi: 10.1016/j.jep.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Bharucha NE. Epidemiology of epilepsy in India. Epilepsia. 2003;44:9–11. doi: 10.1046/j.1528-1157.44.s.1.5.x. [DOI] [PubMed] [Google Scholar]
- 11.Gourie-Devi M, Gururaj G, Satishchandra P, Subbakrishna DK. Prevalence of neurological disorders in Bangalore, India: A community-based study with a comparison between urban and rural Areas. Neuroepidemiology. 2004;23:261–8. doi: 10.1159/000080090. [DOI] [PubMed] [Google Scholar]
- 12.Reddy DS. Pharmacotherapy of catamenial epilepsy. Indian J Pharmacol. 2005;37:288–93. [Google Scholar]
- 13.McDonald RI, Kelly KM. Antiepileptic drugs: Mechanisms of action. Epilepsia. 1993;34:S1-8–20. doi: 10.1111/j.1528-1157.1993.tb05918.x. [DOI] [PubMed] [Google Scholar]
- 14.Ramanjaneyulu R, Ticku MK. Interactions of pentamethylenetetrazole and tetrazole analogues with thepicrotoxinin site of the benzodiazepine-GABA receptor ionophore complex. Eur J Pharmacol. 1984;98:337–45. doi: 10.1016/0014-2999(84)90282-6. [DOI] [PubMed] [Google Scholar]
- 15.Loscher W, Schmidt D. Which animal's models should be used in the search for new antileptic drugs? A proposal based on experimental and clinical consideration. Epilepsy Res. 1988;2:145–81. doi: 10.1016/0920-1211(88)90054-x. [DOI] [PubMed] [Google Scholar]


