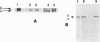Abstract
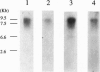
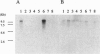
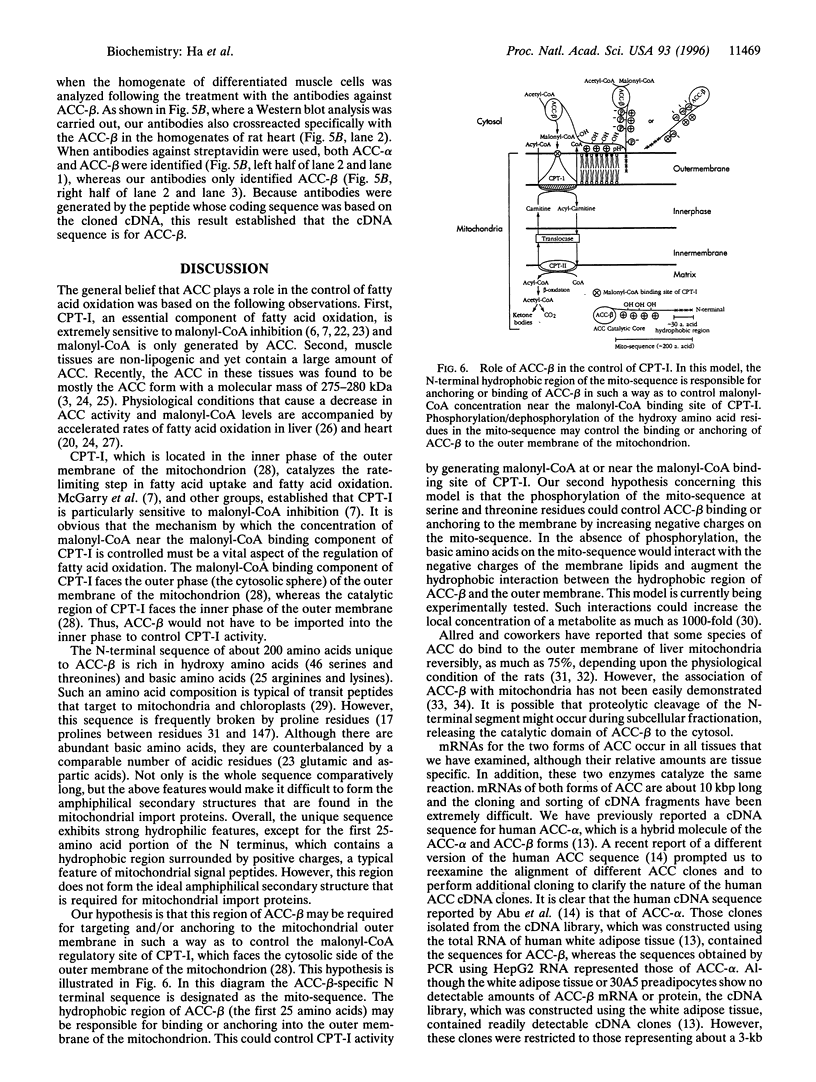
Acetyl-CoA carboxylase, which has a molecular mass of 265 kDa (ACC-alpha), catalyzes the rate-limiting step in the biosynthesis of long-chain fatty acids. In this study we report the complete amino acid sequence and unique features of an isoform of ACC with a molecular mass of 275 kDa (ACC-beta), which is primarily expressed in heart and skeletal muscles. In these tissues, ACC-beta may be involved in the regulation of fatty acid oxidation, rather than fatty acid biosynthesis. ACC-beta contains an amino acid sequence at the N terminus which is about 200 amino acids long and may be uniquely related to the role of ACC-beta in controlling carnitine palmitoyltransferase I activity and fatty acid oxidation by mitochondria. If we exclude this unique sequence at the N terminus the two forms of ACC show about 75% amino acid identity. All of the known functional domains of ACC are found in the homologous regions. Human ACC-beta cDNA has an open reading frame of 7,343 bases, encoding a protein of 2,458 amino acids, with a calculated molecular mass of 276,638 Da. The mRNA size of human ACC-beta is approximately 10 kb and is primarily expressed in heart and skeletal muscle tissues, whereas ACC-alpha mRNA is detected in all tissues tested. A fragment of ACC-beta cDNA was expressed in Escherichia coli and antibodies against the peptide were generated to establish that the cDNA sequence that we cloned is that for ACC-beta.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu-Elheiga L., Jayakumar A., Baldini A., Chirala S. S., Wakil S. J. Human acetyl-CoA carboxylase: characterization, molecular cloning, and evidence for two isoforms. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):4011–4015. doi: 10.1073/pnas.92.9.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguan K., Scott J., See C. G., Sarkar N. H. Characterization and chromosomal localization of the human homologue of a rat AMP-activated protein kinase-encoding gene: a major regulator of lipid metabolism in mammals. Gene. 1994 Nov 18;149(2):345–350. doi: 10.1016/0378-1119(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Allred J. B., Roman-Lopez C. R. Enzymatically inactive forms of acetyl-CoA carboxylase in rat liver mitochondria. Biochem J. 1988 May 1;251(3):881–885. doi: 10.1042/bj2510881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi G., Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- Bertrand C., Largillière C., Zabot M. T., Mathieu M., Vianey-Saban C. Very long chain acyl-CoA dehydrogenase deficiency: identification of a new inborn error of mitochondrial fatty acid oxidation in fibroblasts. Biochim Biophys Acta. 1993 Jan 22;1180(3):327–329. doi: 10.1016/0925-4439(93)90058-9. [DOI] [PubMed] [Google Scholar]
- Bianchi A., Evans J. L., Iverson A. J., Nordlund A. C., Watts T. D., Witters L. A. Identification of an isozymic form of acetyl-CoA carboxylase. J Biol Chem. 1990 Jan 25;265(3):1502–1509. [PubMed] [Google Scholar]
- Coates P. M., Tanaka K. Molecular basis of mitochondrial fatty acid oxidation defects. J Lipid Res. 1992 Aug;33(8):1099–1110. [PubMed] [Google Scholar]
- Cook G. A. Differences in the sensitivity of carnitine palmitoyltransferase to inhibition by malonyl-CoA are due to differences in Ki values. J Biol Chem. 1984 Oct 10;259(19):12030–12033. [PubMed] [Google Scholar]
- Evans J. L., Witters L. A. Quantitation by immunoblotting of the in vivo induction and subcellular distribution of hepatic acetyl-CoA carboxylase. Arch Biochem Biophys. 1988 Jul;264(1):103–113. doi: 10.1016/0003-9861(88)90575-9. [DOI] [PubMed] [Google Scholar]
- Gao G., Widmer J., Stapleton D., Teh T., Cox T., Kemp B. E., Witters L. A. Catalytic subunits of the porcine and rat 5'-AMP-activated protein kinase are members of the SNF1 protein kinase family. Biochim Biophys Acta. 1995 Apr 6;1266(1):73–82. doi: 10.1016/0167-4889(94)00222-z. [DOI] [PubMed] [Google Scholar]
- Ha J., Daniel S., Broyles S. S., Kim K. H. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J Biol Chem. 1994 Sep 2;269(35):22162–22168. [PubMed] [Google Scholar]
- Ha J., Daniel S., Kong I. S., Park C. K., Tae H. J., Kim K. H. Cloning of human acetyl-CoA carboxylase cDNA. Eur J Biochem. 1994 Jan 15;219(1-2):297–306. doi: 10.1111/j.1432-1033.1994.tb19941.x. [DOI] [PubMed] [Google Scholar]
- Iverson A. J., Bianchi A., Nordlund A. C., Witters L. A. Immunological analysis of acetyl-CoA carboxylase mass, tissue distribution and subunit composition. Biochem J. 1990 Jul 15;269(2):365–371. doi: 10.1042/bj2690365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N., Barr A. J., Barr R. L., Desai S., Lopaschuk G. D. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5'-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995 Jul 21;270(29):17513–17520. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- Lopaschuk G. D., Witters L. A., Itoi T., Barr R., Barr A. Acetyl-CoA carboxylase involvement in the rapid maturation of fatty acid oxidation in the newborn rabbit heart. J Biol Chem. 1994 Oct 14;269(41):25871–25878. [PubMed] [Google Scholar]
- López-Casillas F., Bai D. H., Luo X. C., Kong I. S., Hermodson M. A., Kim K. H. Structure of the coding sequence and primary amino acid sequence of acetyl-coenzyme A carboxylase. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5784–5788. doi: 10.1073/pnas.85.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. Characteristics of fatty acid oxidation in rat liver homogenates and the inhibitory effect of malonyl-CoA. Biochim Biophys Acta. 1978 Sep 28;530(3):305–313. doi: 10.1016/0005-2760(78)90150-9. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Stark M. J., Foster D. W. Hepatic malonyl-CoA levels of fed, fasted and diabetic rats as measured using a simple radioisotopic assay. J Biol Chem. 1978 Nov 25;253(22):8291–8293. [PubMed] [Google Scholar]
- McGarry J. D., Woeltje K. F., Kuwajima M., Foster D. W. Regulation of ketogenesis and the renaissance of carnitine palmitoyltransferase. Diabetes Metab Rev. 1989 May;5(3):271–284. doi: 10.1002/dmr.5610050305. [DOI] [PubMed] [Google Scholar]
- McLaughlin S., Aderem A. The myristoyl-electrostatic switch: a modulator of reversible protein-membrane interactions. Trends Biochem Sci. 1995 Jul;20(7):272–276. doi: 10.1016/s0968-0004(00)89042-8. [DOI] [PubMed] [Google Scholar]
- Milatovich A., Plattner R., Heerema N. A., Palmer C. G., Lopez-Casillas F., Kim K. H. Localization of the gene for acetyl-CoA carboxylase to human chromosome 17. Cytogenet Cell Genet. 1988;48(3):190–192. doi: 10.1159/000132623. [DOI] [PubMed] [Google Scholar]
- Murthy M. S., Pande S. V. Malonyl-CoA binding site and the overt carnitine palmitoyltransferase activity reside on the opposite sides of the outer mitochondrial membrane. Proc Natl Acad Sci U S A. 1987 Jan;84(2):378–382. doi: 10.1073/pnas.84.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande S. V., Murthy M. S. Carnitine-acylcarnitine translocase deficiency: implications in human pathology. Biochim Biophys Acta. 1994 Jul 18;1226(3):269–276. doi: 10.1016/0925-4439(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Roman-Lopez C. R., Allred J. B. Acute alloxan diabetes alters the activity but not the total quantity of acetyl CoA carboxylase in rat liver. J Nutr. 1987 Nov;117(11):1976–1981. doi: 10.1093/jn/117.11.1976. [DOI] [PubMed] [Google Scholar]
- Saddik M., Gamble J., Witters L. A., Lopaschuk G. D. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem. 1993 Dec 5;268(34):25836–25845. [PubMed] [Google Scholar]
- Song C. S., Kim K. H. Reevaluation of properties of acetyl-CoA carboxylase from rat liver. J Biol Chem. 1981 Aug 10;256(15):7786–7788. [PubMed] [Google Scholar]
- Tae H. J., Zhang S., Kim K. H. cAMP activation of CAAT enhancer-binding protein-beta gene expression and promoter I of acetyl-CoA carboxylase. J Biol Chem. 1995 Sep 15;270(37):21487–21494. doi: 10.1074/jbc.270.37.21487. [DOI] [PubMed] [Google Scholar]
- Thampy K. G. Formation of malonyl coenzyme A in rat heart. Identification and purification of an isozyme of A carboxylase from rat heart. J Biol Chem. 1989 Oct 25;264(30):17631–17634. [PubMed] [Google Scholar]
- Trumble G. E., Smith M. A., Winder W. W. Purification and characterization of rat skeletal muscle acetyl-CoA carboxylase. Eur J Biochem. 1995 Jul 1;231(1):192–198. doi: 10.1111/j.1432-1033.1995.tb20686.x. [DOI] [PubMed] [Google Scholar]
- Trumble G. E., Smith M. A., Winder W. W. Purification and characterization of rat skeletal muscle acetyl-CoA carboxylase. Eur J Biochem. 1995 Jul 1;231(1):192–198. doi: 10.1111/j.1432-1033.1995.tb20686.x. [DOI] [PubMed] [Google Scholar]
- Verhoeven A. J., Woods A., Brennan C. H., Hawley S. A., Hardie D. G., Scott J., Beri R. K., Carling D. The AMP-activated protein kinase gene is highly expressed in rat skeletal muscle. Alternative splicing and tissue distribution of the mRNA. Eur J Biochem. 1995 Mar 1;228(2):236–243. [PubMed] [Google Scholar]
- Widmer J., Fassihi K. S., Schlichter S. C., Wheeler K. S., Crute B. E., King N., Nutile-McMenemy N., Noll W. W., Daniel S., Ha J. Identification of a second human acetyl-CoA carboxylase gene. Biochem J. 1996 Jun 15;316(Pt 3):915–922. doi: 10.1042/bj3160915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Kim K. H. Glucose activation of acetyl-CoA carboxylase in association with insulin secretion in a pancreatic beta-cell line. J Endocrinol. 1995 Oct;147(1):33–41. doi: 10.1677/joe.0.1470033. [DOI] [PubMed] [Google Scholar]