Abstract
Aims:
We present retrospective analysis of patients of glioblastoma multiforme (GBM) and discuss clinical characteristics, various treatment protocols, survival outcomes, and prognostic factors influencing survival.
Materials and Methods:
From January 2002 to June 2009, 439 patients of GBM were registered in our department. The median age of patients was 50 years, 66.1% were males, and 75% underwent complete or near-total excision. We evaluated those 360 patients who received radiotherapy (RT). Radiotherapy schedule was selected depending upon pre-RT Karnofsky Performance Status (KPS). Patients with KPS < 70 (Group I, n = 48) were planned for RT dose of 30-35 Gy in 10-15 fractions, and patients with KPS ≥ 70 (Group II, n = 312) were planned for 60 Gy in 30 fractions. In group I, six patients and in group II, 89 patients received some form of chemotherapy (lomustine or temozolomide).
Statistical Analysis Used:
Statistical analysis was done using Statistical Package for Social Sciences, version 12.0. Overall survival (OS) was calculated using Kaplan-Meier method, and prognostic factors were determined by log rank test. The Cox proportional hazards model was used for multivariate analysis.
Results:
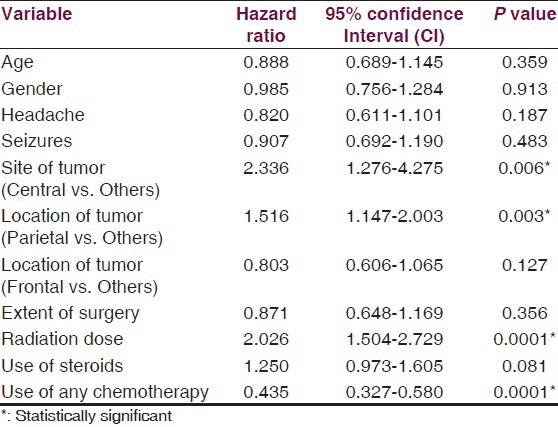
The median follow-up was 7.53 months. The median and 2-year survival rates were 6.33 months and 2.24% for group I and 7.97 months and 8.21% for group II patients, respectively (P = 0.001). In multivariate analysis, site of tumor (central vs. others; P = 0.006), location of tumor (parietal lobe vs. others; P = 0.003), RT dose (<60 Gy vs. 60 Gy; P = 0.0001), and use of some form of chemotherapy (P = 0.0001) were independent prognostic factors for survival.
Conclusions:
In patients with GBM, OS and prognosis remains dismal. Whenever possible, we should use concurrent and/or adjuvant chemotherapy to maximize the benefits of post-operative radiotherapy. Patients with poor performance status may be considered for hypofractionated RT schedules, which have similar median survival rates as conventional RT.
Keywords: Glioblastoma multiforme, prognostic factors, radiotherapy, survival, temozolomide
Introduction
Glioblastoma multiforme (GBM) is regarded as the endpoint of diffusely infiltrating astrocytoma and is grade IV Astrocytoma of World Health Organization (WHO) classification. It is the most common and the most aggressive primary brain tumor in adults, accounts for more than 50% of all malignant astrocytomas and usually presents in sixth or seventh decade of life. The standard treatment includes maximal safe resection and post-operative localized radiotherapy (RT).[1] The median survival of patients after surgery and RT is less than one year with hardly any patient surviving more than two years; and overall survival (OS) at 2-years and 5-years is less than 10% and 2%, respectively.[1,2,3,4] After publication of results of the randomized phase III trial by Stupp et al., post-operative chemo-radiotherapy with concurrent and adjuvant temozolomide (TMZ) has become the new standard of care for patients with GBM.[4,5] However, in a developing country like India where healthcare is not equally accessible, majority of patients with GBM cannot afford costly chemotherapeutic drugs, and radical radiotherapy alone is still the only adjuvant treatment option in these patients. The purpose of the present retrospective analysis was to present and discuss clinical features, various treatment schedules and identify independent prognostic factors that significantly predict survival in a cohort of patients with GBM from routine clinical practice in our institute and to compare the results with literature.
Materials and Methods
From January 2002 to June 2009, 605 patients of high-grade gliomas were registered in our department. The histological diagnosis was provided by the neuropathologist and was graded according to WHO classification. We retrospectively analyzed case records of 439 (72.6%) patients [Figure 1] of GBM who underwent craniotomy and maximal safe resection. All patients were operated at our institute, and only patients with primary GBM were included.
Figure 1.
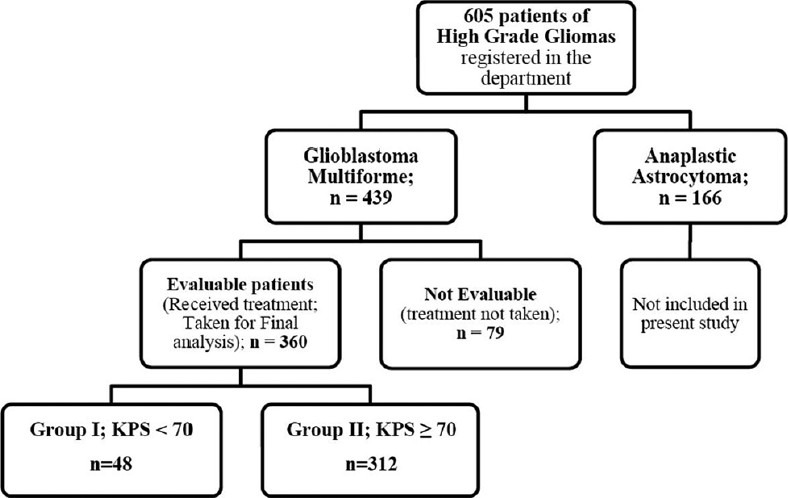
Description of study cohort
Data collection
The following data was collected from the medical records of patients: 1) Demographic profile (age and gender); 2) Presenting symptoms and duration; 3) Site of tumor; 4) Type of surgery; 5) KPS at presentation (before surgery) and before radiation; 6) Type of post-operative treatment (radiotherapy +/- chemotherapy); 7) Treatment related toxicities, and; 8) Follow-up data: Response to treatment (clinical or radiological) and clinical outcomes including OS, which was mainly collected when patients visited the out-patient department or during phone interview with patients and/or relatives.
Statistical analysis
Survival duration was calculated from date of diagnosis to date of death or date of last contact. Data on patients who were alive at the end of study was censored from survival analysis. Statistical analysis was done using Statistical Package for Social Sciences (SPSS), version 12.0. OS was calculated using Kaplan-Meier method, and prognostic factors were determined by log rank test. The Cox proportional hazards model was used for multivariate analysis. A P < 0.05 was considered to indicate statistical significance.
Results
Patient characteristics
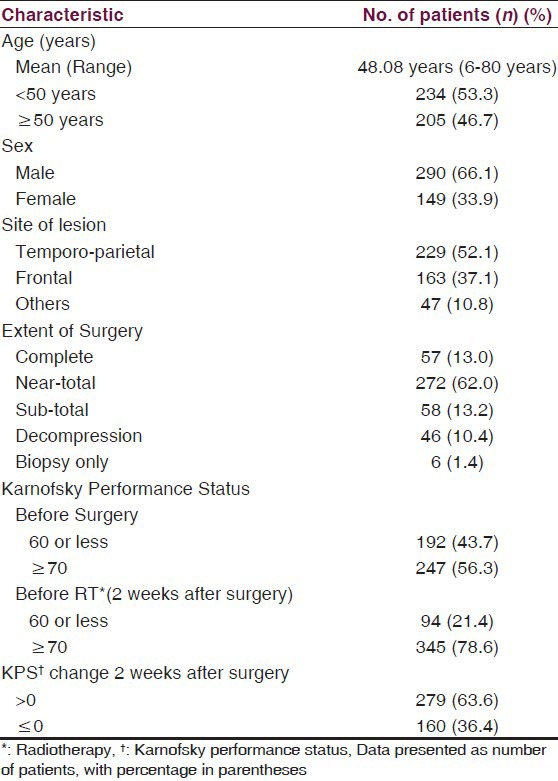
Patient characteristics are presented in Table 1. Three hundred and twenty six (74.3%) patients presented with symptoms of raised intracranial pressure including headache and vomiting, 33% had seizures, while 54.7% had sensorimotor deficits. Most patients had acute onset of symptoms, with median duration of symptoms prior to surgery being two months. Majority of patients (64.7%) had a magnetic resonance (MR) scan prior to surgery, while 28% had computed tomography (CT) scan, and 7.3% had both.
Table 1.
Patient characteristics
Treatment
Standard treatment included surgery and post-operative radiotherapy, with or without concurrent and/or adjuvant chemotherapy.
Surgery
All patients had a surgical intervention with an aim to carry out maximal safe resection while preserving key eloquent sites in brain. If surgical resection was deemed to be associated with relatively high risk of deficits due to extent of disease or proximity to vital structures, an open or stereotactic biopsy was performed to establish histopathological diagnosis. Three-quarters of the patients (n = 329) underwent complete or near-total resection [Table 1]. VP shunt was required in 6.2% patients before definitive surgery to relieve symptoms of raised intracranial pressure.
Radiotherapy
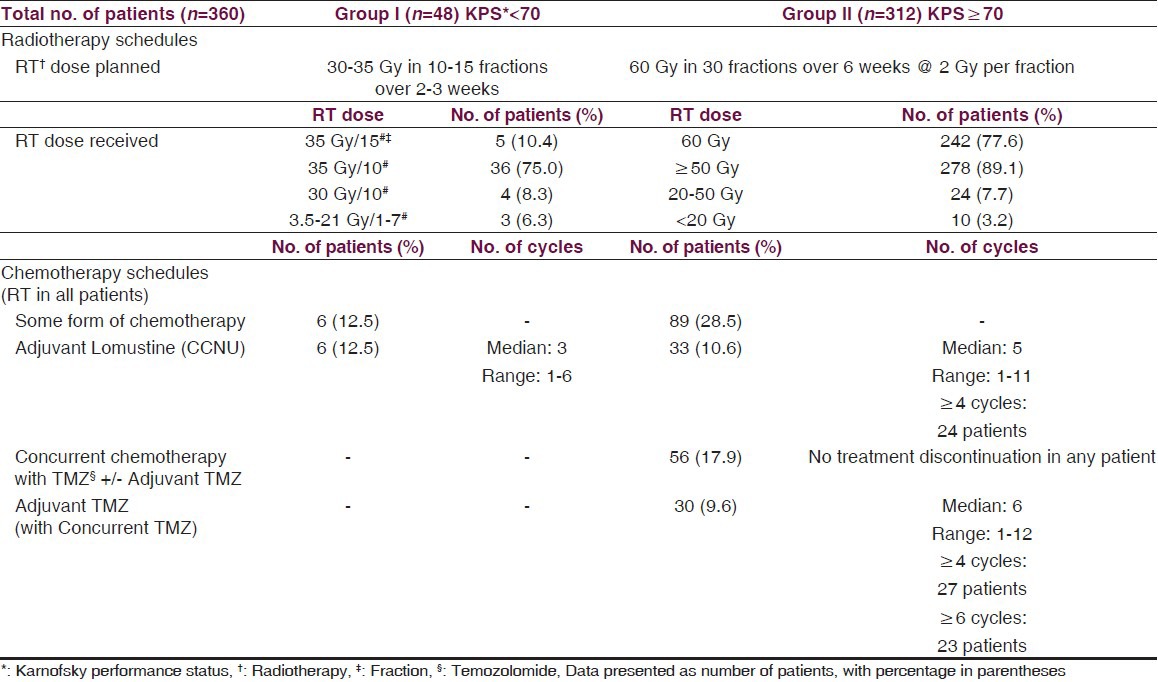
Post-operative radiotherapy was routinely started within one month of surgery. Out of 439 patients of GBM, only those 360 patients who received radiotherapy were evaluable and were taken for final analysis [Figure 1]. Two different schedules of radiotherapy were used in these patients depending upon pre-RT KPS. So, we divided these 360 patients into two groups: I and II. Patients with pre-RT KPS of < 70 (Group I; n = 48) were planned for RT dose of 30-35 Gy in 10-15 fractions over two-three weeks. Patients with KPS ≥ 70 (Group II; n = 312) were planned for RT dose of 60 Gy in 30 fractions treating once daily for five days a week. External beam radiotherapy was delivered using megavoltage telecobalt (Theratron 780 and 780C: Co-60 source) or linear accelerator (Varian CLINAC: 6 MV) machine.
Radiation treatment techniques were individualized according to extent of disease. Group I patients usually received large volume or whole brain RT with a median dose of 35 Gy (Range 3.5 Gy-35 Gy) in a median of 10 fractions (Range: 1-15 fractions). Four patients received whole brain RT dose of 30 Gy in 10 fractions [Table 2]. Group II patients received localized radiotherapy to the contrast-enhancing lesion on the contrast CT/T1-weighted images or the T2/FLAIR sequence MR scan with 2–3 cm margin and were treated by either conventional 2-field (n = 288;92.3%) or 3-dimensional conformal RT (n = 24) technique. A dose of 60 Gy in 30 fractions was planned treating once daily for five days a week. Immobilization masks were used to ensure reproducibility. These patients received a median RT dose of 60 Gy (Range: 2-60 Gy) in a median of 30 fractions (Range: 1-30 fractions) [Table 2]. Forty-two (87.5%) patients in group I and 167 (53.5%) patients in group II required steroids during radiation.
Table 2.
Treatment schedules
Chemotherapy
In group I, six patients received adjuvant chemotherapy with lomustine (CCNU, 110 mg/m2, six-weekly), starting four weeks after completion of RT. In group II, 89 patients received some form of chemotherapy. During the period from 2002-2004, these patients were considered for adjuvant chemotherapy with lomustine (110 mg/m2, six-weekly). After 2004, affording patients were offered concurrent temozolomide (75 mg/m2 daily for six weeks during RT) with or without adjuvant temozolomide (150-200 mg/m2 for five days, four-weekly, starting four weeks after RT completion) or adjuvant lomustine alone [Table 2]. Concurrent oral TMZ was administered on an empty stomach, 1 hour before radiation and in morning, on days without radiation with adequate anti-emetics and weekly blood counts ensuring hemoglobin ≥ 10 gm/dl, TLC > 3,000/mm3, and platelet count >100,000/mm3. Blood counts were also checked before each cycle of adjuvant TMZ or lomustine.
Response to treatment
Clinical and radiological response to treatment was assessed six weeks after completion of RT. In group I, 14 (29.2%) patients had no symptoms while 25 (52.1%) patients had improvement in symptoms after RT. In group II, 178 (57.1%) patients were symptom-free and 100 (32.1%) patients had improvement in symptoms; 11 patients did not show any improvement while 23 patients had clinical deterioration. Radiological response assessment, using Macdonald criteria,[6] was performed by obtaining MR scans in 65 (20.8%) patients only in group II, because of financial constraints. Forty-three patients had no radiological evidence of disease (35 of these had undergone complete surgical resection) while 22 patients showed either no response or progressive disease (all had undergone subtotal resection/decompression).
Recurrence
Data on recurrences was available in 23 (7.4%) patients in group II, who had no radiological evidence of disease after treatment. All these patients had local recurrence after a median duration of 9.6 months (range: 6.7-14.5 months) after surgery, out of which seven underwent re-craniotomy with near-total excision of tumor. All patients with recurrence received therapeutic TMZ (200 mg/m2 D1-5, four-weekly) for a median of five (Range: two-nine) cycles.
Survival
The median follow-up was 7.53 months for evaluable patients (n = 360). The median follow-up for patients in group I was 6.33 months (range: 1-39.5 months), and for group II, it was 9.23 months (range: 2.2-72.1 months).
The median survival for entire cohort was 7.67 months, and 1-year, 1.5-year, and 2-year survival rates were 25.63%, 12.56%, and 7.23%, respectively [Figure 2]. The median, 1-year, 1.5-year, and 2-year survival rates were 6.33 months, 13.46%, 4.49%, and 2.24% for group I patients and 7.97 months, 27.55%, 13.85%, and 8.21% for group II patients, respectively [P = 0.0014; [Figure 3].
Figure 2.
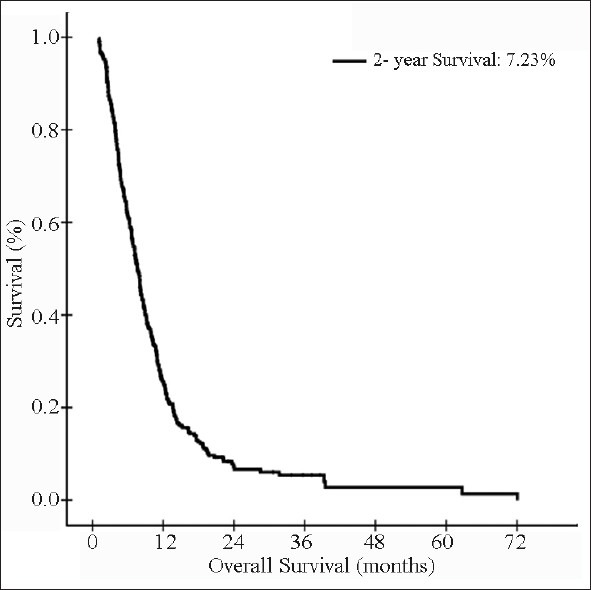
Kaplan-Meier estimates of overall survival in evaluable patients (n = 360)
Figure 3.
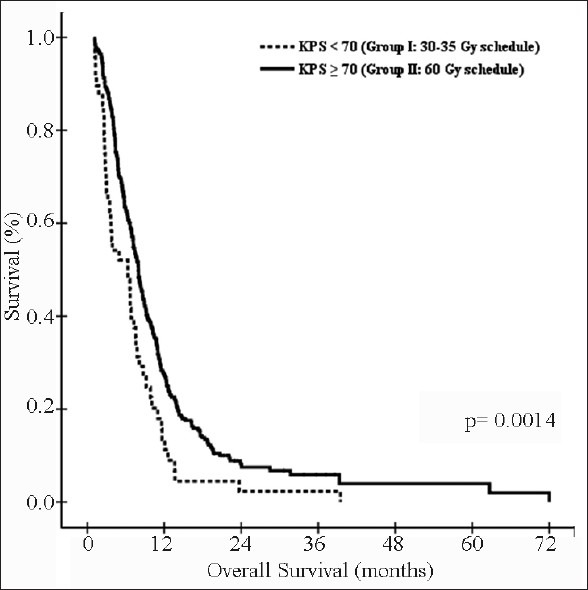
Kaplan-Meier estimates of overall survival: Group I vs. group II
Prognostic factors
The impact of various patient and treatment-related factors on prognosis in group II patients is described in Tables 3 and 4. On univariate analysis, patients who had left-sided or frontal lobe tumors had significantly better survival, and patients with parietal lobe tumors [Figure 4a] had worse survival as compared to others. Also, RT dose of 60 Gy, use of steroids during RT, and use of any form of chemotherapy were the other statistically significant prognostic variables [Figures 4 and 5], whereas the impact of age [≤35 vs. 35-50 vs. >50 years (P = 0.4462); ≤50 vs. >50 (P = 0.2236); ≤60 vs. >60 (P = 0.5551) and <70 vs. ≥70 (P = 0.5961), gender, symptoms of headache and vomiting, extent of surgery, and KPS change after surgery were non-significant [Table 3]. When grouped together, patients with age ≤ 50 years and KPS of 90-100 had a median survival of 8.87 months versus 7.17 months for those with age > 50 years and KPS < 90 (P = 0.028). Using Cox proportional hazards ratio, the central site of tumor, parietal location of tumor, RT dose of 60 Gy, and use of any chemotherapy were found to be independent prognostic factors for OS in multivariate analysis [Table 5].
Table 3.
Predictors of survival in group II patients in univariate analysis
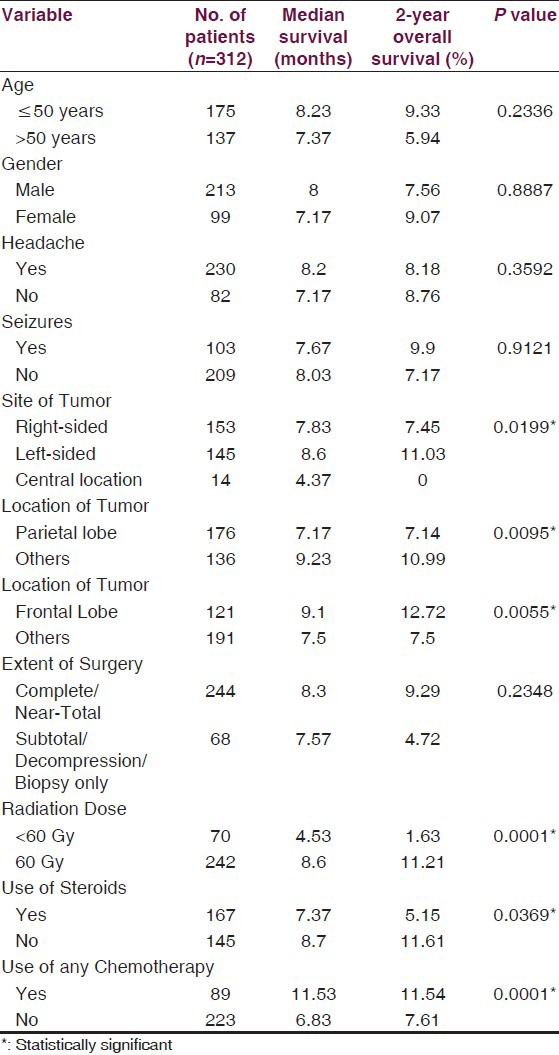
Table 4.
Survival outcome with different treatment schedules
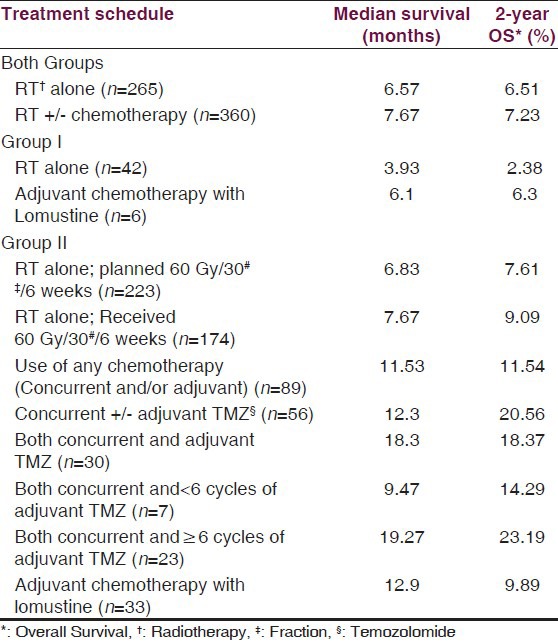
Figure 4.
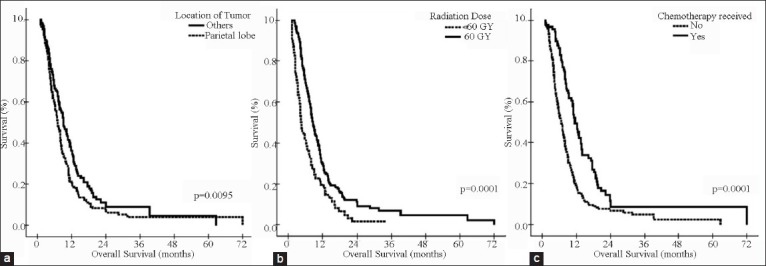
Univariate analysis in group II patients by: (a) Location of tumor: Parietal lobe vs. others; (b) Radiation Dose: 60 Gy vs. < 60 Gy; and (c) Chemotherapy received or not
Figure 5.
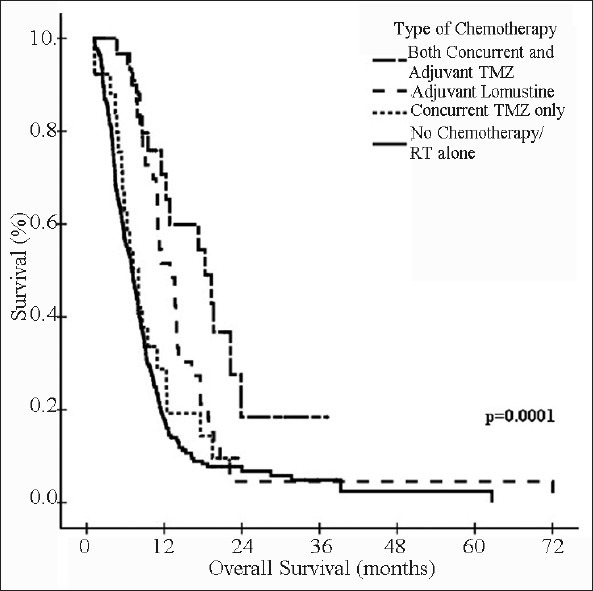
Kaplan-Meier estimates of overall survival in group II patients: No chemotherapy/RT alone vs. Concurrent and/or adjuvant chemotherapy
Table 5.
Predictors of survival in group II patients in multivariate analysis
Toxicity
Toxicity was graded according to Common Terminology Criteria for Adverse Events v3.0. Acute grade 2 and 3 nausea and vomiting were seen in 14 patients in group I and 22 patients in group II, while grade 1 and 2 skin reactions were seen in two patients in group I and 70 patients in group II. Only minor hematological toxicity was seen in patie nts who received concurrent TMZ; no treatment breaks were required. No toxicity was seen in patients who received adjuvant CCNU in either of the groups. In patients of group II, who received adjuvant TMZ, two patients developed grade 1 and one patient developed grade 2 leukopenia; and two patients developed grade1 thrombocytopenia. These patients did not require any treatment for toxicity, and the adjuvant chemotherapy was delayed by one week for recovering from toxicity.
Discussion
The prognosis of patients with GBM has not changed much over the last few decades. Treatment of choice for these patients is surgery followed by adjuvant treatment.[1] Complete resection is not always possible due to infiltration of tumor into the surrounding brain parenchyma or due to closeness of vital structures. Thus, adjuvant radiotherapy became the standard of care post-operatively to take care of this residual/microscopic disease, and it significantly improved survival when compared to surgery alone.[7,8] However, the survival advantage after radiation is small, and OS remains poor.
The addition of nitrosourea-based chemotherapy has also been studied, but the analysis of various randomized trials has provided conflicting results.[9,10] The randomized trial by Stupp et al. reported improved survival for patients treated with concomitant and adjuvant temozolomide and radiotherapy. The median survival with the use of chemo-radiotherapy was 14.6 months versus 12.1 months for radiotherapy alone, and the 2-year and 5-year survival rates were 27.2% and 9.8% versus 10.9% and 1.9% for the two arms, respectively (Hazard ratio 0.6 vs. 1.0).[4,5] This schedule is now considered the new standard of care adjuvant treatment for patients with GBM.
Because of dismal outcomes of GBM, full scale knowledge of prognostic factors from native patients is essential. We undertook this retrospective analysis to determine various factors influencing survival in our group of patients, which constituted an unselected series of consecutive patients who underwent craniotomy and maximal safe resection at our tertiary care institute in north India. Thus, it included a considerable number of patients with unfavorable characteristics as they show up frequently in our radiation oncology department. Also, our study included patients who could not afford costly drugs and are treated with adjuvant radiotherapy alone (73.6% patients). The baseline characteristics of our patients were similar to other reported series with a median age of 50 years and male:female ratio of 2:1.[1,11] In our set-up, the extent of resection is determined from operating neurosurgeon's surgical impression, and post-operative CT or MR scans are usually not obtained, as majority of patients in a resource-limited country like India cannot afford such costly scans frequently.
A dose-response curve exists for GBM with doses <60 Gy yielding an inferior local control.[12,13] The usual dose with adjuvant post-operative radiotherapy is 60 Gy in 30 fractions. Increasing the total dose beyond 60 Gy has not resulted in improved survival.[14,15,16] The advantage of gain in survival time with adjuvant radiotherapy is reduced in a group of patients with poor performance status due to long treatment time of six weeks. In context of palliative nature of treatment of such patients, an abbreviated, palliative treatment course may be more appropriate.[17] Hence, hypofractionated regimen with delivery of higher dose per fraction may be an answer, provided the treatment is well-tolerated and survival gain of post-operative conventional RT is not compromised. Various short palliative hypofractionated RT schedules are described in literature, but there is no standard in terms of dose and fractionation.[18,19]
At our institute, two different radiation dose protocols are followed depending upon pre-RT KPS of the patients. Patients with low KPS, who are incapacitated to come for daily radiation treatment for 6 weeks, are planned for hypofractionated palliative schedule depending upon the volume of irradiation and extent of disease. Patients requiring whole brain RT are treated with radiation to a dose of 30 Gy in 10 fractions, and other patients receive the schedule of 35 Gy in 10-15 fractions. Patients with good KPS are planned for RT dose of 60 Gy in 30 fractions. The planned treatment volume is as per the standard recommendations in these patients.[20] In our study, we have analyzed the two groups (I and II) separately depending upon the planned radiation dose (hypofractionated in group I and conventional in group II).
Age and performance status are the most important variables predictive of patients survival in GBM.[14,21,22] Curran et al. developed a set of classes from a recursive partitioning analysis (RPA) model and found median survival of 18 months in patients with GBM who were < 50 years of age and had KPS of 90-100. Patients with age ≥ 50 years, with low KPS and an abnormal mental status, had a median survival of only five months;[2] similar results were also seen in our study. Stenning presented a multivariate analysis of prognostic factors and confirmed younger age, good performance status, history of seizures, and complete surgical resection of tumor as all being independent favorable prognostic variables.[23]
In our study, median survival for group I patients, with the most important poor prognostic factor of low KPS, was 6.33 months, whereas it was 7.97 months for group II patients (P = 0.0014). In a retrospective analysis of 416 patients of GBM by Lacroix et al., median survival was 10.6 months. Patients with KPS ≤ 70 (25% patients) had median survival of 8.8 months versus 11.2 months for those with KPS > 70.[1] In another study by Lutterbach et al., median survival was 8.8 months for patients with KPS ≥ 70 vs. 6.7 months for KPS < 70 (35% patients).[3] The survival rates of patients with KPS < 70 in our study are similar to those reported in literature,[1,3] but we used hypofractionated RT schedule in these patients, whereas dose schedules in other studies usually include standard fractionation to total dose of 60 Gy. Hulshof et al. reported on the results of a Dutch randomized series, evaluated various hypofractionated RT schedules in poor prognostic subgroup, and reported a median survival of 6.6 months. They argued that the therapeutic efficacy of hypofractionated RT may indeed represent a true radiobiological effect, and such short scheme is more appropriate for patients with intermediate or poor prognosis.[24] So, these schedules can be considered for patients with low KPS as per individual institutions preference, thus decreasing total treatment time and number of hospital visits. The gain of three-four weeks time at home is very valuable in terms of proper care for such patients and has considerable impact on their quality of life.
Hypofractionation with TMZ has also been studied in patients with good KPS. Terasaki et al. analyzed 26 adult patients (39-79 years) who received short course hypofractionated RT (45 Gy in 15 fractions over 3 weeks) with concomitant and adjuvant temozolomide in standard doses. At a median follow-up of 20 months, the median OS was 15.6 months, and it was concluded that adult patients benefited from hypofractionated RT with TMZ, and this procedure might reduce the course of treatment.[25] Similar benefit is not seen in older GBM patients, and for them, a sequential approach (hypofractionated radiotherapy followed by TMZ in responding patients) is preferred.[26] At our center, we use hypofractionation in patients with poor KPS without concurrent TMZ.
We performed univariate analysis of various other prognostic factors in group II patients. In previous studies, decreased survival was associated with older age. In RPA, the most important split was by age, with age of 50 years being the most important break point.[2] The Medical Research Council divided patients into three age groups of < 45 years, 45-59 years, and > 59 years and observed median survival of 12 months, nine months, and less than five months, respectively.[23] We did not find significant difference in survival amongst patients divided into various age groups. Other patient-related factors like gender and presence of symptoms of headache and seizures were also non-significant in predicting survival.
A number of studies have included tumor location in data analysis. In a study by Simpson et al., patients with frontal lobe tumors survived significantly longer (median: 11.4 months) than those with temporal (median: 9.1 months) or parietal (median: 9.6 months) lobe lesions (P = 0.01).[27] The study by Jeremic et al. also confirmed tumor location as an important prognostic factor with best survival rates for frontal lobe tumors (P = 0.00001).[28] Results of current study also found patients with frontal tumors having significantly better survival as compared to other sites (P = 0.0055) and parietal lobe tumors having worse survival (P = 0.0095). Various studies have also correlated site of tumor (left, right, or central) with survival outcomes and found conflicting results. The centrally located tumors or with contra-lateral infiltration usually are associated with worse outcomes.[1,3] In the study by Tait et al., right-sided tumors and tumors confined to one hemisphere were found to be independent predictors of survival, and shorter survival of patients with left-sided tumors was postulated to be due to usual dominance of left hemisphere.[29] But, we observed best survival rates with left-sided tumors as compared to right-sided or centrally located tumors (P = 0.0199).
Extent of surgical resection as a prognostic factor has been debated for decades, but its determination is still imprecise.[14,30] More recently, Sanai et al. analyzed 28 high-grade glioma articles to assess the influence of extent of resection and concluded that there were persistent limitations in the quality of data, but mounting evidence suggested that more extensive surgical resection was associated with longer life expectancy.[31] In another study by Sanai et al., the value of extent of resection (EOR) in improving OS in patients with GBM was analyzed. Aggressive EOR equated to improvement in OS, even at the highest levels of resection, and subtotal resections as low as 78% also corresponded to a survival benefit.[32] In the RPA for patients aged 70 years or older, survival was significantly better in patients who underwent surgical resection as compared to biopsy only.[33] In our study, eight patients were aged ≥ 70 years, all underwent near-total resection and had a median survival of 6.33 months. No correlation between survival and extent of surgery was found in our study. This may be attributable to the fact that we determine EOR from neurosurgeon's operative notes only, and post-operative pre-RT scans are not routinely obtained in the poor patients seen in our out-patient department. As mentioned in Table 1, 75% of our patients underwent complete or near-total resection as per operative findings, which may not be the actual number, as we did not have scans to corroborate these findings.
As already described, doses less than 60 Gy yield inferior local control rate.[12,13] In our study, there was significant difference in survival between the patients who received full dose of 60 Gy vs. < 60 Gy (P = 0.0001). The difference was still significant when we analyzed three subgroup of patients who received RT dose of 60 Gy, 50 Gy to < 60 Gy, and <50 Gy, who had median survival of 8.6 months, 5.77 months, and 3.23 months, respectively (P = 0.0001). Another significant factor was use of steroids during RT with poor outcome in patients who required steroids (P = 0.0369), even in those who underwent complete or near-total resection. It is likely that tumor progression occurred in these patients, which manifested as symptoms of raised intracranial pressure and was the reason for incomplete treatment and poor survival.
In our set up, majority of patients come from poor socio-economic background and cannot afford the standard treatment. In the early part of our study, adjuvant chemotherapy with CCNU was offered to patients with GBM. After 2004, Stupp's therapeutic protocol,[4,5] using concurrent and adjuvant TMZ with radiotherapy, was also offered to group II patients. Because of high cost of this regimen, it is not extensively used in our institution. In our study, use of any form of chemotherapy resulted in statistically significant improvement in survival as compared to RT alone (P = 0.0001), and the advantage was maintained even after excluding the poor prognostic group of patients (who received <60 Gy dose) from analysis (P = 0.0001). The survival results with use of concurrent TMZ with or without adjuvant TMZ was found to be comparable to earlier reported data with similar regimen, where observed median survival was 15-18 months.[4,5,34,35] The best survival rates in our study were seen in patients who received concurrent TMZ followed by six or more cycles of adjuvant TMZ [Table 5]. Patients who received concurrent TMZ alone also had better survival as compared to patients who received RT alone, which is in contradiction to the available data.[36] Also, median survival was superior in patients who received adjuvant lomustine as compared to RT alone (12.9 months vs. 6.83 months). The toxicity rates in patients who received either lomustine or TMZ were low, and treatment discontinuation was not required in any patient. The reason for some patients not continuing with adjuvant TMZ after receiving concurrent TMZ with RT was financial constraints only. Thus, our analysis confirmed the overall good tolerability to chemotherapy in our cohort of patients, both in concurrent and adjuvant setting and concurs well with that of published literature.
Healthcare is not equally accessible in our set-up with majority of the patients not affording the standard but costly chemotherapeutic regimen of concurrent and adjuvant TMZ with radiotherapy. In such patients, radical radiotherapy is still the only adjuvant treatment option as is seen in our study where only 26.4% (95/360; six out of 48 in group I and 89 out of 312 in group II) patients could afford any form of chemotherapy, and only 15.5% patients (56/360) could afford concurrent with or without adjuvant TMZ. In poor patients, the regimen of adjuvant lomustine can be used, which has shown better survival rates as compared to radical radiotherapy alone in our analysis.
Although OS of our GBM patients was poor, individual patient survival was very heterogeneous with 38.6% (139/360) patients dying within 6 months, 78.1% (281/360) patients dying within 12 months, but 3.3% (12/360) patients surviving more than two years. These observations suggest that GBM might not be a single pathological entity, but may encompass several tumor subtypes with different outlook and responsiveness to treatment.
To conclude, this is a valuable retrospective study with considerable number of patients of GBM and full scale analysis. Pre-treatment performance status is an independent prognostic factor for the clinical outcome of GBM. Besides this well-known factor, we identified central tumor location, parietal lobe involvement, RT dose of <60 Gy, and using radiotherapy alone without chemotherapy to have a strong negative influence on survival. Concurrent chemoradiotherapy with TMZ followed by additional cycles of TMZ yield encouraging outcomes, even in our patient population without significant toxicity, validating the published results. We should follow this treatment schedule in all affording patients with good performance status to maximize the benefits of post-operative radiotherapy and consider hypofractionated schedules for patients with poor performance status.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection and survival. J Neurosurg. 2001;95:190–8. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 2.Curran WJ, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma Trials. J Natl Cancer Inst. 1993;85:704–10. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 3.Lutterbach J, Sauerbrei W, Guttenberger R. Multivariate analysis of Prognostic factors in patients with glioblastoma. Strahlenther Onkol. 2003;179:8–15. doi: 10.1007/s00066-003-1004-5. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 6.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–80. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 7.Kristiansen K, Hagen S, Kollevold T, Torvik A, Holme I, Nesbakken R, et al. Combined modality therapy of operated astrocytomas grade III and IV. Confirmation of the value of postoperative irradiation and lack of potentiation of bleomycin on survival time: A prospective multicenter trial of the scandinavian glioblastoma study group. Cancer. 1981;47:649–52. doi: 10.1002/1097-0142(19810215)47:4<649::aid-cncr2820470405>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 8.Laperriere N, Zuraw L, Cairncross G The cancer care ontario practice guidelines initiative neuro-oncology disease site group. Radiotherapy for newly diagnosed malignant glioma in adults: A systematic review. Radiother Oncol. 2002;64:259–73. doi: 10.1016/s0167-8140(02)00078-6. [DOI] [PubMed] [Google Scholar]
- 9.Stewart LA. Chemotherapy in adult high-grade glioma: A systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–8. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 10.Medical research council brain tumor working Party. Randomized trial of procarbazine, lomustine and vincristine in the adjuvant treatment of high-grade astrocytoma: A medical research council trial. J Clin Oncol. 2001;19:509–18. doi: 10.1200/JCO.2001.19.2.509. [DOI] [PubMed] [Google Scholar]
- 11.Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: Data from the glioma outcomes project. J Neurosurg. 2003;99:467–73. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- 12.Walker MD, Alexander E, Jr, Hunt WE, MacCarty CS, Mahaley MS, Jr, Mealey J, Jr, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333–43. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 13.Bleehen NM, Stenning SP. A medical research council trial of two radiotherapy doses in the treatment of 3 and 4 astrocytoma. The medical research council brain tumor working party. Br J Cancer. 1991;64:769–74. doi: 10.1038/bjc.1991.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang CH, Horton J, Schoenfeld D, Salazer O, Perez-Tamayo R, Kramer S, et al. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A joint radiation therapy oncology group and eastern cooperative oncology group study. Cancer. 1983;52:997–1007. doi: 10.1002/1097-0142(19830915)52:6<997::aid-cncr2820520612>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa K, Aoki Y, Fujimaki T, Tago M, Terahara A, Karasawa K, et al. High-dose conformal radiotherapy influenced the pattern of failure but did not improve survival in glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1998;40:1141–9. doi: 10.1016/s0360-3016(97)00911-5. [DOI] [PubMed] [Google Scholar]
- 16.Chan JL, Lee SW, Frass BA. Survival and failure patterns of high grade glioma after three-dimensional conformal radiotherapy. J Clin Oncol. 2002;20:1635–42. doi: 10.1200/JCO.2002.20.6.1635. [DOI] [PubMed] [Google Scholar]
- 17.Kleinberg L, Slick T, Enger C, Grossman S, Brem H, Wharam MD., Jr Short course radiotherapy is an appropriate option for most malignant glioma patients. Int J Radiat Oncol Biol Phys. 1997;38:31–6. doi: 10.1016/s0360-3016(97)00222-8. [DOI] [PubMed] [Google Scholar]
- 18.Lang O, Liebermeister E, Liesegang J, Sautter-Bihl ML. Radiotherapy of glioblastoma multiforme. Feasibility of increased fraction size and shortened overall treatment. Strahlenther Onkol. 1998;174:629–32. doi: 10.1007/BF03038511. [DOI] [PubMed] [Google Scholar]
- 19.Phillips C, Guiney M, Smith J, Hughes P, Narayan K, Quong G. A randomized trial comparing 35Gy in ten fractions with 60Gy in 30 fractions of cerebral irradiation for glioblastoma multiforme and older patients with anaplastic astrocytoma. Radiother Oncol. 2003;68:23–6. doi: 10.1016/s0167-8140(03)00206-8. [DOI] [PubMed] [Google Scholar]
- 20.Manon R, Hui S, Chinnaiyan P, Suh J, Chang E, Timmerman R, et al. The impact of mid-treatment MRI on defining boost volumes in the radiation treatment of glioblastoma multiforme. Technol Cancer Res Treat. 2004;3:303–7. doi: 10.1177/153303460400300308. [DOI] [PubMed] [Google Scholar]
- 21.Gundersen S, Lote K, Hannisdal E. Prognostic factors for glioblastoma multiforme-development of a prognostic index. Acta Oncol. 1996;35:123–7. doi: 10.3109/02841869609098530. [DOI] [PubMed] [Google Scholar]
- 22.Gorlia T, van den Bent MJ, Hegi ME, Mirimanoff RO, Weller M, Cairncross JG, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: Prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008;9:29–38. doi: 10.1016/S1470-2045(07)70384-4. [DOI] [PubMed] [Google Scholar]
- 23.Stenning SP, Freedman LS, Bleehen NM. Prognostic factors for high-grade malignant glioma: Development of a prognostic index. A report of the medical research council brain tumour working party. J Neurooncol. 1990;9:47–55. doi: 10.1007/BF00167068. [DOI] [PubMed] [Google Scholar]
- 24.Hulshof MC, Schimmel EC, Andries Bosch D, Gonzalez Gonzalez D. Hypofractionation in glioblastoma multiforme. Radiother Oncol. 2000;54:143–8. doi: 10.1016/s0167-8140(99)00183-8. [DOI] [PubMed] [Google Scholar]
- 25.Terasaki M, Eto T, Nakashima S, Okada Y, Ogo E, Sugita Y, et al. A pilot study of hypofractionated radiation therapy with temozolomide for adults with glioblastoma multiforme. J Neurooncol. 2011;102:247–53. doi: 10.1007/s11060-010-0306-6. [DOI] [PubMed] [Google Scholar]
- 26.Cao JQ, Fisher BJ, Bauman GS, Megyesi JF, Watling CJ, Macdonald DR. Hypofractionated radiotherapy with or without concurrent temozolomide in elderly patients with glioblastoma multiforme: A review of ten-year single institutional experience. J Neurooncol. 2012;107:395–405. doi: 10.1007/s11060-011-0766-3. [DOI] [PubMed] [Google Scholar]
- 27.Simpson JR, Horton J, Scott C, Curran WJ, Rubin P, Fischbach J, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: Results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993;26:239–41. doi: 10.1016/0360-3016(93)90203-8. [DOI] [PubMed] [Google Scholar]
- 28.Jeremic B, Grujicic D, Antunovic V, Djuric L, Stonjanovic M, Shibamoto Y. Influence of extent of surgery and tumor location on treatment outcome of patients with glioblastoma multiforme treated with combined modality approach. J Neurooncol. 1994;21:177–85. doi: 10.1007/BF01052902. [DOI] [PubMed] [Google Scholar]
- 29.Tait MJ, Petrik V, Loosemore A, Bell BA, Papadopoulos MC. Survival of patients with glioblastoma multiforme has not improved between 1993 and 2004: Analysis of 625 cases. Br J Neurosurg. 2007;21:496–500. doi: 10.1080/02688690701449251. [DOI] [PubMed] [Google Scholar]
- 30.Gehan EA, Walker MD. Prognostic factors for patients with brain tumors. Natl Cancer Inst Monogr. 1977;46:189–95. [PubMed] [Google Scholar]
- 31.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62:753–64. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 32.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115:3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 33.Scott JG, Bauchet L, Fraum TJ, Nayak L, Cooper AR, Chao ST, et al. Recursive partitioning analysis of prognostic factors for glioblastoma patients aged 70 years or older. Cancer. 2012;118:5595–600. doi: 10.1002/cncr.27570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jalali R, Basu A, Gupta T, Munshi A, Menon H, Sarin R, Goel A. Encouraging experience of concomitant Temozolomide with radiotherapy followed by adjuvant Temozolomide in newly diagnosed glioblastoma multiforme: Single institution experience. Br J Neurosurg. 2007;21:583–7. doi: 10.1080/02688690701604574. [DOI] [PubMed] [Google Scholar]
- 35.Demirci U, Buyukberber S, Coskun U, Akmansu M, Yaman E, Baykara M, et al. Long term experience in high grade glial tumors with temozolomide. J Buon. 2012;17:357–62. [PubMed] [Google Scholar]
- 36.Kocher M, Frommolt P, Borberg SK, Ruhi U, Steingraber M, Niewald M, et al. Randomized study of postoperative radiotherapy and simultaneous temozolomide without adjuvant chemotherapy for glioblastoma. Strahlenther Onkol. 2008;184:572–9. doi: 10.1007/s00066-008-1897-0. [DOI] [PubMed] [Google Scholar]


