Abstract
Brain abscess (BA) is defined as a focal infection within the brain parenchyma, which starts as a localized area of cerebritis, which is subsequently converted into a collection of pus within a well-vascularized capsule. BA must be differentiated from parameningeal infections, including epidural abscess and subdural empyema. The BA is a challenge for the neurosurgeon because it is needed good clinical, pharmacological, and surgical skills for providing good clinical outcomes and prognosis to BA patients. Considered an infrequent brain infection, BA could be a devastator entity that easily left the patient into dead. The aim of this work is to review the current concepts regarding epidemiology, pathophysiology, etiology, clinical presentation, diagnosis, and management of BA.
Keywords: Brain abscess, neuroinfection, neurosurgery
Introduction
Central nervous system (CNS) infections and their sequelae still constitute a major source of morbidity.[1] In the recent past, the introduction of newer broad spectrum antibiotics, improved imaging technology, and intensive care facilities have significantly altered the natural history of CNS infections.[1] Brain abscess (BA) is a universal health problem with a high morbidity and mortality rate; thus, the disease today presents a leading public health problem and a major burden on health care facilities all around the world.[2,3] BA is a dynamic focal form of intracranial suppuration and a serious life-threatening emergency.[4] They begin as a localized area of cerebritis and develop into an encapsulated collection of pustular materials presenting as a mass-like lesion, similar to the abscess in other sites.[5]
Currently, in high-income countries the original forms of intracranial suppurative disease (i.e., BA, empyema, and purulent ventriculitis) are so uncommon that most young neurosurgeons are unfamiliar with this form of pathology and recognizing the need at times for judicious, complex, and aggressive surgical management.[6] The infectious origin of the BA causes significant damage to the CNS,[7] because of it's incapability of mounting a sufficient defense against the pyogens, leading to pyogenic abscess.[8]
Advances in surgery, in neuroimaging diagnostic technics and in antibiotics use during the 20th century, have drastically improved the outcomes of these infections, although mortality and morbidity remain high.[9] Especially for the immunocompromized patients such as those who have advanced HIV disease and transplant recipients who are experiencing an increasing incidence of BA notwithstanding those advances, probably due to a growing number of opportunistic infections;[10,11] thus, BA can easily be fatal. For this reason, BA should be regarded as a serious infection and efforts should be focused on continually optimizing diagnosis and management.[9] The aim of this work is to review the current concepts regarding epidemiology, physiopatology, etiology, clinical presentation, diagnosis, and management of BA.
History
Trepanation is known to be the first surgical procedure ever performed.[12] Fortunately, the majority of paleopathological investigations focus on the study of the skull, because it is the most frequently preserved part of the human body recovered from archaeological excavations.[13] Trephinations as ancient as in neolithic people have been described all over the world, but reasons for these procedures however are not always clear.[14] Researchers conclude that at least in some cases such operations have been performed for purely medical reasons.[14] Strikingly, there is evidence that the patients survived months to years after the operations.[15] Unlike structural findings such as skull bone deformity, fractures, and bone tumors, brain parenchymal lesions, like BA, cannot let evidences of its existence thousand years later. The fact those individuals survive months or years after procedure suggest a more benign motive. Nonetheless confirming the astonishing degree of technical skills reached those times without anesthetic, antiseptic, or technologic aids. From the Vesalius’ and Ambroise Paré's writings, and from a biography of Hennry II, Graham Martin,[16] assembled the dead history of Henry II King of France, a dead predicted by Nostradamus 10 years earlier it happens. He died from an orbital wound, which was suffered in a joust, destroying his eye, and leaving behind many splinters. The skull was not penetrated but infection spread intracranially. By autopsy was confirmed that the splinters in the orbit had not pierced the skull, but infection had spread to the brain along the orbital veins, forming an abscess under the cortex. Furthermore, overlying the BA was a subdural effusion of pus. However, as reviewed by Muzumdar et al.,[1,17] Sir Percival Pott was probably the first to recognize and document that infections elsewhere in the body could spread and cause BA.[6,18] The French surgeon Morand in 1768 made the first report of successful surgical treatment of an otitic BA with good recovery.[19]
In 1891, Topuzlu operated on a BA that originated as a complication of a depression fracture of the cranial inner table; this case is highlighted because it was not only the first case of BA to be treated successfully with surgical intervention in the Ottoman Empire, but also one of the first cases of neurological surgery performed using contemporary anesthesiological and surgical techniques, which reveals the importance of neurological examination and cerebral localization techniques in the era before x-rays.[20] In 1893, MacEwan published a monograph, “Pyogenic Infective Disease of the Brain and Spinal Cord,” describing the results of a case series of 19 BA patients in which decalcified chicken bones had been used to drain the pus, with only a 5.3% of mortality (n = 1).[21]
Remarkably, Oscar Wild died in 1900 of an otogenic cerebral abscess,[22] highlighting that even under more favorable circumstances, a successful treatment would not have been possible at that time. After the introduction of antibiotics, organism-isolation techniques, and computed tomography (CT), Rosenblum et al.[23] reported 0% mortality in a series of 20 patients in 1978. The pathology and pathophysiology of BA were more comprehensible when Britt et al.[7,24] created a dog model of BAs and then correlated the pathology with human BAs and CT findings generating the classification of abscess development.
Epidemiology
BA is one of the most serious diseases of the CNS. This condition is more common among men –twice to three times, and morbidity rate is highest in fourth decade of the life[3,25] BA is still associated with high morbidity, including seizures (up to 80%), persistent altered mental status, and focal motor deficits.[26] BA still continues to be a significant problem in the developing world due to large scale poverty, illiteracy, and lack of hygiene.
As pyogenic BA is a kind of infectious disease, the disease is expected to be more common in a setting with poor sanitation and medical facilities and illiteracy.[1] Infectious diseases are usually common in tropical countries.[5] Interestingly, without dependence on the setting of study, the cases are usually elder or pediatric male patients.[27,28,29] However, the trends of decreasing incidents are reported due to the improvement in world sanitation at present.[28] As has been stated previously, although there have been breakthrough advances in neuroimaging, neurosurgical techniques, neuroanesthesia, microbiological isolation techniques, and antibiotic therapy, bacterial BAs can be fatal.[17] The incidence of BAs is approximately 8% of intracranial masses in developing countries and 1-2% in the western countries.[17] At some centers, pediatric cases of BA account for almost 25% of all patients with BA.[30]
Mortality from a BA has recently decreased from about 50% to 20%, mostly as a result of introduction of CT scanning that resulted in earlier diagnosis and accurate localization.[31,32] Further advances in microorganism isolation and identification, superior antimicrobials with greater cerebrospinal fluid (CSF) penetration and stereotactic aspiration has resulted in a contemporary mortality of less than 10%.[33] Mortality is mainly influenced by age and neurological condition at admission; delays in hospitalization, focal neurologic deficits at admission, impaired host immunity, uncontrolled diabetes mellitus, and Glasgow Coma Scale (GCS) <12 are associated with death and permanent neurologic deficits.[3]
In the Nathoo et al.[4] study that constitutes the largest clinical series published to date with 973 BA patients and represents a single tertiary institution's experience with this form of intracranial suppuration, in a developing country, and in the most populous province of South Africa, they found a mean age of 24.36 ± 15.1 years and men were most afflicted (n = 722, 74.2%). Nearly 70% of the patient cohort was in the first three decades of life, and 42.7% were pediatric patients (<18 years), also despite trauma being the commonest cause (32.8%) of which 64.3% were located on the left side. Penetrating cranial injuries were recorded in 91 patients (stab, n = 42; gunshot, n = 16; other weapons, n = 33). Otorhinogenic sepsis was the primary source in 38.5% of patients, occurring predominantly in the first two decades of life. Additional etiologies were other (7.7%), pulmonary (6.8%), cryptogenic (4.6%), postsurgical (3.2%), meningitis (2.8%), cardiac (2.7%), and dental (0.9%); interestingly there were two peaks of traumatic BA corresponding to the levels of political violence in the region.
BA, from where it came?
The origin of BA formation remains elusive (cryptic BA) in up to 40% of cases, but they are a known complication of intracranial surgery.[2,34]
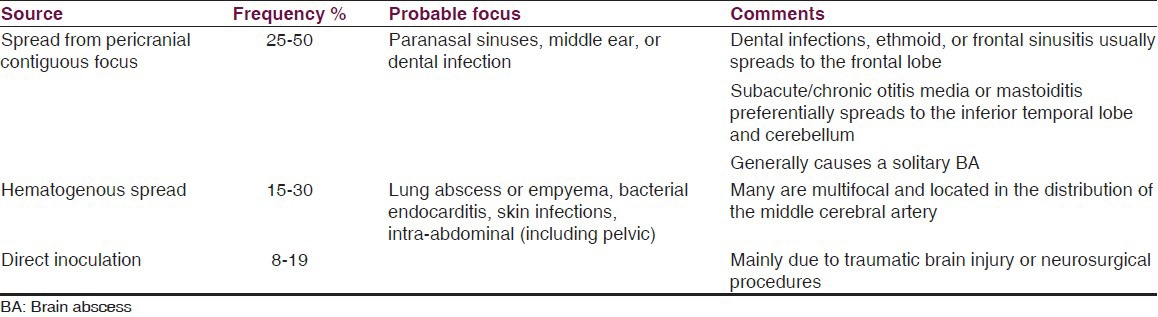
A BA can develop from three sources: First because of spread of infection from pericranial contiguous focus in 25-50% of cases (such as the sinuses, middle ear, or dental infection), interestingly dental infections, ethmoid or frontal sinusitis (usually spreads to the frontal lobe), and subacute or chronic otitis media or mastoiditis (preferentially spreads to the inferior temporal lobe and cerebellum).[2]
The second from is hematogenous spread from a distant focus of infection [such as lung abscess or empyema, bacterial endocarditis, skin infections, and intra-abdominal (including pelvic)] in 15-30% of cases. In some of the patients with cryptogenic BA, it can be possible to find a cardiac source, a congenital heart disease, like a patent foramen ovale (PFO) or a pulmonary arteriovenous fistula,[35,36,37] PFO is a primary contributory factor to BA by permitting infected material to bypass the lungs and enter the systemic circulation. Third, from direct inoculation (such as head trauma or neurosurgery) in 8-19% of cases.[2,4,9,17,38]
Many hematogenous-borne BA are multifocal and located in the distribution of the middle cerebral artery,[2] especially from cyanotic congenital heart disease;[17] those who spread from a contiguous site generally causes a solitary BA.[2] Strikingly, branched hyphal-form fungal infections obstruct large and intermediate size vessel, causing cerebral arterial thrombosis and infarction.[17] Table 1 summarizes specific data about BA sources.
Table 1.
Summary of BA sources characteristics
In contrast to parenchymal BA, a primary intraventricular abscess is a slowly progressing infectious process evolving from an area of cerebritis or ventriculitis. The entry of pathogens to the ventricular system is either hematogenous or through the CSF, unless iatrogenic. Probably bacteria enter the ventricles through the choroid plexus, due to its relative laxity in the blood–brain barrier, once there inflammatory response could generate adhesions and obstruction of the ventricular system. When this happens, the infection is confined to a single ventricle and leads to local abscess formation.[39]
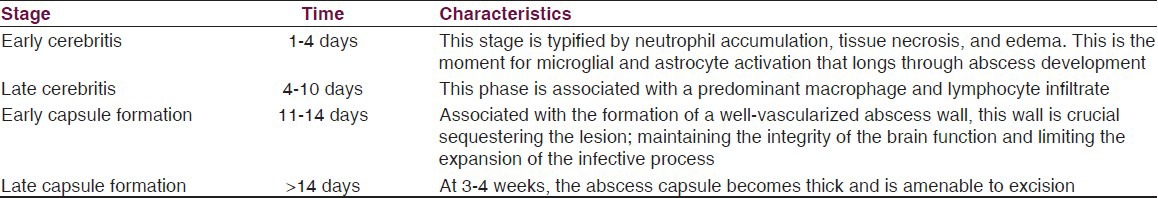
Stages
As mentioned previously, BA pathology have been correlated with CT and magnetic resonance imaging (MRI) findings.[7,40,41] As a result, the BA natural history can be divided into four stages, as listed in Table 2.
Table 2.
Stages of brain abscess formation
BA and neuroinflammation
The immune response elicited can destroy the surrounding normal brain tissue, have been demonstrated that lesions can encompass a large portion of brain tissue, often spreading well beyond the initial focus of infection, this is in part due to the continued release of proinflammatory mediators that could damage the surrounding brain parenchyma.[40]
The healthy CNS is now recognized as a site where immune surveillance occurs.[42] CNS inflammation can be either neuroprotective or destructive depending on:[43]
The type of insult;
the intensity and duration of the inflammatory response; and
the composition of inflammatory infiltrates.
Innate immune activation in the CNS can be triggered by numerous pathways after recognition of invading pathogens and/or tissue damage by pattern recognition receptors; recent researches focus on a two-signal model of recognition, mediated by Toll-like receptors (TLR) and Nod-like receptors (NLR). TLR are the major sensors of invading pathogens[44] by recognizing conserved pathogen-associated molecular patterns (PAMPs) from diverse organisms, including bacteria, viruses, yeast, fungi, and parasites,[45] but also endogenous molecules, referred to as danger-associated molecular patterns (DAMPs), which are typically sequestered from the immune system but released during tissue pathology. These receptors recognize conserved motifs from a wide range of pathogens that are inherently resistant to mutation based on their essential nature for pathogen survival.[46] Especially, TLR2 regulates bacterial burdens, immune infiltrates, and inflammatory mediator production during BA development.[47] All known TLRs with the exception of TLR3 signal through the adaptor protein MyD88 and lead to the activation of the transcription factor nuclear factor kB (NF-kB),[45] which mediates the activation of the production of proinflamatory molecules.
NLR forms the inflammasome, the functional structure responsible for pro-interleukin-1b (pro-IL-1b) and pro-IL-18 processing,[43] which when activated, IL-1b and IL-18, are implicated in the physiopathology of many neurogenerative disorders as well in CNS infection (e.g., bacterial meningitis, HIV-associated dementia, and BA).[48,49,50] Since IL-1 and IL-18 have been shown to have important roles in antibacterial immunity, coupled with the pivotal role of MyD88-dependent pathways in bacterial recognition and the induction of downstream cytokine signaling networks, MyD88 represents a central converging point in the innate inflammatory pathway.[46] IL-1 action is crucial for survival and pathogen containment during bacterial abscess formation in the CNS parenchyma,[51] as has been demonstrated using MyD88 knockout mice, which are exquisitely sensitive to CNS infection with Staphylococcus aureus.[46,52] IL-1 and IL-18 also regulate the induction of adaptive immunity;[53] however, despite advances in defining innate immune pathways elicited during BA development, little information is currently available regarding the functional impact of adaptive immunity in bacterial clearance.[47]
The limited immune surveillance of the CNS makes it crucial that resident cells be able to rapidly recognize and respond to infection. Innate immunity in the CNS depends primarily on the functions of glial cells, astrocytes, and microglia, which are important for the early control of pathogen replication and direct the recruitment and activation of cells of the adaptive immune system required for pathogen clearance.[45]
Microglia cells are considered to be CNS-resident professional macrophages and sensor cells that function as the principal innate immune effector cells. Activated microglia express a range of genes related to inflammation such as pro-inflammatory cytokines, proinflammatory enzymes, and pro-inflammatory adhesion molecules. Recent studies have demonstrated the presence of mRNA and/or protein expression of TLR sets and the co-receptor CD14 in microglia, and have shown that such expression is increased following exposure to bacterial pathogens.[44] The most abundant glial cells, the astrocytes, also have been shown to become activated following challenge with clinically relevant bacterial pathogens, they are highly responsive to gram-positive bacterial species and produce the signature inflammatory cytokines IL-1β, tumor necrosis factor (TNF)-α, and IL-12 p40.[44]
There is evidence of astrocyte functional alteration during the pathogen burden. Normally, astrocytes form syncytial networks within the CNS through gap junctions (GJs) capable of transmitting a wide variety of small molecules (<1 kDa) including glutamate, ATP, glucose, Ca2+, K+, and Na2+, playing a vital role in maintaining ionic and metabolic stability in the CNS parenchyma,[54] but they undergo a series of complex changes in response to a wide variety of pathological insults, i.e., astrogliosis, during which their morphological, electrophysiological, and biochemical properties may be altered. It has been demonstrated that exposure to the gram-positive bacterium S. aureus leads to reductions in astrocyte GJ communication.[55] Karpuk et al.,[54] using a mouse model of BA, founded that astrocyte GJ communication was significantly attenuated in regions immediately surrounding the abscess margins and progressively increased to levels typical of uninfected brain with increasing distance from the abscess proper. These changes are in correlation with alterations in astrocyte resting membrane potential and input conductance, indicating that astrocyte coupling and electrical properties are most dramatically affected near the primary inflammatory site, and thus, affecting the astrocyte function.
Since CNS is not capable of robust regeneration that would be required to resolve the widespread parenchymal damage characteristic of BA, it is essential that the immune response must be tightly regulated to effectively eradicate bacteria while limiting the extent of bystander damage to surrounding brain tissue.[47] Unfortunately, immune engagement can lead to irreparable functional impairment, either resulting from antimicrobial action or as part of an autoimmune pathology.[56]
Etiology
There is a wide range of pathogens that can cause a BA.[2,31,32] Basically, the microbial cause depends on how the BA develops and whether the patient is immunocompromized or not. Streptococci (both aerobic and anaerobic) are the most common pathogens, comprising about 70% of isolates cultured from bacterial BAs.[57] The commonest pathogens related to clinical scenario are summarized in Table 3.
Table 3.
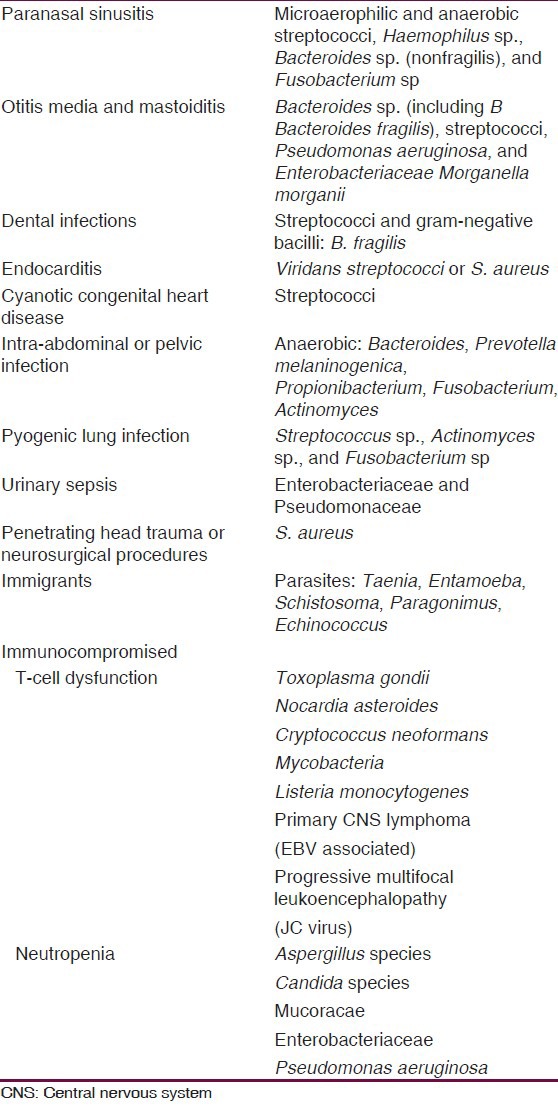
From oral cavity, hemathogenous spread (intra-abdominal/pelvic infections), and from otorhinolaringeal infections the commonest organisms isolated are anaerobic pathogens (Streptococci, Bacteroides spp., Prevotella melaninogenica, Propionibacterium, Fusobacterium, and Actinomyces and aerobic gram-negative rods, like Morganella morganii).[21,58,59,60,61,62,63,64]
In the case of trauma or in patients with prior neurosurgical procedures, the aerobic gram-positive cocci are common (Streptococcus viridans, Streptococcus milleri, and S. aureus), but aerobic gram-negative rods (Klebsiella, Pseudomonas, Escherichia coli, Proteus) can be found also.[17,26]
Peptostreptococcus and Streptococcus (esp. viridians and microaerophilics) are mostly identified in patient with cardiac origin (cyanotic heart disease) and right-to-left shunts.[17]
The growing use of corticosteroid and other imunosupressant drugs had modified the bacterial milieu increasing the frequency of opportunistic pathogens causing BA, and no only due to medicaments, alcoholic patients, those with severe and debilitating neurological conditions (Alzheimer disease, Parkinson disease, or HIV infection/AIDS), are at high risk of suffer opportunistic BA. There is a vast array of pathogens affecting immunocompromised patients, ranging from the usual organisms to other more unusual pathogens including Pesudomonas, Toxoplasma, Listeria, Nocardia, Aspergillus, Cryptococcus, Coccidioides, and other fungal pathogens;[26] in these patients is more likely the hematogenous or metastatic spread.[17] In immigrants or travelers from an underdeveloped part of the world with a BA, parasites should be considered (e.g. cysticercosis, Entamoeba histolytica, Schistosoma, and Paragonimus).[26]
Other group of patients at risk of unusual pathogens are the immigrants from underdeveloped countries, they can have, in addition to those pathogens affecting inmunocompromised patients, other pathogens like Taenia, E. histolytica, Schistosoma, Echinococcus, and Paragonimus.[26,65,66,7,68,69,70,71]
In the Nathoo et al.[4] series (the biggest reported), it was found that majority of patients undergoing surgery (53.2%) had isolation of a single organism, being the commonest the gram-positive organisms: S. aureus and Staphylococcus epidermidis, especially in traumatic BA. Streptococcus milleri isolates were predominant in patients with paranasal sinusitis, whereas Proteus mirabilis was most commonly isolated in otogenic infections.
Recently, Al Masalma et al.[38] used 16S ribosomal DNA amplification and achieved pathogen identification in 9 of 21 (43%) culture-negative BA, identified 44 distinct bacterial species not previously described in BA, and determined polymicrobial infections at a significantly higher rate than by culture. Although it is unclear if those bacteria identified by this technology in BA are clinically significant, because Mycobacteria faucium was identified in four polymicrobial BAs, but all 4four cases recuperated without receiving any specific therapy.[38,72]
Clinical Presentation
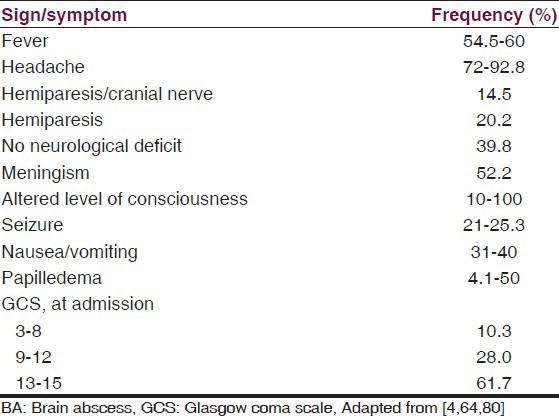
BA can be primarily present in four basic syndromes, viz. focal mass expansion, intra-cranial hypertension, diffuse destruction, and focal neurological deficit.[17] In most cases, predisposing risk factors, such as congenital cyanotic heart disease, decreased immunity, or the presence of a septic focus, can be identified.[73] The frequencies of clinical signs and symptoms in BA are listed in Table 4. The clinical presentation of intracranial abscess is dependent on the origin of infection, site, size, number of lesions, specific brain structures involved, the neighborhood anatomy disturbances involving cisterns, ventricles, and the dural venous sinuses, and any secondary cerebral injury.[32,74,75] Frequently patients present with symptoms of increased intracranial pressure (headache, nausea/vomiting, and altered mental status), focal neurologic deficits, and fever (although fever can be absent in 30-76% of cases).[1,8,75,76] Nathoo et al.[4] reported that headaches, fever, and nuchal rigidity were the commonest clinical presentation. The duration of symptoms ranged from 1 day to 8 weeks (average 11.4 ± 10 days; P = 0.7).
Table 4.
Common presenting signs and symptoms in BA
Unlike other CNS infections, focal deficits are frequently present and papilledema is not uncommon.[26] Pontine abscess may push posteriorly compressing the aqueduct of Sylvius causing obstructive hydrocephalus;[17] Frontal lobe lesions often present subtle clinical manifestations, and are referred to as the “silent area” of the brain.[2,77] The features of frontal lobe abscesses are usually nonspecific, such as fever, nausea, and headache during the initial stages, and signs of increased intracranial pressure, such as altered level of consciousness, become present later. Occipital lesions may rupture into ventricle causing ventriculitis or ependymitis or it may cause septic thrombophlebitis of the transverse sinus causing venous hypertension, edema, seizures, and raised intra-cranial pressure.[17] Common presenting symptomatology[59,78] and raised level of serum markers confer a high degree of clinical suspicion and may be found in up to 75% of cases; nevertheless, the main diagnostic tools today are the imaging modalities.[79] To take into account, is the fact that often the prescription of oral antibiotics, or analgesics, can cause the temporary relief of symptoms and prolongation of the disease course. In view of its early, nonspecific manifestations, the diagnosis of BA, particularly in the pediatric population, can often be delayed.[73]
Patients with BA typically present with one or more symptoms including fever, headache, altered level of consciousness, or focal neurological signs, including seizures, poor balance, dysphagia, or focal sensorimotor deficits.[31]
Diagnosis
Neuroimaging, usually a CT scan with contrast, is essential to diagnose a BA. The typical finding on CT scan or MRI is a hypodense lesion with a contrast-enhancing ring.[26] CT facilitates early detection, exact localization, and accurate characterization, determination of number, size, and staging of the abscess [Figure 1]. It also detects hydrocephalus, raised intracranial pressure (ICP), edema and associated infections like subdural empyema, ventriculitis and thus helps in treatment planning, in the assessment of adequacy of treatment and sequential follow up.[17] To take into account is the fact that corticosteroids decrease enhancement of abscess wall on CT.[17] In the earlier phases, a non-contrast CT may show only low-attenuation abnormalities with mass effect. In later phases, a complete peripheral ring may be seen.[17] On contrast CT, uniform ring enhancement is virtually always present in later phases. Due to de difficulty to visualize de capsule on early phase, double-contrast CT is helpful in defining encapsulation of abscess.[81] MRI features recognize pyogenic abscesses fairly accurately. A central area of liquefaction gives high signals, while the surrounding edematous brain tissue gives low signals on T1-weighted images. On T2-weighted images, the necrosis shows higher signals similar to the gray matter. The maturity of the abscess is indicated by the rim, which is formed probably by the collagen and inflammation due to free radicals and microhemorrhages in the abscess wall. The zone of inflammation is significantly thicker in tubercular as compared to pyogenic abscess in morphometric analysis of histologic sections. MRI findings also depend on the stage of the infection. In the early phase, MRI can have low T1-weighted images (T1WI) signal and high T2-weighted images (T2WI) signal with patchy enhancement. In later phases, the low T1WI signal becomes better demarcated, with high T2WI signal both in the cavity and surrounding parenchyma. Thickness, irregularity, and nodularity of the enhancing ring are suggestive of tumor (majority of cases) or, possibly, fungal infection.[82]
Figure 1.
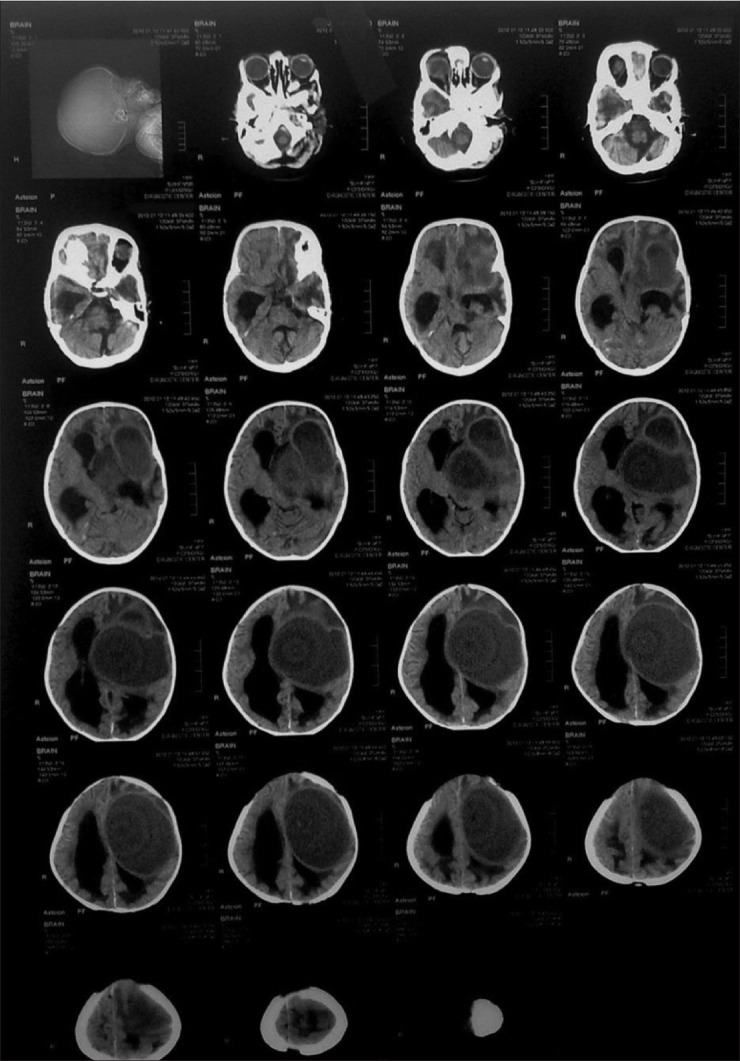
Pediatric brain CT-scan series showing a large hypodense lesion in left fronto-temporal region associated to mass effect and dilatation of ventricular system
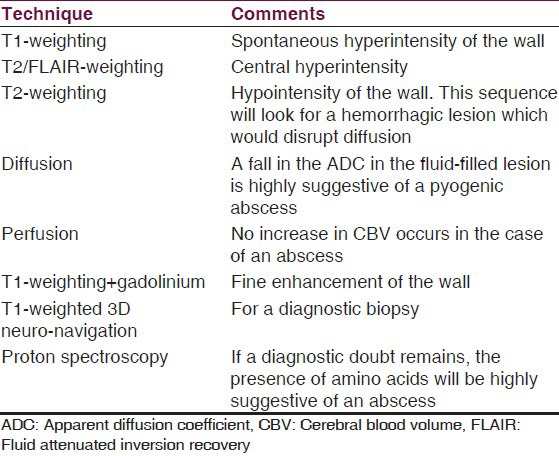
Diagnosis of BA had been revolutionized greatly due to advanced MR technology, making easier to distinguish between the differential diagnosis of BA, like can do diffusion-weighted imaging (DWI), which usually shows restricted diffusion (bright signal) that helps to differentiate abscesses from necrotic neoplasms, which are not usually restricted, although not all abscesses follow this rule.[83,84] Proton MR spectroscopy (1H-MRS) is also a safe, noninvasive imaging modality and can accurately differentiate between necrotic/cystic tumor and cerebral abscesses.[85] DWI has a high sensitivity and specificity, over 90%, for discriminating epidermoid, which has low apparent diffusion coefficient (ADC) from arachnoid cyst (high ADC) and distinguishing abscess (low ADC) from necrotic tumor (high ADC) (1). MR spectroscopy has been shown to be specifically beneficial in differentiating between BAs and other cystic lesions, which can be used to expedite implementation of the appropriate antimicrobial therapy.[85,86] 1H-MRS can also provide valuable information regarding the etiology of an abscess, as well as, its response to any medical or surgical treatment.[1] In Table 5 is listed an exploration protocol when a BA is suspected.
Table 5.
Suggested exploration protocol when a brain abscess is suspected by Auffray-Calvier et al.[87]
BA is the only CNS infection in which a lumbar puncture (LP) is never recommended and may even be contraindicated.[2] In the Nathoo et al.[4] series, pretransfer LP had been performed in 193 patients (19.8%) before consultation, which was followed by neurological deterioration in 26 patients (13.5%), with 7 deaths. CSF analysis revealed nonspecific pleocytosis with increased protein and no organism cultured in 80 patients (41.5%), bacterial meningitis in 71 patients (36.8%), and was normal in 31 patients. No data were available in 11 patients. This advice is not only because an LP does not help in the diagnosis but also because increased ICP is often present as a result of the mass effect, which increases the likelihood of herniation,[18] complicating patient clinical status. In the imagenologic identifications, the predilection of the BA location in relation to etiology were traumatic: Left-sided BA (n = 205, 64.3%); rhinogenic: Frontal (n = 176, 83.8%) and parietal (n = 15, 7.1%); otogenic: Temporal (n = 79, 47.9%), cerebellar (n = 75, 45.5%), and concomitant temporal and cerebellar (n = 4, 2.4%); and finally cardiac: Parietal (n = 15, 57.7%) and frontal (n = 9, 34.6%).[4]
Management
A multidisciplinary approach is paramount to the successful management of BA. The neurosurgeon is the nucleus of the team, working in close association with a neurologist, an infectious disease specialist, and neuroradiologist. This approach include neuroradiological evaluation, surgical intervention, use of antibiotics, and eradication of primary infected foci.[1] Intracranial abscess formation is a direct interplay between the virulence of the offending microorganism and the immune response of the host.[12] BAs usually require drainage in addition to appropriate microbial therapy, so early neurosurgical consultation is recommended.[2]
Although BA is essentially a surgical pathology, Arlotti et al.[75] recommend that choice of patients for a medical approach must be made on an individual basis. These authors consider best candidates for medical treatment to be those with a small abscess (<2.5 cm), in good initial clinical condition (GCS > 12), and for whom the etiology is well-known (microorganism isolated from material other than the abscess pus) recommendation grade C; or in the case of multiple abscesses, after surgery of abscesses >2.5 cm or surgery of abscesses that cause a mass effect, or in patients at serious risk of operation even if in these, the final decision must consider that the prognosis is often bad in any case recommendation grade D.
Antibiotics
The penetration of antimicrobial drugs from the systemic circulation into brain tissue is complex; the physiological properties of the blood–brain barrier and the blood–CSF barrier are distinct. Thus, the penetration of drugs into CSF differs from that into brain tissue or intracranial pus. That is the reason why concentrations of antibiotics in plasma cannot be used to predict the concentrations of these agents in brain tissue or intracranial pus.[88]
With which antibacterial therapy should we start?
Initial therapy should be commenced with broad spectrum antibiotics which can cross blood–brain and blood–CSF barriers in adequate concentrations; the empirical antibiotics should include coverage for anaerobic pathogens, such as a third-generation cephalosporin and metronidazole, plus vancomycin if there is a history of penetrating trauma or a recent neurosurgical procedure;[26] based on predisposing factors they can be administered till the pus is drained and the antibiotic sensitivity reports become available, specific bactericidal agents for the organism cultured should be administered.[1] Regard to this, Arlotti et al.[75] recommends (Grade D) that sample from the BA should be made without antibiotic therapy or, at least within not more than 3 days of the start of therapy!
If the culture is negative, then the broad spectrum antibiotics should be continued according to the likely predisposing cause (primary source; see “etiology”) and the anatomic location of abscess.[89] Penicillin, ampicillin, cefuroxime, chloramphenicol, co-trimoxazole, ceftazidime, and metronidazole have been shown to achieve therapeutic concentrations in intracranial pus, and have been administered successfully as treatment in various combinations.[88] In different experiences, penicillin and chloramphenicol have long been the main stay of empiric antimicrobial therapy. They have now been replaced by cefotaxime/ceftriaxone/ceftazidime, vancomycin, and metronidazole.[17,90,91,92]
Treatment according the complexity of the flora
The complexity of microbial flora in BA necessitates empirical antibiotic therapy against both aerobic and anaerobic organisms, remembering that more than one third otogenic and metastatic abscesses are polymicrobial (aerobic and/or anaerobic).[17]
Bacteroides, Peptostreptococcus, and Fusobactrium are common anaerobes and are sensitive to metronidazole.[88] We can take into account that rhinogenic abscesses are generally streptococcal; Staphylococcus is common in posttraumatic and postoperative cases; in infants and neonates, post-meningitic abscess is caused by gram-negative organisms.[17] Sulpha drugs are most effective in Nocardia and vancomycin against Staphylococcus.[17,91]
What happen with those patients who have immune function defects?
Infection with Nocardia asteroides or Toxoplasma gondii is common in patients with reduced lymphocytic function; in them sulfonamide and pyrimethanium are most effective. In the specific case of T-lymphocytic defect, Candida neoformans is common; thus 5 flucytosine and amphotericin-B can be used; in patients with leukemia and lymphoma, Pseudomonas infection is common and aminoglycosides are most effective; in renal transplant recipients, patients with blood cancer and those on steroid therapy, Listeria is common and ampicillin is most effective.[17]
Which antibiotic regimen?
Duration of antimicrobial therapy should be determined individually, based on the size of abscess, combination of surgical treatment, causative organism, and response to treatment. However, Arlotti et al.[75] consider (grade C) prudent a period of 4-6 weeks of treatment for surgically treated abscesses, and 6-8 weeks for intravenous treatment for BA treated solely medically and in the case of multiple BA when larger ones are treated surgically.
Usually, “triple high dose” antibiotics intravenously for 2 weeks followed by 4 weeks of oral therapy is recommended; in the case of immunocompromised patients, antimicrobial drugs are given for 3-12 months.[17] Metronidazole readily penetrates BAs; intralesional concentrations have been found to be 40 mg/ml; it has excellent bactericidal activity against many anaerobes but is not active against aerobic organisms. According to these characteristics, many experts recommend this agent to most patients with BA.[17] Canal et al.[93] performed the largest study of carbapenems for the treatment of BA ever published in the world literature, they founded that carbapenem monotherapy proved to be an effective alternative to the standard combination of IV cefotaxime + metronidazole when used in conjunction with neurosurgery to treat bacterial BA. However, the nonrandomised design of the study might have introduced a bias to the results. Meropenem and imipenem also penetrate into the CSF and show excellent antimicrobial activity in meningitis caused by different microorganisms.[94,95,96,97] Imipenem has been shown to be present in high concentrations in brain pus and, probably meropenem should behave similarly.[93,97,98,99]
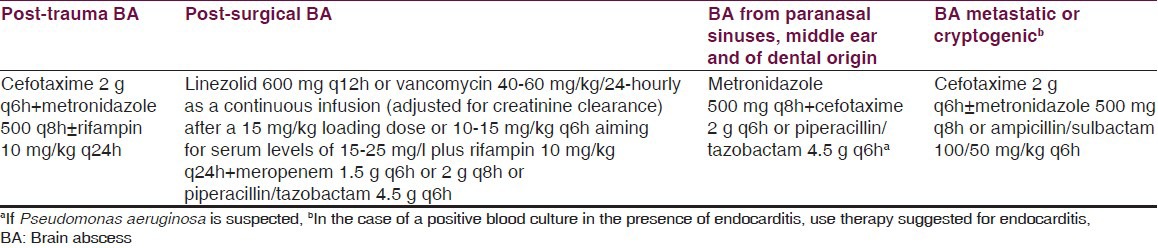
Arlotti et al.,[75] in their systematical review of the literature regarding controversial aspects of BA management, found that there is insufficient evidence to make specific recommendations for antibacterial treatment for BA. But they suggested drug choices related to the source of the BA [Table 6] based on the limited clinical data and on pharmacokinetic/pharmacodynamic considerations.
Table 6.
Suggested drug choices and scheduled regimens by Arlotti et al.[75]
Steroids
There is no well-controlled, randomized clinical study evaluating the use of corticosteroids for controlling the cerebral edema surrounding BA; nevertheless, corticosteroids are recommended perioperatively for reducing intracranial pressure and avoiding acute brain herniation,[100] but only in those patients that demonstrate signs of meningitis or disproportionate cytotoxic edema posing a life-threatening problem.[17]
Anticonvulsants
Seizures can be the initial manifestation of BA in up to 25-43% of cases.[101,102] Legg et al.[103] followed BA patients for long periods (up to 30 years) and founded an incidence of subsequent seizures after BA near 70%. They advocated anticonvulsant therapy for 5 years to all patients with BA.[103] Some authors consider discontinuation of antiepileptic drugs when patient is seizure free for at least 2 years after surgery and electroencephalogram (EEG) shows no epileptic activity,[17] other recommends seizure prophylaxis or antiepileptic medication in every case and continued for extended periods.[104] In the case of children, anticonvulsants are recommended in those who have developed seizures to potentially prevent further episodes.[105] The duration should be individualized and guided by EEG studies in the follow-up phase of disease. Most authors recommend providing at least 3 months of prophylaxis if no more seizures have occurred.[105]
Surgical Treatment
Sargent, in 1928, was the first to report successful excision of BA.[6] The historical methods of surgical drainage have been tube drainage, marsupialization, the migration method of Kahn, and Dandy's sequential tapping of a chronic BA to prevent brain fungus coinfection.[6] Currently, the principals methods for surgical management are open evacuation, excision, and aspiration through a bur hole and more recently stereotactic.[6,32,33,106,107]
According to the “Infection in Neurosurgery’ Working Party Of The British Society For Antimicrobial Chemotherapy,” the guiding principles for surgical management are:[88]
To urgently reduce raised intracranial pressure by aspiration of the cavity using image guidance;
to confirm the diagnosis;
to obtain pus for microbiological diagnosis;
to enhance the efficacy of antibiotic therapy; and
to avoid iatrogenic spread of infection into the ventricles.
Thus, for almost any BA, emergent drainage is indicated both therapeutically and for diagnosis to establish the causative offending pathogen since high rates of negative cultures.
In most instances, aspiration of the purulent material is sufficient to initiate the healing of abscess. However, surgical excision becomes mandatory if the pus is thick and in multiloculated abscesses. Urgent evacuation of abscess is required for subdural empyema and cerebellar abscess.[1]
Operative and nonoperative cases
Nonresponse BA to only medical management (i.e., evidence of growing abscess while on antibiotics or no change in size at 2-3 weeks), will necessitate surgical drainage.[4,108] Traumatic BA may require craniotomy to remove foreign material or bone chips.[1] Cerebellar or brain stem abscesses are often indication for posterior fossa craniotomy due to the high risk of brain herniation.[3,25] Periventricular BA often requires craniotomy given the risk of intraventricular rupture. A ventriculostomy placement is indicated for significantly elevated intracranial pressure.[1]
Multiple abscesses are best treated by aspiration of the largest one for diagnosis and of others if they are causing mass effect. Where a peripherally placed abscess fails to respond to aspiration consideration should be given to craniotomy and excision;[88]
Nonoperative treatment has been proposed, even for BAs with chronic encapsulation, provided they are less than 2 cm in diameter, for multiple small abscesses and for patients who are extremely poor surgical candidates.[6]
Complications considerations
Primary excision of a BA carries the risk of serious damage to the surrounding brain with increased potential for neurological sequelae and epilepsy,[4,58,109] because the capsule often has anchor extensions into the surrounding white matter, with the surgical procedure may be caused unplanned extensive damage to adjacent viable cerebral tissue.[33] Furthermore, small, deep, and multiple abscesses are generally not suitable for excision.
Today, aspiration of BA has become the preferred method of drainage,[6] providing rapid relief from raised intracranial pressure for single or multiple, deep- and/or “eloquent area”-located lesions, it is considered easy to perform; main disadvantages of aspiration are repeat procedures and, in up to 70% of patients, there is possibility of iatrogenic puncture of the ventricle and subarachnoid leakage of pus leading to meningitis and/or ventriculitis.[33]
Surgical approaches comparison
In 2009, Mut et al.[110] compared the efficacies of aspiration of the BA cavity versus excision of BA capsule and their impact on postoperative antibiotic use and the length of hospital stay. Nine patients underwent the capsule excision and 11 patients to BA aspiration. They found no differences in terms of age, sex, location, and radiographic features. There were three residual/recurrence cases in the aspiration group, who needed a second aspiration, whereas no residual/recurrence was observed in the excision group. Postoperative use of antibiotics was significantly less in the excision group (mean: 26.7 days in the excision group vs. 46.6 days in aspiration group). Length of hospital stay for the purpose of IV antibiotic administration was significantly shorter in the excision group.
In 2012, Sarmast et al.[111] investigated the efficacies of aspiration versus excision for the management of large solitary encapsulated pyogenic BA located in superficial noneloquent areas comparing the impact on length of hospital stay, duration of postoperative antibiotic use, improvement in neurological status, and morbidity and mortality. They included 47 patients with pyogenic BA from a total of 114 patients evaluated over a period of 10 years (October 2001 to October 2011). Aspiration was performed in 29 patients (61.7%), of whom 7 patients needed second aspiration and 18 patients underwent excision (38.3%) of the abscess capsule. The mean duration of antibiotic use in the excision group was significantly shorter at 2.7 weeks (SD ± 1.1) compared to the aspiration group at 3.8 weeks (SD ± 1.3) (P = 0.006). Similarly, mean length of hospital stay was significantly shorter in the excision group at 18.1 days (SD ± 7.7) compared to the aspiration group at 24.9 days (SD ± 6.6) (P = 0.002). In addition, significantly earlier improvement in neurological function (P = 0.025) and significantly lower rate of resurgery (P = 0.0238) were found in the excision group compared to the aspiration group.
In 2010, Tan et al.[112] compared the burr hole approach versus craniotomy used as treatment for superficial BA and its outcome in terms of radiological clearance on brain CT, improvement of neurological status, the need for repeated surgery, and survival and morbidity at three months after surgery. Fifty-one cases were included in the study during a period of four years (2004-2007): 28 patients (54.9%) had undergone craniotomy and excision of abscess, and the rest had undergone burr hole aspiration as their first surgical treatment. They found that patients who had undergone craniotomy and excision of abscess showed a significantly earlier improvement in neurological function, better radiological clearance, and lower rate of resurgery as compared to the burr hole aspiration group (P < 0.05), but with no significant difference between the two surgical methods regarding to neurological improvement at 3 months, morbidity, and mortality.
Unfortunately, the studies designs are not the best to acquire strong conclusions regarding the surgical technique. These findings can only be confirmed by a prospective randomised series, but excision have been better than aspiration regard to duration of antibiotic use, length of hospital stay, and overall cost of treatment is, with no significant difference in morbidity and mortality.
In the Nathoo et al.[4] experience, surgical drainage was performed in 945 patients (97.1%). Preoperative hydrocephalus was detected in 79 of 104 (76%) patients with meningitis/ventriculitis/ventricular rupture and in 69 patients (68.3%) with cerebellar abscesses. Frame-based stereotactic drainage for deep-seated BA was necessary in 31 patients (3.2%), of whom 16 (51.6%) were HIV positive, with 16 patients experiencing a poor outcome (13 deaths, 41.9%). In cases of otorhinogenic BA were needed repeated drainages more frequently when compared to traumatic BA (49.9% vs. 33.5%) (P ≤ 0.01). As complications, two patients died (ventriculitis; secondary intracranial hypertension), and three patients (16.7%) developed postoperative seizures. Only 6 of 129 patients (4.7%) had complete resolution of their cerebritis process after primary source eradication and intravenous antibiotic therapy.
Complications, Outcomes and Prognosis
Many poor prognostic indicators have been described, which include delayed diagnosis, rapidly progressing disease, coma, multiple lesions, intraventricular rupture, and fungal etiology.[102,113] Obviously, we can find all these prognosis indicators in the immunocompromised patients. Outcome is poorer in the newborn and the elderly.[102] Focal neurological deficits and mental retardation are recognized complications of BA,[102] especially when occurs during childhood;[60] Nathoo reported as complications, two deaths, one due to ventriculitis and the other due to secondary intracranial hypertension, and three patients (16.7%) developed postoperative seizures.
Mortality rate is directly related to the rate of disease progression and the neurological condition of the patient on admission.[33] Before 1970, overall mortality due to BA could be as high as up to 60%; fortunately new antibacterial approaches and the use of new imaginological technologies have contributed to diminish the mortality, being between 8% and 25% in the large cases series.[4,27,80] Intraventricular rupture and posterior fossa location where there can be obstruction in the flow of CSF are also associated with a poor prognosis, and with a mortality rate near to 80%,[33,39] and of 90% if the etiological pathogen is Aspergillus.[114] The fact to have a comparable mortality rate, around 13% in developed or developing countries, indicates the necessity of an aggressive surgical approach to BA for the optimal outcome.[33] In the Nathoo et al.[4] experience, operative drainage of BA >2.5 cm in diameter and antibiotic therapy resulted in a mortality rate of 13%, a poor outcome in 5%, and a good outcome in 81% of patients, being similar in other retrospective studies in Europe and Asia.[31,115,116]
Conclusions
In conclusion, BA still continues to be a formidable challenge despite introduction of newer and effective antimicrobial chemotherapy and radiological and neurosurgical technology. It is important to advise the fact that older and immunocompromised population are increasing, the latter either due to immunosuppressive medication or to illness, this circumstance will lead to a more difficult diagnosis and management if arcane concepts regard to BA are not abandoned. BA is not only an entity of undeveloped countries, it is also present in the entire world although in different frequencies. Further researches must be conducted to clarify specific aspects, such as anticonvulsant prophylaxis/therapy, and also for the improvement of microbiological diagnosis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Muzumdar D. Central nervous system infections and the neurosurgeon: A perspective. Int J Surg. 2011;9:113–6. doi: 10.1016/j.ijsu.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;25:763–79. doi: 10.1086/515541. [DOI] [PubMed] [Google Scholar]
- 3.Menon S, Bharadwaj R, Chowdhary A, Kaundinya DV, Palande DA. Current epidemiology of intracranial abscesses: A prospective 5 year study. J Med Microbiol. 2008;57:1259–68. doi: 10.1099/jmm.0.47814-0. [DOI] [PubMed] [Google Scholar]
- 4.Nathoo N, Nadvi SS, Narotam PK, Van Dellen JR. Brain Abscess: Management and Outcome Analysis of a Computed Tomography Era Experience with 973 Patients. World Neurosurg. 2011;75:716–26. doi: 10.1016/j.wneu.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 5.Wiwanitkit S, Wiwanitkit V. Pyogenic brain abscess in Thailand. N Am J Med Sci. 2012;4:245–8. doi: 10.4103/1947-2714.97200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathoo N, Narotam PK, Nadvi S, Van Dellen JR. Taming an old enemy: A profile of intracranial suppuration. World Neurosurg. 2012;77:484–90. doi: 10.1016/j.wneu.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Britt RH, Enzmann DR, Yeager AS. Neuropathological and computerized tomographic findings in experimental brain abscess. J Neurosurg. 1981;55:590–603. doi: 10.3171/jns.1981.55.4.0590. [DOI] [PubMed] [Google Scholar]
- 8.Manzar N, Manzar B, Kumar R, Bari ME. The study of etiologic and demographic characteristics of intracranial brain abscess: A consecutive case series study from Pakistan. World Neurosurg. 2011;76:195–200. doi: 10.1016/j.wneu.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Saeed H, Alamri A, Crocker M, Dave J. Twenty years of intracranial abscesses: Prognostic indicators and treatment review. J Infect. 2011;63:491–2. [Google Scholar]
- 10.Britt RH, Enzmann DR, Remington JS. Intracranial infection in cardiac transplant recipients. Ann Neurol. 1981;9:107–19. doi: 10.1002/ana.410090203. [DOI] [PubMed] [Google Scholar]
- 11.Vieira J, Frank E, Spira TJ, Landesman SH. Acquired immune deficiency in Haitians: Opportunistic infections in previously healthy Haitian immigrants. N Engl J Med. 1983;308:125–9. doi: 10.1056/NEJM198301203080303. [DOI] [PubMed] [Google Scholar]
- 12.Chauvet D, Sainte-Rose C, Boch AL. The mystery of prehistoric trepanations: Is neurosurgery the world eldest profession? Neurochirurgie. 2010;56:420–5. doi: 10.1016/j.neuchi.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Gerszten PC, Gerszten E, Allison MJ. Diseases of the skull in pre-Columbian South American mummies. Neurosurgery. 1998;42:1145–51. doi: 10.1097/00006123-199805000-00114. [DOI] [PubMed] [Google Scholar]
- 14.Piek J, Lidke G, Terberger T. The Neolithic skull from Bölkendorf-evidence for Stone Age neurosurgery? Cent Eur Neurosurg. 2011;72:42–3. doi: 10.1055/s-0029-1246133. [DOI] [PubMed] [Google Scholar]
- 15.Alfieri A, Strauss C, Meller H, Stoll-Tucker B, Tacik P, Brandt S. The woman of Pritschoena: An example of the German neolithic neurosurgery in Saxony-Anhalt. J Hist Neurosci. 2012;21:139–46. doi: 10.1080/0964704X.2011.575117. [DOI] [PubMed] [Google Scholar]
- 16.Martin G. The death of Henry II of France: A sporting death and post-mortem. ANZ J Surg. 2001;71:318–20. doi: 10.1046/j.1440-1622.2001.02102.x. [DOI] [PubMed] [Google Scholar]
- 17.Muzumdar D, Jhawar S, Goel A. Brain abscess: An overview. Int J Surg. 2011;9:136–44. doi: 10.1016/j.ijsu.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Stone JL, Hockley AD. Percivall Pott and the miners of Cornwall. Br J Neurosurg. 2002;16:501–6. doi: 10.1080/0268869021000030348. [DOI] [PubMed] [Google Scholar]
- 19.Morand S. Paris: Despress; 1768. Surgery Opuscules. [Google Scholar]
- 20.Mut M, Dinç G, Naderi S. On the report of the first successful surgical treatment of brain abscess in the Ottoman Empire by Dr. Cemil Topuzlu in 1891. Neurosurgery. 2007;61:869–72. doi: 10.1227/01.NEU.0000298917.17001.88. [DOI] [PubMed] [Google Scholar]
- 21.MacEwan W. Glasgow, Scotland: Maclehose Press; 1893. Pyogenic infective diseases of the brain and spinal cord. [Google Scholar]
- 22.Feldmann H, Oscar Wilde. Medical observations on the 100 th anniversary of his death 30 November 2000. Laryngorhinootologie. 2000;79:698–702. doi: 10.1055/s-2000-8300. [DOI] [PubMed] [Google Scholar]
- 23.Rosenblum ML, Hoff JT, Norman D, Weinstein PR, Pitts L. Decreased mortality from brain abscesses since advent of computerized tomography. J Neurosurg. 1978;49:658–68. doi: 10.3171/jns.1978.49.5.0658. [DOI] [PubMed] [Google Scholar]
- 24.Britt RH, Enzmann DR. Clinical stages of human brain abscesses on serial CT scans after contrast infusion. Computerized tomographic, neuropathological, and clinical correlations. J Neurosurg. 1983;59:972–89. doi: 10.3171/jns.1983.59.6.0972. [DOI] [PubMed] [Google Scholar]
- 25.Kaczorowska B, Chmielewski H, Pawełczyk M, Przybyła M, Błaszczyk B, Chudzik W. The case of multiple brain abscesses conservatively treated. Pol Merkur Lekarski. 2007;22:150–3. [PubMed] [Google Scholar]
- 26.Mace SE. Central nervous system infections as a cause of an altered mental status? What is the Pathogen growing in your central Nervous system? Emerg Med Clin North Am. 2010;28:535–70. doi: 10.1016/j.emc.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Gutiérrez-Cuadra M, Ballesteros MA, Vallejo A, Miñambres E, Fariñas-Alvarez C, García-Palomo JD, et al. Brain abscess in a third-level hospital: Epidemiology and prognostic factors related to mortality. Rev Esp Quimioter. 2009;22:201–6. [PubMed] [Google Scholar]
- 28.Sharma R, Mohandas K, Cooke RP. Intracranial abscesses: Changes in epidemiology and management over five decades in Merseyside. Infection. 2009;37:39–43. doi: 10.1007/s15010-008-7359-x. [DOI] [PubMed] [Google Scholar]
- 29.Roche M, Humphreys H, Smyth E, Phillips J, Cunney R, McNamara E, et al. A twelve-year review of central nervous system bacterial abscesses; presentation and aetiology. Clin Infect Dis. 2003;9:803–9. doi: 10.1046/j.1469-0691.2003.00651.x. [DOI] [PubMed] [Google Scholar]
- 30.Woods CR. Brain abscess and other intracranial suppurative complications. Adv Pediatr Infect Dis. 1995;10:41–79. [PubMed] [Google Scholar]
- 31.Carpenter J, Stapleton S, Holliman R. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur J Clin Microbiol Infect Dis. 2007;26:1–11. doi: 10.1007/s10096-006-0236-6. [DOI] [PubMed] [Google Scholar]
- 32.Yang SY. Brain abscess: A review of 400 cases. J Neurosurg. 1981;55:794–9. doi: 10.3171/jns.1981.55.5.0794. [DOI] [PubMed] [Google Scholar]
- 33.Hall WA, Truwit CL. The surgical management of infections involving the cerebrum. Neurosurgery. 2008;62(Suppl 2):519–30. doi: 10.1227/01.neu.0000316255.36726.5b. [DOI] [PubMed] [Google Scholar]
- 34.Moorthy RK, Rajshekhar V. Management of brain abscess: An overview. Neurosurg Focus. 2008;24:E3. doi: 10.3171/FOC/2008/24/6/E3. [DOI] [PubMed] [Google Scholar]
- 35.Kawamata T, Takeshita M, Ishizuka N, Hori T. Patent foramen ovale as a possible risk factor for cryptogenic brain abscess: Report of two cases. Neurosurgery. 2001;49:204–6. doi: 10.1097/00006123-200107000-00032. [DOI] [PubMed] [Google Scholar]
- 36.Schuchlenz HW, Saurer G, Weihs W, Rehak P. Persisting eustachian valve in adults: Relation to patent foramen ovale and cerebrovascular events. J Am Soc Echocardiogr. 2004;17:231–3. doi: 10.1016/j.echo.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Horiuchi Y, Kato Y, Dembo T, Takeda H, Fukuoka T, Tanahashi N. Patent foramen ovale as a risk factor for cryptogenic brain abscess: Case report and review of the literature. Intern Med. 2012;51:1111–4. doi: 10.2169/internalmedicine.51.7133. [DOI] [PubMed] [Google Scholar]
- 38.Al Masalma M, Lonjon M, Richet H, Dufour H, Roche PH, Drancourt M, et al. Metagenomic analysis of brain abscesses identifies specific bacterial associations. Clin Infect Dis. 2012;54:202–10. doi: 10.1093/cid/cir797. [DOI] [PubMed] [Google Scholar]
- 39.Gadgil N, Chamoun RB, Gopinath SP. Intraventricular brain abscess. J Clin Neuroscie. 2012;19:1314–6. doi: 10.1016/j.jocn.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 40.Kielian T. Immunopathogenesis of brain abscess. J Neuroinflammation. 2004;1:16. doi: 10.1186/1742-2094-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta RK, Srivastava S, Saksena S, Rathore RK, Awasthi R, Prasad KN, et al. Correlation of DTI metrics in the wall and cavity of brain abscess with histology and immunohistochemistry. NMR Biomed. 2010;23:262–9. doi: 10.1002/nbm.1448. [DOI] [PubMed] [Google Scholar]
- 42.Greenwood J, Heasman SJ, Alvarez JI, Prat A, Lyck R, Engelhardt B. Review: Leucocyte-endothelial cell crosstalk at the blood-brain barrier: A prerequisite for successful immune cell entry to the brain. Neuropathol Appl Neurobiol. 2011;37:24–39. doi: 10.1111/j.1365-2990.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 43.Hanamsagar R, Hanke ML, Kielian T. Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol. 2012;33:333–42. doi: 10.1016/j.it.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konat GW, Kielian T, Marriott I. The role of Toll-like receptors in CNS response to microbial challenge. J Neurochem. 2006;99:1–12. doi: 10.1111/j.1471-4159.2006.04076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carpentier PA, Duncan DS, Miller SD. Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain Behav Immun. 2008;22:140–7. doi: 10.1016/j.bbi.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garg S, Nichols JR, Esen N, Liu S, Phulwani NK, Syed MM, et al. MyD88 expression by CNS-resident cells is pivotal for eliciting protective immunity in brain abscesses. ASN Neuro. 2009;1 doi: 10.1042/AN20090004. pii: e00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vidlak D, Mariani MM, Aldrich A, Liu S, Kielian T. Roles of Toll-like receptor 2 (TLR2) and superantigens on adaptive immune responses during CNS staphylococcal infection. Brain Behav Immun. 2011;25:905–14. doi: 10.1016/j.bbi.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jha S, Srivastava SY, Brickey WJ, Iocca H, Toews A, Morrison JP, et al. The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J Neurosci. 2010;30:15811–20. doi: 10.1523/JNEUROSCI.4088-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Griffin WS, Mrak RE. Interleukin-1 in the genesis and progression of and risk for development of neuronal degeneration in Alzheimer's disease. J Leukoc Biol. 2002;72:233–8. [PMC free article] [PubMed] [Google Scholar]
- 50.Zwijnenburg PJ, Van der Poll T, Florquin S, Roord JJ, Van Furth AM. IL-1 receptor type 1 gene-deficient mice demonstrate an impaired host defense against pneumococcal meningitis. J Immunol. 2003;170:4724–30. doi: 10.4049/jimmunol.170.9.4724. [DOI] [PubMed] [Google Scholar]
- 51.Kielian T, Bearden ED, Baldwin AC, Esen N. IL-1 and TNF-alpha play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuropathol Exp Neurol. 2004;63:381–96. doi: 10.1093/jnen/63.4.381. [DOI] [PubMed] [Google Scholar]
- 52.Kielian T, Phulwani NK, Esen N, Syed MM, Haney AC, McCastlain K, et al. MyD88-dependent signals are essential for the host immune response in experimental brain abscess. J Immunol. 2007;178:4528–37. doi: 10.4049/jimmunol.178.7.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanamsagar R, Torres V, Kielian T. Inflammasome activation and IL-1β/IL-18 processing are influenced by distinct pathways in microglia. J Neurochem. 2011;119:736–48. doi: 10.1111/j.1471-4159.2011.07481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karpuk N, Burkovetskaya M, Fritz T, Angle A, Kielian T. Neuroinflammation leads to region-dependent alterations in astrocyte gap junction communication and hemichannel activity. J Neurosci. 2011;31:414–25. doi: 10.1523/JNEUROSCI.5247-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Esen N, Shuffield D, Syed MM, Kielian T. Modulation of connexin expression and gap junction communication in astrocytes by the gram-positive bacterium S. aureus. Glia. 2007;55:104–17. doi: 10.1002/glia.20438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanisch UK, Johnson TV, Kipnis J. Toll-like receptors: Roles in neuroprotection? Trends in Neurosciences. 2008;31:176–82. doi: 10.1016/j.tins.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 57.Derber CJ, Troy SB. Head and Neck emergencies: Bacterial Meningitis, encephalitis, brain abscess, upper airway obstruction, and jugular septic thrombophlebitis. Med Clin North Am. 2012;96:1107–26. doi: 10.1016/j.mcna.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Beller AJ, Sahar A, Praiss I. Brain abscess. Review of 89 cases over a period of 30 years. J Neurol Neurosurg Psychiatry. 1973;36:757–68. doi: 10.1136/jnnp.36.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhatia R, Tandon PN, Banerji AK. Brain abscess-an analysis of 55 cases. Int Surg. 1973;58:565–8. [PubMed] [Google Scholar]
- 60.Nielsen H, Harmsen A, Gyldensted C. Cerebral abscess. A long-term follow-up. Acta Neurol Scand. 1983;67:330–7. doi: 10.1111/j.1600-0404.1983.tb03150.x. [DOI] [PubMed] [Google Scholar]
- 61.Patil AB, Nadagir SD, Lakshminarayana S, Syeda FM. Morganella morganii, subspecies morganii, biogroup A: An unusual causative pathogen of brain abscess. J Neurosci Rural Pract. 2012;3:370–2. doi: 10.4103/0976-3147.102631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sims L, Lim M, Harsh GR., IV Review of Brain Abscesses. Oper Tech Neurosurg. 2004;7:176–81. [Google Scholar]
- 63.Tonon E, Scotton PG, Gallucci M, Vaglia A. Brain abscess: Clinical aspects of 100 patients. Int J Infect Dis. 2006;10:103–9. doi: 10.1016/j.ijid.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 64.Calfee DP, Wispelwey B. Brain abscess. Semin Neurol. 2000;20:353–60. doi: 10.1055/s-2000-9397. [DOI] [PubMed] [Google Scholar]
- 65.Lakshmi V, Umabala P, Anuradha K, Padmaja K, Padmasree C, Rajesh A, et al. Microbiological spectrum of brain abscess at a tertiary care hospital in South India: 24-year data and review. Patholog Res Int 2011. 2011 doi: 10.4061/2011/583139. 583139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Britton PN, Chaseling R. Brain abscess in a recent immigrant. J Paediatr Child Health. 2013;49:E176–8. doi: 10.1111/jpc.12044. [DOI] [PubMed] [Google Scholar]
- 67.Abdel Razek AA, Watcharakorn A, Castillo M. Parasitic diseases of the central nervous system. Neuroimaging Clin N Am. 2011;21:815–41. doi: 10.1016/j.nic.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 68.Finsterer J, Auer H. Parasitoses of the human central nervous system. J Helminthol. 2013;4(Suppl 1):s257–70. doi: 10.1017/S0022149X12000600. [DOI] [PubMed] [Google Scholar]
- 69.Maldonado-Barrera CA, Campos-Esparza Mdel R, Muñoz-Fernández L, Victoria-Hernández JA, Campos-Rodríguez R, Talamás-Rohana P, et al. Clinical case of cerebral amebiasis caused by E. histolytica. Parasitol Res. 2012;110:1291–6. doi: 10.1007/s00436-011-2617-8. [DOI] [PubMed] [Google Scholar]
- 70.Sayhan Emil S, Altinel D, Bayol U, Ozcolpan OO, Tan A, Ganiusmen O. Amebic cerebral abscess mimicking bacterial meningitis. Indian J Pediatr. 2008;75:1078–80. doi: 10.1007/s12098-008-0182-7. [DOI] [PubMed] [Google Scholar]
- 71.Ozüm U, Karadağ O, Eğilmez R, Engin A, Oztoprak I, Ozçelik S. A case of brain abscess due to Entamoeba species, Eikenella corrodens and Prevotella species. Br J Neurosurg. 2008;22:596–8. doi: 10.1080/02688690801894646. [DOI] [PubMed] [Google Scholar]
- 72.Al Masalma M, Armougom F, Scheld WM, Dufour H, Roche PH, Drancourt M, et al. The expansion of the microbiological spectrum of brain abscesses with use of multiple 16S ribosomal DNA sequencing. Childs Nerv Syst. 2009;48:1169–78. doi: 10.1086/597578. [DOI] [PubMed] [Google Scholar]
- 73.Sankararaman S, Riel-Romero RM, Gonzalez-Toledo E. Brain abscess from a peritonsillar abscess in an immunocompetent child: A case report and review of the literature. Pediatr Neurol. 2012;47:451–4. doi: 10.1016/j.pediatrneurol.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 74.Chun CH, Johnson JD, Hofstetter M, Raff MJ. Brain abscess. A study of 45 consecutive cases. Medicine. 1986;65:415–31. [PubMed] [Google Scholar]
- 75.Arlotti M, Grossi P, Pea F, Tomei G, Vullo V, De Rosa FG, et al. Consensus document on controversial issues for the treatment of infections of the central nervous system: Bacterial brain abscesses. Int J Infect Dis. 2010;14(Suppl 4):S79–92. doi: 10.1016/j.ijid.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 76.Ratnaike TE, Das S, Gregson BA, Mendelow AD. A review of brain abscess surgical treatment-78 years: Aspiration versus excision. World Neurosurg. 2011;76:431–6. doi: 10.1016/j.wneu.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 77.Shachor-Meyouhas Y, Bar-Joseph G, Guilburd JN, Lorber A, Hadash A, Kassis I. Brain abscess in children-epidemiology, predisposing factors and management in the modern medicine era. Acta Paediatr. 2010;99:1163–7. doi: 10.1111/j.1651-2227.2010.01780.x. [DOI] [PubMed] [Google Scholar]
- 78.Carey ME, Chou SN, French LA. Experience with brain abscesses. J Neurosurg. 1972;36:1–9. doi: 10.3171/jns.1972.36.1.0001. [DOI] [PubMed] [Google Scholar]
- 79.Loeser E, Scheinberg L. Brain abscesses; A review of ninety-nine cases. Neurology. 1957;7:601–9. doi: 10.1212/wnl.7.9.601. [DOI] [PubMed] [Google Scholar]
- 80.Helweg-Larsen J, Astradsson A, Richhall H, Erdal J, Laursen A, Brennum J. Pyogenic brain abscess, a 15 year survey. BMC Infect Dis. 2012;12:332. doi: 10.1186/1471-2334-12-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salzman C, Tuazon CU. Value of the ring-enhancing sign in differentiating intracerebral hematomas and brain abscesses. Arch Intern Med. 1987;147:951–2. [PubMed] [Google Scholar]
- 82.Haimes AB, Zimmerman RD, Morgello S, Weingarten K, Becker RD, Jennis R, et al. MR imaging of brain abscesses. AJR Am J Roentgenol. 1989;152:1073–85. doi: 10.2214/ajr.152.5.1073. [DOI] [PubMed] [Google Scholar]
- 83.Guzman R, Barth A, Lövblad KO, El-Koussy M, Weis J, Schroth G, et al. Use of diffusion-weighted magnetic resonance imaging in differentiating purulent brain processes from cystic brain tumors. J Neurosurg. 2002;97:1101–7. doi: 10.3171/jns.2002.97.5.1101. [DOI] [PubMed] [Google Scholar]
- 84.Liao KH, Chang CK, Chang HC, Chang KC, Chen CF, Chen TY, et al. Clinical practice guidelines in severe traumatic brain injury in Taiwan. Surg Neurol. 2009;72(Suppl):S66–73. doi: 10.1016/j.surneu.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 85.Fountas KN, Kapsalaki EZ, Gotsis SD, Kapsalakis JZ, Smisson HF, 3rd, Johnston KW, et al. In vivo Proton Magnetic Resonance Spectroscopy of Brain Tumors. Stereotact Funct Neurosurg. 2000;74:83–94. doi: 10.1159/000056467. [DOI] [PubMed] [Google Scholar]
- 86.Burtscher IM, Holtås S. In vivo proton MR spectroscopy of untreated and treated brain abscesses. AJNR Am J Neuroradiol. 1999;20:1049–53. [PMC free article] [PubMed] [Google Scholar]
- 87.Auffray-Calvier E, Toulgoat F, Daumas-Duport B, Lintia Gaultier A, Desal H. Infectious and metabolic brain imaging. Diagn Interv Imaging. 2012;93:911–34. doi: 10.1016/j.diii.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 88.Infection in Neurosurgery Working Party of the British Society for Antimicrobial Chemotherapy. The rational use of antibiotics in the treatment of brain abscess. Br J Neurosurg. 2000;14:525–30. doi: 10.1080/02688690020005527. [DOI] [PubMed] [Google Scholar]
- 89.De Louvois J. Bacteriological examination of pus from abscesses of the central nervous system. J Clin Pathol. 1980;33:66–71. doi: 10.1136/jcp.33.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Le Moal G, Landron C, Grollier G, Bataille B, Roblot F, Nassans P, et al. Characteristics of brain abscess with isolation of anaerobic bacteria. Scand J Infect Dis. 2003;35:318–21. doi: 10.1080/00365540310000265. [DOI] [PubMed] [Google Scholar]
- 91.Hakan T, Ceran N, Erdem I, Berkman MZ, Göktaş P. Bacterial brain abscesses: An evaluation of 96 cases. J Infect. 2006;52:359–66. doi: 10.1016/j.jinf.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 92.Tonon E, Scotton PG, Gallucci M, Vaglia A. Brain abscess: Clinical aspects of 100 patients. Int J Infect Dis. 2006;10:103–9. doi: 10.1016/j.ijid.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 93.Martin-Canal G, Saavedra A, Asensi JM, Suarez-Zarracina T, Rodriguez-Guardado A, Bustillo E, et al. Meropenem monotherapy is as effective as and safer than imipenem to treat brain abscesses. Int J Antimicrob Agents. 2010;35:301–4. doi: 10.1016/j.ijantimicag.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 94.Meis JF, Groot-Loonen J, Hoogkamp-Korstanje JA. A brain abscess due to multiply-resistant Enterobacter cloacae successfully treated with meropenem. Clin Infect Dis. 1995;20:1567. doi: 10.1093/clinids/20.6.1567. [DOI] [PubMed] [Google Scholar]
- 95.Asensi V, Carton JA, Maradona JA, Asensi JM, Pérez F, Redondo P, et al. Imipenem therapy of brain abscesses. Eur J Clin Microbiol Infect Dis. 1996;15:653–7. doi: 10.1007/BF01691152. [DOI] [PubMed] [Google Scholar]
- 96.Asensi V, Cartón JA, Maradona JA, Asensi JM, Pérez F, Redondo P, et al. Therapy of brain abscess with imipenem-a safe therapeutic choice? J Antimicrob Chemother. 1996;37:200–3. doi: 10.1093/jac/37.1.200. [DOI] [PubMed] [Google Scholar]
- 97.Baldwin CM, Lyseng-Williamson KA, Keam SJ. Meropenem: A review of its use in the treatment of serious bacterial infections. Drugs. 2008;68:803–38. doi: 10.2165/00003495-200868060-00006. [DOI] [PubMed] [Google Scholar]
- 98.Modai J, Vittecoq D, Decazes JM, Meulemans A. Penetration of imipenem and cilastatin into cerebrospinal fluid of patients with bacterial meningitis. J Antimicrob Chemother. 1985;16:751–5. doi: 10.1093/jac/16.6.751. [DOI] [PubMed] [Google Scholar]
- 99.Linden P. Safety profile of meropenem: An updated review of over 6,000 patients treated with meropenem. Drug Saf. 2007;30:657–68. doi: 10.2165/00002018-200730080-00002. [DOI] [PubMed] [Google Scholar]
- 100.Lee TH, Chang WN, Su TM, Chang HW, Lui CC, Ho JT, et al. Clinical features and predictive factors of intraventricular rupture in patients who have bacterial brain abscesses. J Neurol Neurosurg Psychiatry. 2007;78:303–9. doi: 10.1136/jnnp.2006.097808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garibi JM. Prophylactic antiepileptic treatment of cerebral aggressions. Rev Neurol. 2002;34:446–8. [PubMed] [Google Scholar]
- 102.Ananth Ramakrishnan K, Levin M, Faust SN. Bacterial meningitis and brain abscess. Medicine. 2009;37:567–73. [Google Scholar]
- 103.Legg NJ, Gupta PC, Scott DF. Epilepsy following cerebral abscess. A clinical and EEG study of 70 patients. Brain. 1973;96:259–68. doi: 10.1093/brain/96.2.259. [DOI] [PubMed] [Google Scholar]
- 104.Lu CH, Chang WN, Lui CC. Strategies for the management of bacterial brain abscess. J Clin Neurosci. 2006;13:979–85. doi: 10.1016/j.jocn.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 105.Sáez-Llorens X. Brain abscess in children. Semin Pediatr Infect Dis. 2003;14:108–14. doi: 10.1053/spid.2003.127227. [DOI] [PubMed] [Google Scholar]
- 106.Maurice-Williams RS. Open evacuation of pus: A satisfactory surgical approach to the problem of brain abscess? J Neurol Neurosurg Psychiatry. 1983;46:697–703. doi: 10.1136/jnnp.46.8.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maurice-Williams RS. Experience with “open evacuation of pus” in the treatment of intracerebral abscess. Br J Neurosurg. 1987;1:343–51. doi: 10.3109/02688698709023776. [DOI] [PubMed] [Google Scholar]
- 108.Rosenblum ML, Mampalam TJ, Pons VG. Controversies in the management of brain abscesses. Clin Neurosurg. 1986;33:603–32. [PubMed] [Google Scholar]
- 109.Jooma OV, Pennybacker JB, Tutton GK. Brain abscess: Aspiration, drainage, or excision? J Neurol Neurosurg Psychiatry. 1951;14:308–13. doi: 10.1136/jnnp.14.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mut M, Hazer B, Narin F, Akalan N, Ozgen T. Aspiration or capsule excision? Analysis of treatment results for brain abscesses at single institute. Turk Neurosurg. 2009;19:36–41. [PubMed] [Google Scholar]
- 111.Sarmast A, Showkat H, Kirmani A, Bhat A, Patloo A, Ahmad S, et al. Aspiration versus excision: A single center experience of Forty-Seven patients with brain abscess over 10 years. Neurol Med Chir (Tokyo) 2012;52:724–30. doi: 10.2176/nmc.52.724. [DOI] [PubMed] [Google Scholar]
- 112.Tan WM, Adnan JS, Mohamad Haspani MS. Treatment outcome of superficial cerebral abscess: An analysis of two surgical methods. Malays J Med Sci. 2010;17:23–9. [PMC free article] [PubMed] [Google Scholar]
- 113.Sharma R. Fungal infections of the nervous system: Current perspective and controversies in management. Int J Surg. 2010;8:591–601. doi: 10.1016/j.ijsu.2010.07.293. [DOI] [PubMed] [Google Scholar]
- 114.Lee JC, Lim DJ, Ha SK, Kim SD, Kim SH. Fatal case of cerebral aspergillosis: A case report and literature review. J Korean Neurosurg Soc. 2012;52:420–2. doi: 10.3340/jkns.2012.52.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsou TP, Lee PI, Lu CY, Chang LY, Huang LM, Chen JM, et al. Microbiology and epidemiology of brain abscess and subdural empyema in a medical center: A 10-year experience. J Microbiol Immunol Infect. 2009;42:405–12. [PubMed] [Google Scholar]
- 116.Tseng JH, Tseng MY. Brain abscess in 142 patients: Factors influencing outcome and mortality. Surg Neurol. 2006;65:557–62. doi: 10.1016/j.surneu.2005.09.029. [DOI] [PubMed] [Google Scholar]


