Abstract
Background and Purpose
This study examined whether overall cerebral blood flow was associated with known vascular risk factors, including cardiometabolic risk factors that comprise the metabolic syndrome, carotid artery intima-media thickness, and the Framingham risk score.
Methods
Three separate samples were available for analysis. Two comparable samples were combined to form a primary sample of middle-aged participants (n=576, 30 to 55 years of age) that completed both a risk factor assessment and a resting brain scan. Samples were recruited via mailings and advertisements within an urban area. Quantitative measures of cerebral blood flow were derived from Arterial Spin Labeled magnetic resonance imaging in this and a validation/generalization sample (n=76, 30 to 55 years).
Results
Cerebral blood flow was inversely associated with cardiometabolic risk indices, i.e., associated with lower waist circumference, systolic blood pressure, glucose, and triglyceride and higher high density lipoprotein. Moreover, cerebral blood flow was also related to Framingham Risk and carotid intima-media thickness. In the validation sample, which employed a slightly different brain imaging technique, significant relationships were replicated for cardiometabolic risk, but not for Framingham risk.
Conclusions
Reduced cerebral blood flow appears to be a correlate of vascular disease risk factors associated with cardio-metabolic dysregulation. Cerebral blood flow may provide a valid imaging biomarker for cardiovascular risk.
Introduction
Resting cerebral blood flow (CBF) shows substantial stability due to autoregulation,1 but decrements in CBF with age and vascular disease may underlie cognitive impairments and dementia.2 This raises the question of whether vascular risk factors relate to cerebrovascular circulation prior to old age.
This question remains largely open because total CBF is not routinely assessed due to the non-quantitative nature of typical magnetic resonance imaging (MRI) measures, the expense of positron emission tomography assessment, and a research focus on elderly samples. Early work with small samples using xenon inhalation and single photon tomography did provide estimates of CBF and suggested a decline in CBF with age, with trends toward exacerbation of this decline by cardiovascular risk factors.3–10 One larger, longitudinal study verified the CBF decline with age as well as demonstrating a CBF decline with overt cerebrovascular disease.9 A small number of more current studies variously estimating CBF also suggest a relationship to vascular risk factors. Among elderly men but not women, blood pressure is negatively related to single photon emission computed tomography regional CBF estimates.11 Hypertension among patients with atherosclerosis has been shown over 5 years of follow up to reduce CBF estimated from arterial measures.12 Effective treatment of hypertension has been shown, however, to enhance CBF velocity.13 Less CBF velocity has also been related to the presence of Type 2 diabetes and inflammatory indices.14 These arterial velocity measures also show lower estimated CBF to relate to greater number of white matter hyperintensities.14, 15 Framingham Risk has been related to longitudinal reductions in regional flow to visceromotor and viscerosensory brain areas using relative measures from positron emission tomography,16 to middle cerebral artery flow,17 and to cerebral vasoreactivity to hypercapnia, but not to overall CBF, as assessed with arterial spin labeling (ASL).18 In short, quantitative resting CBF has rarely been examined relative to cardiovascular risk though various less direct techniques and related measures suggest that a relationship will be observed.
Moreover, virtually all evidence to date bearing on this issue has been from elderly participants (mean ages 65 years or greater). Vascular risk factors emerge over time and are useful to identify prior to frank cerebrovascular disease. The current study cross-sectionally examined in midlife samples (30–55 years of age) whether components of the metabolic syndrome (MetS)19 would relate to total cerebral blood flow (CBF). Additional analyses examined the relationship to carotid intima thickness20, 21 and the Framingham Risk score.22 The use of ASL, an MRI technique yielding quantitative CBF estimates, permitted examination of a reasonably large sample.23–26 Total CBF was expected to relate inversely to risk. Emphasis was placed on a quantitative combination of MetS components give the expected low prevalence of the complete MetS in the midlife. We tested our hypothesis in the primary sample, and examined the robustness of our results in an independent, more racially diverse sample imaged by pseudocontinuous ASL rather than pulsed ASL MRI sequence. This sample also had measures of white matter hyperintensity available. We sought in the ‘validation’ sample to replicate relationships between CBF and MetS components despite any variance due to the differing ASL sequences.
Methods
Participants
We report on 576 participants with acceptable brain imaging and risk data drawn from two projects. Table 1 presents participant characteristics separately for the two samples combined and third, validation sample. Inclusion criteria are listed in the online supplement; those with cardiovascular disease or medicated for this, diabetes, or lipids were excluded.
Table 1.
Characteristics of Three Samples
| Primary Sample: (n=436) | Primary Sample: (n=140) | Generalization Sample (n=76) | |
|---|---|---|---|
| Mean | Mean | Mean | |
| Age (Years) | 42.6 ± 7.3 | 40.7 ± 6.2 | 46.4 ± 7.3 |
| Gender (% male) | 47 | 53 | 47 |
| Race (% Caucasian) | 83 | 73 | 63 |
| Education (years) | 16.9± 2.8 | 17.2± 3.2 | 14.9±2.8 |
| BMI1 | 26.9 ± 5.2 | 27.0 ± 4.9 | 27.8 ± 5.9 |
| SBP2 (mmHg) | 114.9 ± 11.2 | 121.4 ± 9.3 | 115.4 ± 11.5 |
| DBP3 (mmHg) | 72.2 ± 8.1 | 73.0 ± 8.7 | 76.2 ± 7.5 |
| Glucose (mg/dl) | 98.1 ± 9.8 | 88.5 ± 12.8 | 90.7 ± 14.2 |
| Waist (cm) | 90.3 ± 14.0 | 90.4 ± 13.0 | 94.5 ± 14.3 |
| Triglyceride (mg/dl) | 109.0 ± 67.2 | 93.0 ± 59.7 | 106.9 ± 50.9 |
| HDL4 (mg/dl) | 55.4 ± 14.4 | 49.9 ± 16.4 | 56.7 ± 15.1 |
| Insulin (mg/dl) | 12.4 ± 6.3 | 8.2 ± 5.9 | 12.4 ± 8.5 |
| HOMA5 | 3.1 ± 1.8 | 2.0 ± 1.9 | 3.0 ± 3.0 |
| Metabolic Syndrome (%prevalence) | 13 | 16 | 15 |
| Years in School | 16.9 ± 2.8 | 17.2 ± 3.2 | 15.0 ± 2.8 |
| Alcohol (Drinks Weekly) | 3.2 ± 4.7 | 3.0 ± 4.4 | 2.4 ± 1.2 |
| Nicotine Use (%) | 15 | 14 | 28 |
Note.
BMI is body mass index,
SBP is systolic blood pressure,
DBP is diastolic blood pressure,
HDL is high density lipoprotein,
HOMA is homeostatic model assessment, N varies slightly between measures due to technical loss of data.
The validation sample included participants collected to date from a study of middle-aged participants with normal to pre-hypertensive levels of blood pressure. Seventy six participants were available. Other than the blood pressure inclusion, inclusion criteria were essentially identical to the other studies. Cardiometabolic risk (CMR) measures were assessed using the same methods as in the primary sample. All studies used an ASL technique, but the studies in the primary sample used the pulsed ASL MRI sequence and validation sample used pseudo-continuous ASL. Psychosocial and psychophysiological predictors of cardiovascular risk were the focus of the studies in the primary sample and progression to hypertension the focus of the validation study. Further minor differences in age and racial composition were adventitious. Samples were recruiting via mailings and advertisements within an urban area, mass mailings to targeted areas and campus and city newspaper advertisements. Participants in all studies provided informed consent and all procedures were approved by the University of Pittsburgh Institutional Review Board.
Design
Participants performed multi session protocols for the primary samples: Initial medical and demographic data collection, electrocardiogram and ultrasound measures, a neuropsychological and personality test session and the brain imaging session. A fasting blood draw was performed in the morning of the initial session.
MRI method
MRI data were collection on a Siemens 3T magnet. A resting scan used a pulsed ASL sequence to obtain quantitative measures of CBF. Images were preprocessed to control for movement, align scans, and normalize to a standard brain space. Previously established analytic software23–26 adjusted for our scan parameters assessed CBF. The online supplement provides details on the MR technique and processing.
Metabolic risk
Both the presence or absence of the MetS as well as a quantitative index of cardiometabolic risk (CMR) were defined. According to NCEP criteria,27 the MetS is defined as the presence of three or more of the following: (1) serum triglycerides ≥ 150 mg/dL; (2) fasting serum glucose ≥ 100 mg/dL; (3) waist circumference ≥102 cm in men or ≥ 88 cm in women; (4) SBP ≥ 130 or DBP ≥ 85 mm Hg; (5) serum HDL cholesterol < 40 mg/dL in men or < 50 mg/dL in women. The scope of our study did not permit assessment of important possible etiological correlates of metabolic syndrome, e.g. hepatic steatosis.
A composite index of cardiometabolic risk was calculated from the criteria that define the MetS: 28 BP, waist circumference, HDL-cholesterol, triglycerides and glucose. Use of cardiometabolic risk as a continuous measure better predicts future CVD events 29 and would be expected in middle-aged samples such as the current one to be a better predictor than the use of threshold values to calculate the syndrome as present or absent. The 5 risk factors (using systolic BP as the BP factor) were each standardized and HDL was multiplied by −1. The 5 measures were summed and labeled as “cardiometabolic risk score” (CMR).
The Framingham Risk score was available as an additional index of known risk. This score is an accumulation of points assigned separately by gender for age, total cholesterol, smoking status, high density lipoprotein, and systolic blood pressure. 22
Carotid and white matter hyperintensity measurement
Carotid artery IMT was assessed by duplex (B-mode) ultrasonography using an Acuson Antares scanner (Acuson-Siemens, Malvern, PA). Four locations spanning the interior and exterior carotid in right and left arteries were assessed and mean IMT from these areas formed the primary dependent variable currently employed. (See further detail in online supplement.)
This measure was unavailable for the validation sample. This sample though assessed white matter hyperintensities using a validated, automated technique. 30 Total voxel counts of those showing a white matter hyperintensity were analyzed.
Analysis
Descriptive bivariate correlations between CBF and risk were followed by multiple regression modeling. The modeling first adjusted for demographic influences on CBF and risk factor was added as a second step. At initial step, age, race, current smoking status, gender, and total brain volume were input. The second step added the CMR measure, MetS or the Framingham index. Age and smoking status are included in the Framingham index so in the second step for the Framingham index separate age and smoking indices were not included. This basic analysis was repeated in the validation sample. Depending on the availability of the measure in a sample, carotid IMT or white matter hyperintensity measures were also assessed in step 2.
Results
Participant characteristics are presented in Table 1 for the primary and validation samples. The samples are quite comparable with reasonably typical values for their age cohort. The validation sample differs somewhat in mean age and racial composition in addition to the use of the pseudocontinuous ASL measure.
Carotid IMT and Metabolic risk related to CBF
Males, those with relatively higher body mass index, and those possessing the metabolic syndrome had lower CBF. Race and nicotine use were unrelated. Table 1 of the online supplement details these results for categorical participant characteristics. Table 2 presents the bivariate correlations with participant characteristics as well as the relation to the components of the syndrome and related insulin and HOMA indices. Table 2 also illustrates the similarity of CBF and regional flow correlations using regions selected to approximate the watershed areas for the primary cerebral arteries, i.e. anterior cerebral artery (medial frontal, superior parietal, and cingulate areas), middle cerebral artery (frontal, temporal, and inferior parietal), and posterior cerebral artery (occipital and inferior temporal). (Note that the correlations based on brain regions are only available for the larger of the two samples that were combined.)
Table 2.
Pearson Correlations between overall and regional CBF1, Demographic Cardiometabolic Risk Factors
| Cerebral blood flow | CMR2 | Age | Carotid IMT3 | SBP4 | Glucose | Waist5 | Tri- Glyceride | HDL6 | Insulin | HOMA7 |
|---|---|---|---|---|---|---|---|---|---|---|
| Global Cerebral Blood Flow | −0.28 | −.11 | −0.21 | −.28 | −0.16 | −0.24 | −0.19 | 0.28 | −0.09 | −0.14 |
| Anterior Cerebral Artery | −0.26 | −.11 | −0.22 | −.17 | −0.16 | −0.22 | −0.16 | 0.17 | −0.08 | −0.10 |
| Middle Cerebral Artery | −0.23 | −.12 | −0.23 | −.20 | −0.14 | −0.25 | −0.17 | 0.18 | −0.13 | −0.15 |
| Posterior Cerebral Artery | −0.18 | −.07 | −0.18 | −.18 | −0.19 | −0.35 | −0.20 | 0.29 | −0.24 | −0.26 |
Note. Correlations greater than the absolute value of r=.09 are significant at p<.05.
Regional correlations available only for the initial samples in the combined sample (n=439).
CMR is cardiometabolic risk,
IMT is intima-media thickness,
SBP is systolic blood pressure,
Waist is waist circumference,
HDL is high density lipoprotein, and
HOMA is homeostatic model assessment. Cardiometabolic score is sum of standardized measures of the MetS factors.
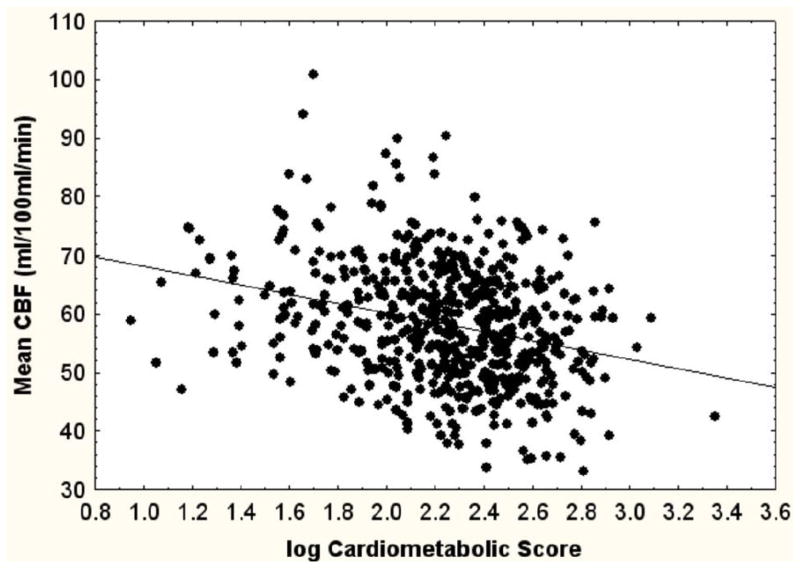
Figure 1 presents the scatter diagrams showing the correlations of overall CBF with CMR and carotid IMT. The correlation with CMR accounts for 8% of the variance in CBF while that with carotid IMT, 4%.
Figure 1.
Scatterdiagrams showing bivariate relationship between CBF and a cardiometabolic score (left) and carotid intima-media thickness (right). The product-moment correlation for the cardiometabolic score and CBF was r=−.28, p<.0001 for carotid intima-media thickness r=−.21, p<.001, n for each=576.
Multivariate relations: CBF, IMT, and CMR
Table 3 shows the results for the basic model and for the models adding risk factors. Each risk factor was added individually to assess its specific relationship to CBF as a second step. Parallel models are presented for the basic sample as well as the validation sample. Separate metabolic variables were not separately tested but subsumed within the CMR and MetS variables.
Table 3.
Regression Weights of Indices of Vascular Risk 1
| Primary Sample (n=576) | ||||
|---|---|---|---|---|
| Variable | Beta | Standard Error | T | p-value |
| Age | −.17 | .038 | −4.57 | <.001 |
| Brain Volume | .08 | .052 | 1.62 | Not Significant |
| Race | .04 | .039 | .99 | Not Significant |
| Sex | .49 | .050 | 9.63 | <.001 |
| Current Smoking | −.01 | .038 | −.43 | Not Significant |
|
| ||||
| STEP 2 | ||||
|
| ||||
| Cardiometabolic Risk | −.11 | .042 | −2.63 | .009 |
| MetS | −.05 | .038 | −1.36 | Not Significant |
| Framingham Risk2 | −.15 | .060 | −2.51 | .01 |
| Carotid IMT | −.12 | .047 | −2.44 | .01 |
| Validation Sample (n=76) | ||||
|
| ||||
| Age | −.07 | .116 | −.59 | Not Significant |
|
| ||||
| Brain Volume | −.27 | .183 | −1.48 | Not Significant |
|
| ||||
| Race | −.09 | .121 | −.72 | Not Significant |
|
| ||||
| Current Smoking | .07 | .119 | .63 | Not Significant |
|
| ||||
| Sex | .14 | .179 | .79 | Not Significant |
| STEP 2 | ||||
|
| ||||
| Cardiometabolic Risk | −.36 | .113 | −3.20 | .002 |
|
| ||||
| MetS | −.32 | .112 | −2.84 | .006 |
|
| ||||
| Framingham Risk2 | −.13 | .216 | −.62 | Not Significant |
|
| ||||
| White Matter Hyperintensities | −.06 | .127 | −.48 | Not Significant |
Model initially shows demographic and CBF result, then in Step 2 showing effect of adding either CMR or other risk factor, e.g., Framingham Risk score. Beta is standardized weight.
Model included total Framingham Risk score, race, and total brain volume.
In the initial model Age and Gender showed directionally consistent influences on CBF, but these factors were not significant in the smaller validation sample. Brain size, smoking, and race did not show significant relationships in either sample.
Adding CMR to the model demonstrated significant relationships with CBF in both the primary and validation samples. The relationship is modest, but consistent across samples. The dichotomized MetS variable relates to CBF significantly in the validation sample and is only directionally consistent with this in the primary sample. The Framingham Risk score in contrast was significantly related to CBF in the primary sample, but only directionally consistent in the validation sample. Carotid IMT was available only in the primary sample, in which it was related significantly to CBF. Importantly, in the validation sample, automated voxel counts of white matter hyperintensities were available, but these were unrelated to CBF.
Discussion
The current results establish that CBF is related to cardio metabolic risk in midlife. The relationship is consistent, though modest, across the primary sample and the validation sample. CBF seems closely related to each of the components of the metabolic syndrome—a known risk for both diabetes and cardiovascular disease.21, 22 Furthermore, CBF was independently related to carotid IMT and Framingham risk in the primary sample. The degree of relationship between CBF and vascular risk though modest is approximately half of the risk computed in this sample between being male or overweight/obese and carotid IMT, both accepted vascular risk factors. Our results are largely consistent with earlier work in older samples suggesting decrements in CBF with age and vascular risk, e.g.15
Our cross-sectional observations do not allow any causal inferences. Metabolic factors could induce reductions in CBF or cerebrovascular changes could precede metabolic changes. Longitudinal observations might determine whether CBF is modified before, after, or in conjunction with increases in risk via metabolic syndrome factors. Relationships of CBF to risk were generally consistent across samples despite variation in ASL imaging; the variation though limits inferences about factors not related consistently across the primary and validation samples. This as well, as sampling variability, may contribute to the seemingly stronger relationship between CBF and MetS components in the smaller, validation sample. Although carotid IMT and Framingham Risk were independently related to CBF in our primary sample, the relationship with metabolic factors was most consistent. CBF was also related significantly and similarly to each of the factors composing the metabolic syndrome. Microcirculatory change more closely related to metabolic factors, such as hyperglycemia, are known to influence the structure and function of larger vessels, potentially altering large vessel flow, i.e. CBF.31 Factors that we did not measure may, of course, have pathogenic influences on CBF and cardio metabolic disease, but the current concomitance of CBF and metabolic syndrome factors argues for initially understanding this linkage. Our prior work has suggested that hypertension, one factor of the metabolic syndrome, may have early effects on the brain that are not readily reversed by successful pharmacological treatment of hypertension32. Examining metabolic factors taken together and atherosclerosis seems, however, to be equally or more important.
Reductions in CBF in conjunction with vascular risk might signal early atherosclerotic influences on the brain vasculature. Given the absence of regional specificity in our results, systemic factors should be considered. CBF varies as a function of the number of neurons and their overall activity, most primarily the energetic demand of post synaptic potential changes.33, 34 Early atherogenic and vasoconstrictive effects of likely pathogenetic excesses of, e.g., angiotensin II, may impact CBF by reducing metabolic demand through neuronal cell death as well as tonically constricting the vasculature.19, 35–37 The latter effect may impact cerebral autoregulation of blood flow,38 i.e., challenge regulatory capacity. We examined white matter hyperintensities as a possible indicant of cortical arteriosclerosis.39 White matter hyperintensities were not related to CBF in the validation study although this is not definitive given the relatively young mean age of this sample. As noted previously, CBF has been related to white matter lesions in older samples14, 15 although ASL assessed CBF was unrelated to Framingham Risk in a small sample of older adults (mean age 71.2, n=33, 15 with mild cognitive deficit).18 Of greater relevance, white matter disruption in a middle aged sample with high blood pressure was recently reported using diffusion tensor imaging to detect damage in white matter tracts (a relationship not present for white matter hyperintensities).40 Others have suggested that the MetS impairs brain capillary vasodilation through insulin resistance.41 Our data cannot specifically test this mechanism. In short, although reasonable alternatives exist, specific pathophysiological processes related to cardiovascular disease in the samples examined could not be identified; the mechanism underlying the observed relationship remains unknown.
Summary/Conclusions
Overall, the results confirm early observations that suggested that CBF might be reduced with age and vascular risk. The availability of an MRI sequence suitable for quantifying CBF made it possible to confirm these observations. The modest but consistent relationship of cardiometabolic risk to CBF is supportive of the involvement of cerebrovascular factors in risk at midlife. We have not been able to draw any specific implications of differences in CBF for cortical function, however. Finding the relationship at midlife in metabolic risk factors, likely themselves mediated by modifiable health behaviors, does strengthen the potential value of preventive measures taken well prior to old age. Progression of CBF changes with age may be of particular importance given observations of regional CBF relationships with preclinical indicants of vascular disease at older ages.42 Further examination of central regulation disruption as a component of cardiometabolic risk seems required.
Supplementary Material
Figure 2.
Acknowledgments
We thank Brittney Gidwitz for her assistance in data collection.
Sources of Funding. The project described was supported by the NIH through Grant Numbers R01-HL089850, R01-HL101421, HL101959, HL040962, UL1RR024153 and UL1TR000005
Footnotes
Conflicts of Interest. None.
References
- 1.Hall JE. Guyton and hall textbook of medical physiology. Philadelphia: Saunders/Elsevier; 2011. [Google Scholar]
- 2.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claus JJ, Breteler MM, Hasan D, Krenning EP, Bots ML, Grobbee DE, et al. Regional cerebral blood flow and cerebrovascular risk factors in the elderly population. Neurobiol Aging. 1998;19:57–64. doi: 10.1016/s0197-4580(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 4.Naritomi H, Meyer JS, Sakai F, Yamaguchi F, Shaw T. Effects of advancing age on regional cerebral blood flow. Studies in normal subjects and subjects with risk factors for atherothrombotic stroke. Arch Neurol. 1979;36:410–416. doi: 10.1001/archneur.1979.00500430040005. [DOI] [PubMed] [Google Scholar]
- 5.Nobili F, Rodriguez G, Marenco S, DeCarli F, Gambaro M, Castello C, et al. Regional cerebral blood flow in chronic hypertension. A correlative study. Stroke. 1993;24:1148–1153. doi: 10.1161/01.str.24.8.1148. [DOI] [PubMed] [Google Scholar]
- 6.Grotta J, Ackerman R, Correia J, Fallick G, Chang J. Whole blood viscosity parameters and cerebral blood flow. Stroke. 1982;13:296–301. doi: 10.1161/01.str.13.3.296. [DOI] [PubMed] [Google Scholar]
- 7.Rogers RL, Meyer JS, Shaw TG, Mortel KF. Reductions in regional cerebral blood flow associated with chronic consumption of alcohol. J Am Geriatr Soc. 1983;31:540–543. doi: 10.1111/j.1532-5415.1983.tb02198.x. [DOI] [PubMed] [Google Scholar]
- 8.Rogers RL, Meyer JS, Shaw TG, Mortel KF, Hardenberg JP, Zaid RR. Cigarette smoking decreases cerebral blood flow suggesting increased risk for stroke. Jama. 1983;250:2796–2800. [PubMed] [Google Scholar]
- 9.Shaw TG, Mortel KF, Meyer JS, Rogers RL, Hardenberg J, Cutaia MM. Cerebral blood flow changes in benign aging and cerebrovascular disease. Neurology. 1984;34:855–862. doi: 10.1212/wnl.34.7.855. [DOI] [PubMed] [Google Scholar]
- 10.Gur RC, Gur RE, Obrist WD, Skolnick BE, Reivich M. Age and regional cerebral blood flow at rest and during cognitive activity. Arch Gen Psychiatry. 1987;44:617–621. doi: 10.1001/archpsyc.1987.01800190037006. [DOI] [PubMed] [Google Scholar]
- 11.Waldstein SR, Lefkowitz DM, Siegel EL, Rosenberger WF, Spencer RJ, Tankard CF, et al. Reduced cerebral blood flow in older men with higher levels of blood pressure. J Hypertens. 2010;28:993–998. doi: 10.1097/hjh.0b013e328335c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller M, van der Graaf Y, Visseren FL, Mali WP, Geerlings MI, Group SS. Hypertension and longitudinal changes in cerebral blood flow: The SMART-MR study. Ann Neurol. 2012;71:825–833. doi: 10.1002/ana.23554. [DOI] [PubMed] [Google Scholar]
- 13.Lipsitz LA, Gagnon M, Vyas M, Iloputaife I, Kiely DK, Sorond F, et al. Antihypertensive therapy increases cerebral blood flow and carotid distensibility in hypertensive elderly subjects. Hypertension. 2005;45:216–221. doi: 10.1161/01.HYP.0000153094.09615.11. [DOI] [PubMed] [Google Scholar]
- 14.Novak V, Last D, Alsop DC, Abduljalil AM, Hu K, Lepicovsky L, et al. Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes Care. 2006;29:1529–1534. doi: 10.2337/dc06-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Vrooman HA, Hofman A, et al. Total cerebral blood flow and total brain perfusion in the general population: The Rotterdam scan study. J Cereb Blood Flow Metab. 2008;28:412–419. doi: 10.1038/sj.jcbfm.9600526. [DOI] [PubMed] [Google Scholar]
- 16.Beason-Held LL, Thambisetty M, Deib G, Sojkova J, Landman BA, Zonderman AB, et al. Baseline cardiovascular risk predicts subsequent changes in resting brain function. Stroke. 2012;43:1542–1547. doi: 10.1161/STROKEAHA.111.638437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pase MP, Grima NA, Stough CK, Scholey A, Pipingas A. Cardiovascular disease risk and cerebral blood flow velocity. Stroke. 2012;43:2803–2805. doi: 10.1161/STROKEAHA.112.666727. [DOI] [PubMed] [Google Scholar]
- 18.Glodzik L, Rusinek H, Brys M, Tsui WH, Switalski R, Mosconi L, et al. Framingham cardiovascular risk profile correlates with impaired hippocampal and cortical vasoreactivity to hypercapnia. J Cereb Blood Flow Metab. 2011;31:671–679. doi: 10.1038/jcbfm.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farooqui AA, Farooqui T, Panza F, Frisardi V. Metabolic syndrome as a risk factor for neurological disorders. Cell Mol Life Sci. 2012;69:741–762. doi: 10.1007/s00018-011-0840-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: A meta-analysis. Atherosclerosis. 2012;220:128–133. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 21.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2010;56:e50–e103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the 3rd Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Wang DJ, Detre JA. Test-retest reliability of arterial spin labeling with common labeling strategies. J Magn Reson Imaging. 2011;33:940–949. doi: 10.1002/jmri.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Detre JA, Wang J, Wang Z, Rao H. Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Curr Opin Neurol. 2009;22:348–355. doi: 10.1097/WCO.0b013e32832d9505. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Aguirre GK, Rao H, Wang J, Fernandez-Seara MA, Childress AR, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26:261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu WC, Fernandez-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med. 2007;58:1020–1027. doi: 10.1002/mrm.21403. [DOI] [PubMed] [Google Scholar]
- 27.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 28.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2006;21:1–6. doi: 10.1097/01.hco.0000200416.65370.a0. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal S, Jacobs DR, Jr, Vaidya D, Sibley CT, Jorgensen NW, Rotter JI, et al. Metabolic syndrome derived from principal component analysis and incident cardiovascular events: The multi ethnic study of atherosclerosis (MESA) and health, aging, and body composition (Health ABC) Card Res Pract. 2012:919425. doi: 10.1155/2012/919425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu M, Rosano C, Butters M, Whyte E, Nable M, Crooks R, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006;148:133–142. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Safar ME, Struijker-Boudier HA. Cross-talk between macro- and microcirculation. Acta Physiol. 2010;198:417–430. doi: 10.1111/j.1748-1716.2009.02073.x. [DOI] [PubMed] [Google Scholar]
- 32.Jennings JR, Zanstra Y. Is the brain the essential in hypertension? Neuroimage. 2009;47:914–921. doi: 10.1016/j.neuroimage.2009.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drake CT, Iadecola C. The role of neuronal signaling in controlling cerebral blood flow. Brain Lang. 2007;102:141–152. doi: 10.1016/j.bandl.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Karbowski J. Scaling of brain metabolism and blood flow in relation to capillary and neural scaling. PLoS ONE. 2011;6:e26709. doi: 10.1371/journal.pone.0026709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Putnam K, Shoemaker R, Yiannikouris F, Cassis LA. The renin-angiotensin system: A target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H1219–1230. doi: 10.1152/ajpheart.00796.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saavedra JM. Angiotensin II AT(1) receptor blockers as treatments for inflammatory brain disorders. Clin Sci (Lond) 2012;123:567–590. doi: 10.1042/CS20120078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saavedra JM. Angiotensin II AT(1) receptor blockers ameliorate inflammatory stress: A beneficial effect for the treatment of brain disorders. Cell Mol Neurobiol. 2012;32:667–681. doi: 10.1007/s10571-011-9754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baumbach GL, Heistad DD. Heterogeneity of brain blood flow and permeability during acute hypertension. Am J Physiol. 1985;249:H629–H637. doi: 10.1152/ajpheart.1985.249.3.H629. [DOI] [PubMed] [Google Scholar]
- 39.Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, et al. Heterogeneity of small vessel disease: A systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82:126–135. doi: 10.1136/jnnp.2009.204685. [DOI] [PubMed] [Google Scholar]
- 40.Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, et al. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: A cross-sectional study. Lancet Neurol. 2012;11:1039–1047. doi: 10.1016/S1474-4422(12)70241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of metabolic syndrome on cognition and brain: A selected review of the literature. Arterioscler Thromb Vasc Biol. 2012;32:2060–2067. doi: 10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sojkova J, Najjar SS, Beason-Held LL, Metter EJ, Davatzikos C, Kraut MA, Zonderman AB, Resnick SM. Intima-Media Thickness and Regional Cerebral Blood Flow in Older Adults. Stroke. 2010;41:273–279. doi: 10.1161/STROKEAHA.109.566810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.