Abstract
Bone is unique of all the tissues in the vertebrate organism. When injured, it heals by formation of new bone. Bone morphogenetic proteins (BMPs) are powerful inductors of the osteogenic activity during the embryologic bone formation phase and in cases of bone healing. They have proliferative effects on different cellular types, showing chemotactic properties and are able to induce mesenchymal cells differentiation into osteoblastic and chondroblastic line cells. Both primary cells and cell lines have been shown to respond to BMPs. Further the ability of embryonic cells to respond to BMPs by differentiating into cartilage and bone cells suggests that they are involved in the development of embryonic skeletal system. In addition, these proteins can also promote the angiogenesis, regulate the activity of some growth factors, and affect the production of these growth factors, which is helpful for the osteogenesis. BMPs have been considered as the most potent growth factors that can promote the bone regeneration. Thus, the aim of this review is to emphasize on the unique nature of the BMP molecules regarding their structure, classification, signaling mechanism, etc., as BMPs are the only molecules which show such deviation from the normal order, type. This will further help in understanding the role of BMPs and their potential advances which are necessary to facilitate the process of regeneration in periodontics.
Keywords: Bone, growth factors, healing, repair
INTRODUCTION
Bone morphogenetic proteins (BMPs) are a group of growth factors and cytokines which were originally discovered by their ability to induce formation of bone and cartilage, but are now considered to constitute a group of pivotal morphogenetic signals, orchestrating tissue architecture throughout the body. The ability of BMPs to induce a cellular response resulting in new bone tissue formation was first observed and researched extensively by an orthopedic surgeon, Dr. Marshall Urist.[1] Urist was the director of the bone research laboratory at the University of California and he was practicing orthopedic surgery. His unique work included implantation of HCl-decalcified homogeneous diaphyseal bone, excised from adult rabbits, other laboratory animals, and humans, into different intramuscular sites of rabbits, rats, mice, and pigs. Within a few weeks after implantation, new cartilage and bone development appeared in or around the donor bone matrix surfaces. Urist characterized the process observed as new-bone formation by autoinduction in which both the inductor cell and the induced cells are derived from ingrowing cells of the host bed. This phenomenon was attributed to the presence of substance in bone matrix, which he later named BMPs.[1] BMPs play a role in the differentiation, proliferation, growth inhibition, and arrest of maturation of a wide variety of cells, depending on the cellular microenvironment and the interactions with other regulatory factors.[2] Bone matrix contains a variety of protein components, including an array of growth and differentiation factors. Thus, it remained elusive whether the observed BMP activity resulted from the combined activities of already identified factors, or was inherent to a new protein factor.
When BMPs bind to their cell surface receptors on mesenchymal cell, a BMP signaling cascade is activated. Signals are sent via specific proteins to the cell nucleus. This results in the expression of genes that lead to the synthesis of macromolecules involved in cartilage and bone formation, and the mesenchymal cell becomes a chondrocyte or an osteoblast. Implantation of this protein component of bone matrix resulted in a complex series of cellular events [Figure 1] including mesenchymal cell infiltration, cartilge formation, vascularization, bone formation, and ultimately remodeling of the new bone tissue along with population by hematopoietic bone marrow elements.[3] Endochondral bone induction is the result of the combinatorial action of BMPs and the complementary substratum that delivers the osteogenic activity of the soluble molecular signal.[4] In addition to differentiation of cells into the chondrocyte lineage, BMPs have been shown to directly differentiate into cells of the osteoblast phenotype.
Figure 1.
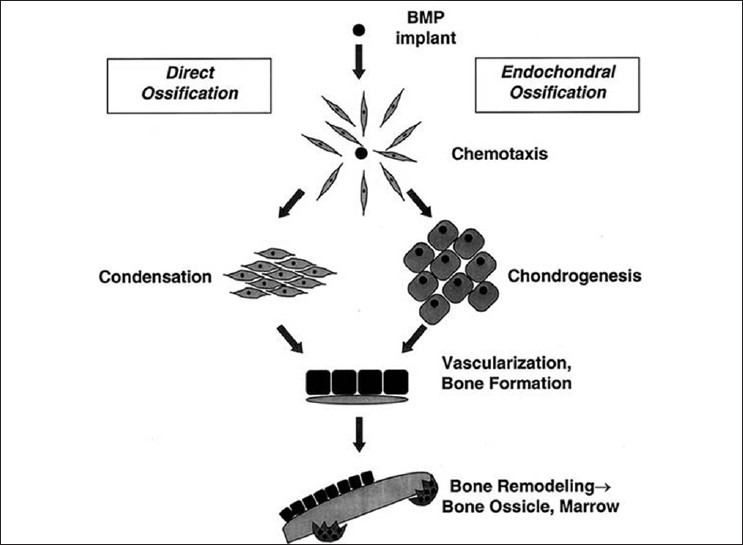
Cellular events after implantation of bone morphogenetic proteins (BMPs)
CHEMICAL STRUCTURE OF BONE MORPHOGENETIC PROTEINS
Bone morphogenetic proteins are members of TGF-β (Transforming growth factor-β) super family, a large family of growth factors. The TGF-β was so named because of its ability to transform cultured fibroblasts.[5] At their carboxy-terminal ends, all BMPs posses a region containing seven cysteine residues that is conserved among all the reported members of the TGF-β superfamily.[6] BMPs are synthesized inside the cell in a precursor form with a hydrophobic stretch of about 50-100 amino acids. Prior to secretion, BMPs consists of a signal peptide, pro-domain, and mature peptide. Following cleavage of the signal peptide, the precursor protein undergoes glycosylation and dimerization. On secretion of the mature bioactive dimeric BMP by the cell, the pro-domain is cleaved. The mature BMP derives from the carboxy terminal region by proteolytical cleavage and are secreted as either heterodimers or homodimers [Figure 2].
Figure 2.
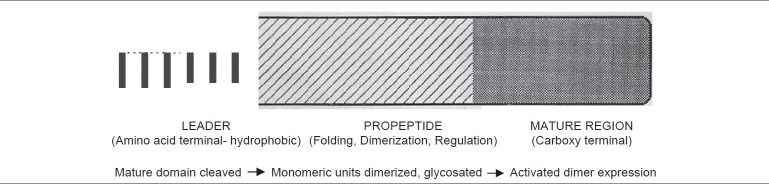
Chemical structure of BMPs
Structural and chemical differences between the homodimeric and heterodimeric forms may be responsible for variations of their biologic potential and binding characteristics.
Signaling mechanism of bone morphogenetic proteins
Receptors for BMPs are complexes of two different types of membrane-bound serine/threonine kinases: Type I BMP receptors, BMPR-1A and BMPR- 1B, and type II receptors.[7]
Ligand binding → Heterotetramer complex → Signaling cascade.
After ligand binding, the type II receptor phosphorylates the type I receptor. The activated type I receptor then phosphorylates a member of the Smad family of intracellular proteins, which are the functional signal transducers of the TGF-β/BMP family.
At the receptor level itself, the oligomerization mode of the receptors determines the specificity of the activation of the signaling pathway. Into intracellular compartment, the signal can be modulated by the activation of inhibitory Smad proteins (I-Smads). In the nucleus, there is a number of co-activators needed for the activation of specific target genes and their transcription can be inhibited by co-repressors. After binding of a BMP to its receptor, Smad 1 and 5 (class I Smads) form heteromeric Smad–Smad complexes with Smad 4 (class II Smad). The complexes regulate molecular transcriptional responses directly. Smads 6 and 7 (class III Smads) are inhibitors of TGFb/BMP signaling.[8]
Classification of bone morphogenetic proteins
The human genome encodes 20 BMPs.[9]
Comparisons among the derived amino acid sequences of the BMPs found in osteoinductive extracts of bone indicate that they fall into three subclasses[10] [Table 1].
Table 1.
Sub-classes of BMPs
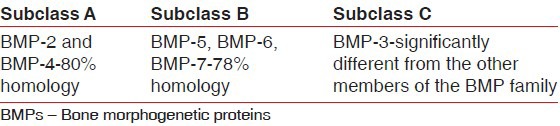
The first subclass contains BMP-2 and BMP-4, highly related molecules that differ mainly in the amino terminal region, with BMP-2 containing a heparin-binding domain. In the second subclass are BMP-5, BMP-6, and BMP-7, also known as osteogenic protein-1 (OP-1), and BMP-8 (OP-2). These are slightly larger proteins than BMP-2 and BMP-4, and there is an approximate 78% amino acid identity between the subgroups. In the third subclass, and more distantly related to these factors, is BMP-3, also called osteogenin. Proof that these proteins were responsible for the bone inductive activity in bone matrix was found in the recombinant expression of each of these proteins.[11]
By the use of molecular biology techniques and sequence information from the bovine molecular clones, the human homologs of each BMP coding sequence were obtained. Mammalian cells were engineered to express each protein by inserting the coding sequence (gene) for each protein into an expression vector and producing stable cell lines. Each cell line thus produces a single BMP molecule that is secreted into the medium wherein the cells are growing. The protein then can be tested by implantation in the in vivo bone induction assay system. Using this process, BMP-2 was first shown to induce the formation of new cartilage and bone tissues, demonstrating that members of this class of molecules were necessary and sufficient for osteoinduction. Subsequently, it was demonstrated that other BMP proteins also induce bone formation.[12]
The nomenclature of BMPs is complex including BMPs, growth and differentiation factors (GDFs), and others depending on the method used to identify them [Table 2].
Table 2.
Characteristics of various BMPs
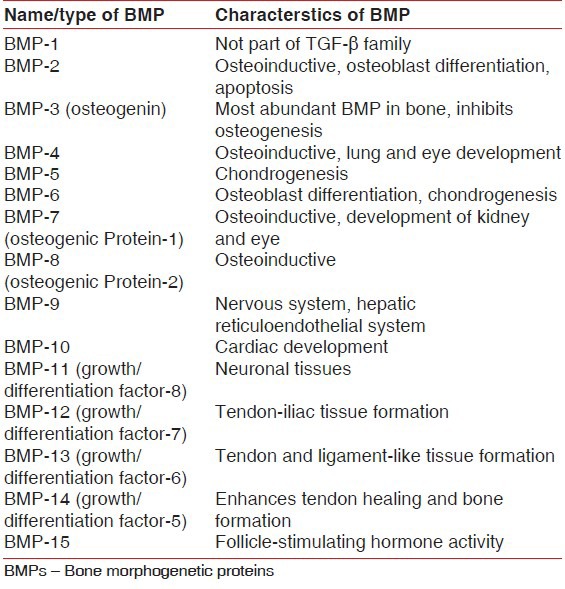
Bone morphogenetic proteins in wound healing
BMPs directly affect wound healing process.[13]
BMPs directly affect the healing in the following sequential phases
INJURY → inflammatory response → complement ensues → Extravasation and cell signaling
PROLIFERATION → granulation tissue → binding of growth factors to collagens
REMODELING → activation-resorption formation→Osteoclasts resorptive pits.
FACTORS AFFECTING BONE MORPHOGENETIC PROTEINS ACTIVITY
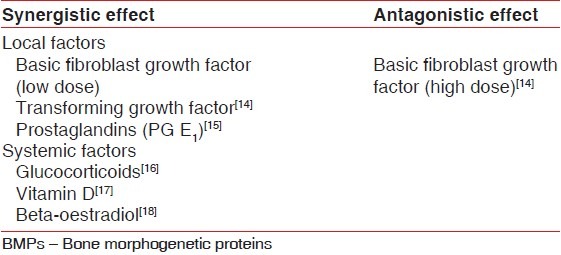
There are various local and systemic factors affecting BMP activity [Table 3].
Table 3.
Local and systemic factors affecting BMP activity
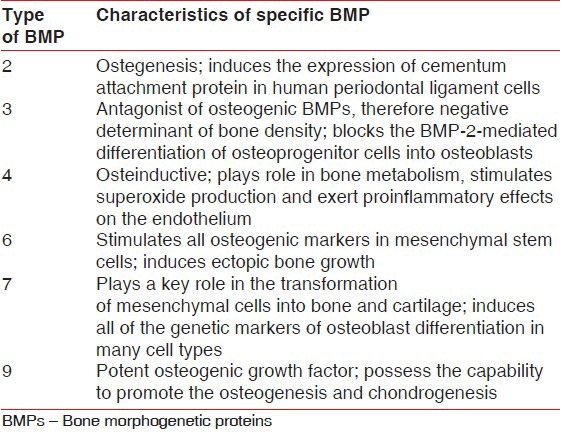
Characteristics of specific bone morphogenetic proteins useful in periodontal regeneration
In the field of periodontal regeneration, much of the research interest has focused on BMP-2, BMP-3 (osteogenin), and BMP-7 (OP-1) [Table 4]. Bowers et al.[19] did the first human study using single application of BMP-3 (osteogenin) combined with demineralized bone allograft in a submerged tooth model to demonstrate periodontal regeneration Crude preparations of BMP-2 and BMP-3 have also been applied in surgically induced furcation defects and have shown to stimulate periodontal regeneration.[4] Recent studies have utilized recombinant human BMP to determine their potential for correcting intrabony, supra-alveolar, furcation, and fenestration defects.[20] However, in most of these studies, histologic analysis has revealed periodontal regeneration with areas of ankylosis. Contrary to these findings, BMP-7 augmentation resulted in a significant increase in periodontal regeneration without any ankylosis; so most of the recent research utilizing recombinant human BMP-7 has been involved in the preparation of implant site for ossteointeration.[21]
Table 4.
Characteristics of specific BMPs in periodontal regeneration
CONCLUSION
The presence of the structural activity of bone morphogenetic proteins amongst soluble osteogenic molecular signals indicates a therapeutic significance in clinical contexts. The challenge lies in applying these drugs with consistent success in various applications. Further, studies are needed for development of carrier materials that have mechanical properties and surgical practicality appropriate for controlled release of bone morphogenetic proteins.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Urist MR, Nakata N, Felser JM. An osteosarcoma cell and matrix retained morphogen for normal bone formation. Clin Orthop. 1977;124:251–66. [PubMed] [Google Scholar]
- 2.Wang EA. Bone morphogenetic proteins (BMPs): Therapeutic potential in healing bony defects. Trends Biotechnol. 1993;11:379–83. doi: 10.1016/0167-7799(93)90096-R. [DOI] [PubMed] [Google Scholar]
- 3.Reddi AH. Cell biology and biochemistry of endochondral bone development. Coll Relate Res. 1981;1:209–26. doi: 10.1016/s0174-173x(81)80021-0. [DOI] [PubMed] [Google Scholar]
- 4.Ripamonti U, Renton L. Bone morphogenetic proteins and the induction of periodontal tissue regeneration. Periodontol 2000. 2006;41:73–87. doi: 10.1111/j.1600-0757.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 5.Rengachary SS. Bone morphogenetic proteins: Basic concepts. Neurosurg Focus. 2002;13:1–6. doi: 10.3171/foc.2002.13.6.3. [DOI] [PubMed] [Google Scholar]
- 6.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, et al. Novel regulators of bone formation: Molecular clones and activities. Science. 1988;242:1528–34. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 7.Sakou T. Bone morphogenetic proteins: From basic studies to clinical approaches. Bone. 1998;22:591–603. doi: 10.1016/s8756-3282(98)00053-2. [DOI] [PubMed] [Google Scholar]
- 8.Padgett RW, Cho SH, Evangelista C. Smads are the central component in transforming growth factor-beta signaling. Pharmacol Ther. 1998;78:47–52. doi: 10.1016/s0163-7258(97)00166-6. [DOI] [PubMed] [Google Scholar]
- 9.Reddi AH. Regulation of cartilage and bone differentiation by bone morphogenetic protein. Curr Opin Cell Biol. 1992;4:850–5. doi: 10.1016/0955-0674(92)90110-x. [DOI] [PubMed] [Google Scholar]
- 10.Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nature Biotechnol. 2003;21:1025–32. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 11.Wang EA, Rosen V, D’Alessandro JS. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci USA. 1990;87:2220–4. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampath TK, Maliakal JC, Hauschka PV. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem. 1992;267:20352–62. [PubMed] [Google Scholar]
- 13.Hollinger JO, Buck DC, Bruder SP. Biology of bone healing: Its impact on clinical therapy. In: Lynch, Samuel E, Marx, Robert E, editors. Tissue Engineering: Applications in Oral and Maxillofacial Surgery and Periodontics. 2nd ed. Quintessence Publishing; 1998. pp. 30–40. [Google Scholar]
- 14.Hanada K, Dennis JE, Caplan AI. Stimulatory effects of basic fi Stimula growth factor and bone morphogenetic protein-2 on osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. J Bone Mineral Res. 1997;12:1606–14. doi: 10.1359/jbmr.1997.12.10.1606. [DOI] [PubMed] [Google Scholar]
- 15.Ono I, Inoue M, Kuboki Y. Promotion of the osteogenetic activity of recombinant human bone morphogenetic protein by prostaglandin E₁. Bone. 1996;19:581–8. doi: 10.1016/s8756-3282(96)00282-7. [DOI] [PubMed] [Google Scholar]
- 16.Mayer H, Scutt AM, Ankenbauer T. Subtle differences in the mitogenic effects of recombinant human bone morphogenetic proteins-2 to -7 on DNA synthesis in primary bone-forming cells and identification of BMP-2/4 receptor. Calcif Tissue Int. 1996;58:249–55. doi: 10.1007/BF02508644. [DOI] [PubMed] [Google Scholar]
- 17.Amédée J, Bareille R, Rouais F, Cunningham N, Reddi H, Harmand MF. Osteogenin (bone morphogenic protein 3) inhibits proliferation and stimulates differentiation of osteoprogenitors in human bone marrow. Differentiation. 1994;58:157–64. [PubMed] [Google Scholar]
- 18.Takuwa Y, Ohse C, Wang EA, Wozney JM, Yamashita K. Bone morphogenetic protein-2 stimulates alkaline phosphatase activity and collagen synthesis in cultured osteoblastic cells, BMPs and bone regeneration. Biochem Biophys Res Commun. 1991;174:96–101. doi: 10.1016/0006-291x(91)90490-x. [DOI] [PubMed] [Google Scholar]
- 19.Bowers G, Felton F, Middleton C, Glynn D, Sharp S, Mellonig J. Histologic comparison of regeneration in human intrabony defects when osteogenin is combined with demineralized freeze-dried bone allograft and with purified bovine collagen. J Periodontol. 1991;62:690–702. doi: 10.1902/jop.1991.62.11.690. [DOI] [PubMed] [Google Scholar]
- 20.Saito A, Saito E, Handa R, Honma Y, Kawanami M. Influence of residual bone on recombinant human bone morphogenetic protein-2-induced periodontal regeneration in experimental periodontitis in dogs. J Periodontol. 2009;80:961–8. doi: 10.1902/jop.2009.080568. [DOI] [PubMed] [Google Scholar]
- 21.Sasikumar KP, Elavarasu S, Gadagi JS. Recombinant human bone morphogenetic protein-2 for peri-implant bone regeneration: A case report. J Periodontol. 2011;82:1212–8. doi: 10.1902/jop.2011.100626. [DOI] [PubMed] [Google Scholar]


