Abstract
Background:
Although autografts are the standard procedure for bone grafting, the use of bone regeneration by means of dental pulp stem cell is an alternative that opens a new era in this field. Rigenera Protocol is a new technique able to provide the surgeon autologous pulp micro-grafts.
Materials and Methods:
At the Department of Oral Surgery, Don Orione Hospital, Bergamo, Italy, one patient underwent sinus lift elevation with pulp stem micro-grafts gentle poured onto collagen sponge. A CT scan control was performed after 4 months and DICOM data were processed with medical imaging software which gives the possibility to use a virtual probe to extract the bone density. Pearson's Chi-square test was used to investigate difference in bone density (BD) between native and newly formed bone.
Results:
BD in newly formed bone is about the double of native bone.
Conclusion:
This report demonstrated that micro-grafts derived from dental pulp poured onto collagen sponge are a useful method for bone regeneration in atrophic maxilla.
Keywords: Bone, homograft, jaw, reconstruction, resorption, stem cell
INTRODUCTION
Today the main problem in transferring the experimental protocols of tissue engineering in the routine clinical practice is the identification of accessible sites where an adequate amount of stem cells are collected.[1,2]
Although within the adult human body there are several “loci” or “niches” inhabited by a significant number of stem cells,[3,4,5] often these loci are not easy to access and have high residual morbidity of the anatomical site.
In the dental-maxillo-facial area, the dental pulp represents a niche housing neural-crest-derived stem cells that display plasticity and multipotential capability.[6] This niche is easily accessible and there is limited morbidity of the anatomical site after collection of the pulp.[7,8,9,10]
Several studies have been performed on dental pulp stem cells (DPSCs) and it is mainly found that these cells are multipotent stromal cells that can be safely cryopreserved, used with several scaffolds, that can extensively proliferate, have a long lifespan and build in vivo an adult bone with Havers channels and an appropriate vascularization.[1,2]
DPSCs can be cultured by two methods: The first is the enzyme-digestion method[11] in which the pulp tissue is collected under sterile conditions, digested with appropriate enzymes, and then the resulting cell suspensions are seeded in culture dishes containing a special medium supplemented with necessary additives and then incubated. Finally, the resulting colonies are subcultured before confluence and the cells are stimulated to differentiate. The second method for isolating DPSCs is the explant outgrowth method[12] in which the extruded pulp tissues are cut, anchored via microcarriers onto a suitable substrate, and directly incubated in culture dishes containing the essential medium with supplements. Up to 2 weeks are needed to allow a sufficient number of cells to migrate out of the tissue.
From a clinical point of view, despite the tremendous potential of the pulp, both these methods are not allowed for therapeutical application, due to extensive manipulation of the pulp tissue. In this case report, we present a new method for clinical management of the human pulp tissue, called Rigenera Protocol. With this innovative approach the pulp is treated as any other connective tissue subjected to grafts, with a phase of collection and a phase of mechanical disaggregation of the tissue without manipulating the matrix. Rigenera produces millions of viable micro-grafts and filters them with a cut-off of 50 microns, in order to promote the discharging of old differentiated cells and the enrichment of young progenitors cells contained within the pulp. In the current study, we performed a sinus lift augmentation using these micro-grafts derived from dental pulp and the patient was evaluated after 4 months by using CT scan and a special computer program[13] to evaluate bone density in order to evaluate the suitability of this new bone as dental implant site.
MATERIALS AND METHODS
Patient
A male M.B., 45 years old, was selected in the Department of Oral Surgery, Don Orione Hospital, Bergamo, Italy. Informed written consent approved by the local Ethics Committee was obtained from patient to use his data for research purpose.
The patient had an unremarkable medical history, no other oral diseases, and he wanted to rehabilitate the upper right maxilla with dental implant-prosthetic therapy. He was taking no medications and denied any allergies. Before the surgery he underwent sextant scaling with ultrasonic and hand instruments. The same patient presented a third molar with advanced bone resorption but healthy for caries. We decided to extract the upper right third molar and extract the pulp to produce micro-gratfs for bone tissue regeneration of the upper left maxilla.
Surgical procedure and dental pulp stem cells collection
After the extraction of the third molar the crown was separated from the roots following the enamel-cementum line in order to open the pulp chamber and expose the soft connective pulpar tissue. The pulp was gently collected using a Gracey curette and dissociated using Rigenera System (HBW srl, Turin, Italy) in 1.2 ml of physiologic solution. After 30 seconds of agitation the cellular suspension was collected from the system and gentle poured onto collagen sponge (Gingistat, GABA, Italy).
The pharmacological profylaxis was prescribed as follows: Dexamethason (Decadron, Visufarma spa, Roma, Italy) 4.5 mg 6 h before the surgery, Nimesulide (Doc Generici srl, Milano, Italy) 100 mg 2 h before the surgery, amoxicillin 875 mg plus 125 clavulanic acid (Augmentin, Glaxosmithkline spa, Verona, Italy) 12 h and 1 h before the surgery. Before the surgery the oral decontamination was obtained using 60 seconds of mouth rinsing with 0.2% Chlorhexidine washing (Forhans, Torino, Italy). The local anesthetic used was articain 2% 1:100000 epinephrin (Espe, Norristown, PA, USA).
The incisions were performed and a full thickness flap elevated according the lateral approach technique; a bony window of square shape with 25 mm side was obtained to access the sinus using a 1.5 mm bur.
Then, the sinus cavity was filled with a mix of Gingistat and dental pulp stem cells. The implant was positioned (Camlog, Alta-tech, Vicenza, Italy). The sinus access was closed and sutured.
Postsurgical medication were prescribed as follows: Antibiotic therapy (875 amoxicillin plus 125 clavulanic acid) twice a day for 7 days; dexamethason 3 mg for the first day; and 1.5 mg for the second day after the surgery; Nimesulide 100 mg twice a day for 5 days; 0.12% Chlorhexidine rinse twice a day for 15 days. The patient was advised against blowing his nose and was asked to return in 1 week for suture removal.
The post-surgical course was uneventful.
Data collection
Radiographs were taken before and after the surgery.
After 4 months follow-up radiographic examinations were done with the use of CT scans.
The Digital Imaging and Communications in Medicine data were processed with medical imaging software (3Diagnosys 3.0-3DIEMME, Milano, Italy) which gives the possibility to use a virtual probe to extract the bone density values in the desired regions and export them in Excel tables for statistical analysis. The virtual probes were set in the following regions in the “after surgery” CT scans:
Native bone
Grafted zone
Airways (calibration probe).
An area of 1 mm thickness was extracted with this probe and exported to Excel for further analysis. The data extracted is expressed in Hounsfield Units, being the processed CT scans calibrated according to water-based phantoms.
Statistical analysis
Pearson's Chi-square test was used to investigate difference in bone density between native and FB.
RESULTS
The Figure 1a and b shows the Radiographs taken in the regenerated site before and after the surgery. The Figure 2a–c shows the sample preparation. Figure 3 shows the positioning of the collagen sponge with micro-grafts suspension within the maxillary sinus.
Figure 1.
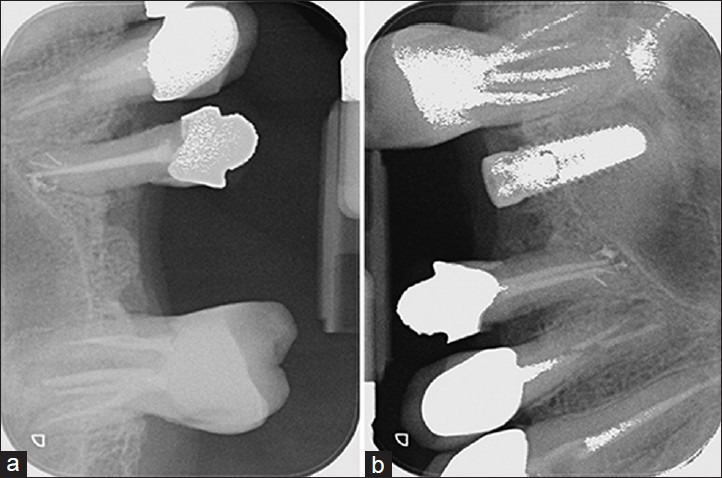
Endoral radiograph taken before (a) and after (b) the surgery
Figure 2.
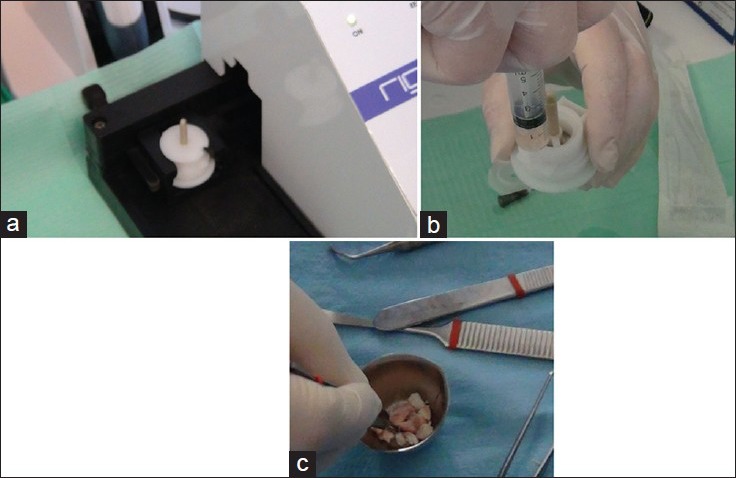
The Rigenera Protocol: (a) the Rigeneracons disposable medical device are positioned within the Rigenera Machine; (b) The micro-graft suspension collected from the Rigeneracons after the disaggregation of the dental pulp tissue; (c) The Micro-graft suspension is poured on collagen sponges
Figure 3.
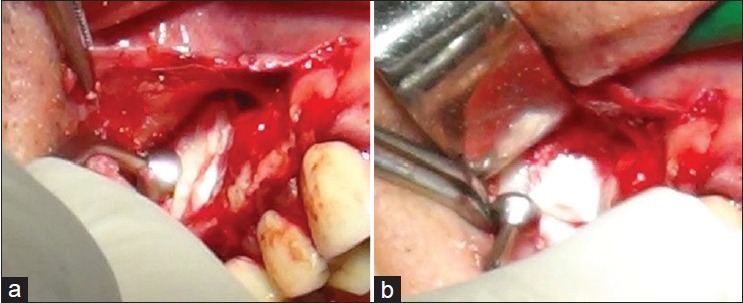
The biocomplex constituted by a collagen sponge as carrier of pulp micro-grafts is put within the maxillary sinus (a) and gently pressed (b)
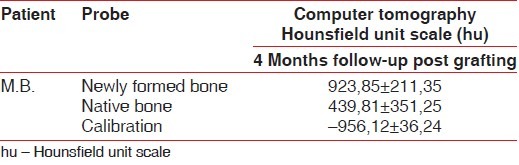
Table 1 reports the median bone density (BD) of the patient: In columns are reported the BD whereas in rows are reported the type of bone (grafted and native). BD of newly formed bone is about the double compare to native BD.
Table 1.
Results of Computer tomography evaluation by Hounsfield unit scale
DISCUSSION
The tissue engineering opens new challenges for clinical dentistry. The use of adult stem/progenitor cells can be extensive, since stem/progenitor cells can be harvested from various tissues such as adipose tissue, bone marrow, dental pulp, and periodontal ligament.[14] Dental and periodontal tissues represent a possible source of stem cells because approachable niches contain a high number of stem cells compared to equal volumes with the bone marrow. DPSCs display different antigenic patterns and noticeable plasticity that is explained by their neural-crest origin.[5,15] This is of great importance to understand the extraordinary plasticity displayed by these cells, which differentiate not only toward osteoblasts and adipocytes.[5,15] but also toward neurons or myocytes. Moreover, the use of these cells is of great interest because dental pulp can be collected easily and pulpectomy itself is a therapy in some cases.[16] With the Rigenera Protocol the dental pulp tissue can have a new clinical use and open new surgical strategies.
The use of appropriate biomaterial scaffolds combined with selected growth factors can significantly improve the survival and differentiation of the transplanted stem/progenitor cells.[17,18,19,20,21,22] Dental stem/progenitor cells collected from dental pulp can be differentiated in vitro and then transplanted with biomaterial scaffolds into the host without immunologic rejection.[23,24] Graziano et al.[25] observed DPSCs performances on different scaffolds, such as PLGA or poly(lactic-co-glycolic acid) 85:15, hydroxyapatite chips, (HA) and titanium. Results showed that stem cells exerted a different response, depending on the different type of textured surface. Actually, stem cells challenged with concave surfaces differentiated quicker and showed nuclear polarity, an index of secretion, cellular activity, and matrix formation. Moreover, bone-specific proteins were significantly expressed and the obtained bone tissue was of significant thickness. Thus, cells cultured on the concave textured surface had better cell-scaffold interactions and were induced to secrete factors that, due to their autocrine effects, quickly lead to osteodifferentiation, bone tissue formation, and vascularization.
The quality and quantity of regenerated bone formed by DPSCs was demonstrated in vitro and in vivo experiments using stem cells and biomaterials.[5,23,24,26] Thus, dental pulp could be considered as an interesting and potentially important source of autologous stem/progenitor cells that are ready for use for therapeutic purposes, such as the repair/regeneration of craniofacial bones.
Although this is only one case report, it is extremely encouraging for dentistry that new therapeutical approaches can be developed from the transferring of biological science into clinical practice, using the enormous regenerative potential of the human bone.
ACKNOWLEDGMENT
This work was supported by FAR from the University of Ferrara (FC), Ferrara, Italy, and from PRIN 2008 (F.C.), and from Regione Emilia Romagna, Programma di Ricerca Regione Università, 2007-2009, Area 1B: Patologia osteoarticolare: Ricerca pre-clinica e applicazioni cliniche della medicina rigenerativa, Unità Operativa n. 14.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sinanan AC, Hunt NP, Lewis MP. Human adult craniofacial muscle-derived cells: Neural-cell adhesion-molecule (NCAM; CD56)-expressing cells appear to contain multipotential stem cells. Biotechnol Appl Biochem. 2004;40:25–34. doi: 10.1042/BA20030185. [DOI] [PubMed] [Google Scholar]
- 2.Papaccio G, Graziano A, d’Aquino R, Graziano MF, Pirozzi G, Menditti D, et al. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: A cell source for tissue repair. J Cell Physiol. 2006;208:319–25. doi: 10.1002/jcp.20667. [DOI] [PubMed] [Google Scholar]
- 3.Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–21. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 4.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 6.Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, et al. A new population of human adult dental pulp stem cells: A useful source of living autologous fibrous bone tissue (LAB) J Bone Miner Res. 2005;20:1394–402. doi: 10.1359/JBMR.050325. [DOI] [PubMed] [Google Scholar]
- 7.Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, et al. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184:105–16. doi: 10.1159/000099617. [DOI] [PubMed] [Google Scholar]
- 8.Jo YY, Lee HJ, Kook SY, Choung HW, Park JY, Chung JH, et al. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13:767–73. doi: 10.1089/ten.2006.0192. [DOI] [PubMed] [Google Scholar]
- 9.Lensch MW, Daheron L, Schlaeger TM. Pluripotent stem cells and their niches. Stem Cell Rev. 2006;2:185–201. doi: 10.1007/s12015-006-0047-2. [DOI] [PubMed] [Google Scholar]
- 10.Mitsiadis TA, Barrandon O, Rochat A, Barrandon Y, De Bari C. Stem cell niches in mammals. Exp Cell Res. 2007;313:3377–85. doi: 10.1016/j.yexcr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J Endod. 2008;34:166–71. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito T, Ogawa M, Hata Y, Bessho K. Acceleration effect of human recombinant bone morphogenetic protein-2 on differentiation of human pulp cells into odontoblasts. J Endod. 2004;30:205–8. doi: 10.1097/00004770-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Tarnow DP, Wallace SS, Testori T, Froum SJ, Motroni A, Prasad HS. Maxillary sinus augmentation using recombinant bone morphogenetic protein-2/acellular collagen sponge in combination with a mineralized bone replacement graft: A report of three cases. Int J Periodontics Restorative Dent. 2010;30:139–49. [PubMed] [Google Scholar]
- 14.d’Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, et al. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75–83. doi: 10.22203/ecm.v018a07. [DOI] [PubMed] [Google Scholar]
- 15.Laino G, Graziano A, d’Aquino R, Pirozzi G, Lanza V, Valiante S, et al. An approachable human adult stem cell source for hard-tissue engineering. J Cell Physiol. 2006;206:693–701. doi: 10.1002/jcp.20526. [DOI] [PubMed] [Google Scholar]
- 16.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graziano A, d’Aquino R, Laino G, Papaccio G. Dental pulp stem cells: A promising tool for bone regeneration. Stem Cell Rev. 2008;4:21–6. doi: 10.1007/s12015-008-9013-5. [DOI] [PubMed] [Google Scholar]
- 18.Srisawasdi S, Pavasant P. Different roles of dexamethasone on transforming growth factor-beta1-induced fibronectin and nerve growth factor expression in dental pulp cells. J Endod. 2007;33:1057–60. doi: 10.1016/j.joen.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Iohara K, Zheng L, Ito M, Tomokiyo A, Matsushita K, Nakashima M. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells. 2006;24:2493–503. doi: 10.1634/stemcells.2006-0161. [DOI] [PubMed] [Google Scholar]
- 20.Ueno A, Yamashita K, Miyoshi K, Horiguchi T, Ruspita I, Abe K, et al. Soluble matrix from osteoblastic cells induces mineralization by dental pulp cells. J Med Invest. 2006;53:297–302. doi: 10.2152/jmi.53.297. [DOI] [PubMed] [Google Scholar]
- 21.Hosoya A, Nakamura H, Ninomiya T, Hoshi K, Yoshiba K, Yoshiba N, et al. Hard tissue formation in subcutaneously transplanted rat dental pulp. J Dent Res. 2007;86:469–74. doi: 10.1177/154405910708600515. [DOI] [PubMed] [Google Scholar]
- 22.Otaki S, Ueshima S, Shiraishi K, Sugiyama K, Hamada S, Yorimoto M, et al. Mesenchymal progenitor cells in adult human dental pulp and their ability to form bone when transplanted into immunocompromised mice. Cell Biol Int. 2007;31:1191–7. doi: 10.1016/j.cellbi.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 23.d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: A pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14:1162–71. doi: 10.1038/sj.cdd.4402121. [DOI] [PubMed] [Google Scholar]
- 24.Graziano A, d’Aquino R, Laino G, Proto A, Giuliano MT, Pirozzi G, et al. Human CD34+stem cells produce bone nodules in vivo. Cell Prolif. 2008;41:1–11. doi: 10.1111/j.1365-2184.2007.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graziano A, d’Aquino R, Cusella-De Angelis MG, De Francesco F, Giordano A, Laino G, et al. Scaffold's surface geometry significantly affects human stem cell bone tissue engineering. J Cell Physiol. 2008;214:166–72. doi: 10.1002/jcp.21175. [DOI] [PubMed] [Google Scholar]
- 26.d’Aquino R, De Rosa A, Laino G, Caruso F, Guida L, Rullo R, et al. Human dental pulp stem cells: From biology to clinical applications. J Exp Zool B Mol Dev Evol. 2009;312B:408–15. doi: 10.1002/jez.b.21263. [DOI] [PubMed] [Google Scholar]


