Abstract
Allogeneic hematopoietic stem cell transplantation (alloSCT) is a potentially curative therapy for many hematologic and immunologic diseases. Further, partial or full donor hematopoietic chimerism following alloSCT may be sufficient to guarantee immunologic tolerance to solid organs from the same donor, obviating any requirement for prolonged pharmacologic immunosuppression. Despite alloSCT’s potential, the procedure is beset by two major limitations. The first relates to the procedure’s toxicity including conditioning regimen toxicity, graft-versus-host disease (GVHD), and infection. The second limitation is the lack of histocompatible donors. A human leukocyte antigen (HLA)-matched sibling or unrelated donor cannot be identified expeditiously for up to 40% of patients. Historically, alloSCT from partially HLA-mismatched or HLA-haploidentical relatives has been complicated by unacceptably high incidences of graft rejection, severe GVHD, and non-relapse mortality. Recently, our groups have developed a method to selectively deplete alloreactive cells in vivo by administering high doses of cyclophosphamide in a narrow window after transplantation. Using high-dose, post-transplantation cyclophosphamide (PT/Cy), crossing the HLA barrier in alloSCT is now feasible and donors can be found for nearly all patients. This review discusses the history of HLA-haploidentical SCT, recent clinical results and immunologic mechanisms of action of high-dose PT/Cy for prevention of graft rejection and GVHD.
Graft failure, graft-versus-host disease, and infection: the Devil’s Triangle of HLA-haploidentical stem cell transplantation
HLA-haploidentical SCT refers to the situation where a donor and recipient have inherited the same set of HLA-alleles on one chromosome 6, but differ at one or more HLA loci on the unshared chromosome 6. When high resolution typing is performed for HLA-A, -B, -C, -DRB1, and –DQB1, HLA-haploidentical first degree relatives differ in the expression of one to five HLA genes. The fundamental clinical obstacle to crossing the HLA barrier in hematopoietic stem cell transplantation (HSCT) arises from intense, bi-directional responses from T cells responding to allogeneic HLA molecules resulting in unacceptably high incidences of graft rejection or graft-versus-host disease. The initial attempts at HLA-haploidentical HSCT employed lethal conditioning and transplantation of T cell-replete bone marrow.1-4 These reports documented high incidences of graft failure and GVHD, and twelve of thirty five patients transplanted by Powles et al.4 died from a syndrome of pulmonary edema with or without fever, fluid retention, and renal failure, similar to what has now been described as “engraftment syndrome” following autologous or allogeneic HSCT.5 Although there were a few long-term survivors in these studies, most patients died from either graft failure, GVHD, or overwhelming infections. Importantly, Beatty et al. found increasing HLA mismatch between donor and recipient was associated with worse overall survival.1 Although a single HLA antigen mismatch between donor and recipient was tolerated without a compromise in survival compared to HLA –matched sib transplants, long-term survival was <10% when donor and recipient differed by more than one HLA antigen. Subsequent studies confirmed the poor outcomes of transplants using related donors mismatched for 2 or 3 HLA antigens, although such transplants were associated with a decreased risk of relapse for patients with advanced, poor risk hematologic malignancies. 6 After it was discovered that T cell depletion of the donor graft prevents GVHD after alloSCT in mice, there was substantial interest in preventing GVHD after HLA-haploidentical SCT using ex vivo T cell depletion.7 While this technique was indeed successful in reducing the incidence and severity of GVHD, there was a compensatory increase in the risk of graft failure and disease relapse.8 To lower the risk of graft failure after T cell-depleted, HLA-haploidentical HSCT, Aversa and colleagues employed intensive conditioning of the recipient including total body irradiation, thiotepa, fludarabine, and anti-thymocyte globulin.9 T cells were depleted from donor by soybean agglutination and E-rosetting,9 and later by positive selection of CD34+ cells using magnetic columns.10 Using intensive conditioning of the recipient and rigorous T cell depletion of the donor graft, nearly all patients achieved sustained engraftment of donor cells, and the cumulative incidences of acute and chronic GVHD were both <10%. However, non-relapse mortality ranged between 35–40%, mostly from infection. Interestingly, the loss of a T cell-mediated “graft-versus-leukemia”, or GVL effect, was compensated for by an anti-leukemia effect of alloreactive natural killer cells.11 Another trial employed intensive conditioning and transplantation of HLA-haploidentical bone marrow depleted of T cells ex vivo using antibodies. While engraftment occurred in 98% of 201 patients and the incidence of grades II-IV acute GVHD was only 13%, the 5-year cumulative incidence of non-relapse mortality was 51%, with the majority of deaths occurring from infection (n=45) or organ toxicity (n=34).12 These results of HLA-haploidentical HSCT, obtained over three decades, illustrate that T cell alloreactivity is the major barrier to success. If donor T cells are left in the graft unaltered, then morbidity and mortality from GVHD is unacceptably high. If donor T cells are removed from the graft, then graft failure is too frequent. If the recipient is lymphodepleted through intense conditioning and then given a T cell-depleted graft, then immune reconstitution is poor and mortality is high from infection and organ toxicity. There are only two ways out of this morass. First, one could supplement a T cell-depleted graft with non-alloreactive T cells to provide effective immune reconstitution. Alternatively, one could give a graft selectively depleted of alloreactive T cells, or deplete them selectively in vivo. Examples of the former strategy include supplementing T cell-depleted grafts with pathogen specific13 or allodepleted T cells,14 or infusing regulatory T cells along with conventional T cells to suppress the alloreactive population.15,16 Examples of the latter strategy include induction of anergy in alloreactive T cells via costimulatory blockade17, or selective in vivo allodepletion using high dose, post-transplantation cyclophosphamide. Many of these strategies have been reviewed recently, and will not be discussed here.18 We will focus on in vivo tolerance induction with high-dose post-transplantation cyclophosphamide.19
Cyclophosphamide-induced immunologic tolerance
High-dose, post-transplantation cyclophosphamide (PT/Cy) is an attractive approach for crossing the HLA barrier in allogeneic HSCT because the treatment is cheap, strikingly effective, and requires no special expertise beyond IV chemotherapy administration. Still, the use of PT/Cy in the clinic represents the culmination of over 50 years of painstaking research dedicated to inducing a state of immunologic tolerance in the adult. Cyclophosphamide-induced tolerance is an example of the larger phenomenon of drug-induced immunologic tolerance, first demonstrated in 1959 by Schwartz and Dameshek.20 These investigators showed that rabbits developed an immunologic mechanism to clear human serum albumin (HSA) from their blood following repeated IV injections of the protein, and that this mechanism could be disabled in naïve rabbits by injecting them with HSA and then with daily injections of 6-mercaptopurine for two weeks. Since rabbits make antibodies to an injected foreign protein, and since those antibodies enhance clearance of the protein, Schwartz and Dameshek effectively showed that antigen followed by 6-MP disabled the mechanism of antibody formation. In 1963, Berenbaum and Brown were the first to study the effect of cyclophosphamide on the allograft response.21 Cyclophosphamide 200 mg/kg intraperitoneally was given in a single dose to mice at defined intervals before or after placement of a major histocompatibility complex (MHC)-mismatched skin graft. Cy was chosen for this experiment because it was found to have the highest therapeutic index of several drugs tested for suppression of antibody responses in rats.22 While control mice rejected the allogeneic skin after a mean of 14.1 days, mice treated with cyclophosphamide exhibited delayed skin graft rejection. Interestingly, the drug was found to be maximally effective in prolonging graft survival if given any time from shortly after grafting to about the 4th day afterwards. Median graft survival ranged from 19.9–23.5 days if a single dose of Cy was given between days 0 to 4, 14.4–17.7 days if given between days −4 to 0, and 16.8 days if given on day 6. These results suggested that the effect of Cy in prolonging skin graft survival was not solely due to global, antigen non-specific immunosuppression, and was dependent upon the interval between grafting and drug administration. While Cy delayed but did not completely prevent rejection of MHC-mismatched grafts, it completely prevented the production of lytic antibodies to sheep red blood cells (SRBCs) in mice given SRBCs followed by four days of Cy. 23 According to the study authors, it seemed plausible that “the metabolic alterations preparatory to cell division render the antigen-stimulated, haemolysin-producing cells particularly susceptible to cyclophosphamide”; in other words, Cy is selectively toxic to dividing cells.
These early studies of Cy-induced tolerance (or also called “cells-followed-by-Cy system”) triggered substantial interest in using this method to achieve immunologic tolerance of transplanted solid organs. Unfortunately, as mentioned above, MHC-mismatched skin transplantation followed by Cy administration failed to induce complete tolerance. However, it is possible to induce tolerance to MHC-matched grafts that differed only in expression of non-MHC, “minor” histocompatibility antigens.24,25 One of the first observations in transplantation immunology was that natural26 or induced27,28 hematopoietic chimeras are tolerant of solid organ grafts from the same donor. In light of these observations, transplantation immunologists became interested in developing simple, relatively non-toxic methods of inducing durable hematopoietic chimerism as a precursor to solid organ transplantation without maintenance immunosuppression. Several of these methods incorporated PT/Cy. The simplest of these methods, which works for induction of tolerance to minor but not to major histocompatibility antigens, consists of the intravenous injection of a large number of allogeneic donor spleen cells followed in 48–72 hours by the administration of high-dose cyclophosphamide. This protocol of cyclophosphamide-induced immunologic tolerance to minor histocompatibility antigens has been developed and studied extensively by Hisanori Mayumi, Kikuo Nomoto, and colleagues.29 The requirements for tolerance in this model system include: 1) no MHC incompatibility between allogeneic donor and recipient; 2) IV injection of ≥50 million donor spleen cells, roughly equivalent to 2.5 × 109 nucleated cells/kg in the human; and 3) administration of Cy ≥150 mg/kg IP between 48 and 72 hours after cell infusion. The timing of drug administration was critical to the achievement of tolerance, as giving cyclophosphamide either 24 hours or 96 hours after cell infusion failed to induce tolerance of allogeneic cells. Cyclophosphamide-induced tolerance was blocked in animals concomitantly treated with the calcineurin inhibitor cyclosporine A,30 which blocks antigen-driven T cell proliferation. This result provides support for the idea that antigen-driven proliferation sensitizes a T cell to be killed by Cy. Tolerance could not be induced if the recipient had been immunized previously to donor cells. When tolerance was induced by giving Cy two days after the infusion of cells from a donor that had been previously immunized against cells of the recipient, full donor chimerism and lethal graft-versus-host disease ensued.29 This result reinforces the observation that Cy can only induce transplantation tolerance in a narrow window of time after first antigen exposure
While the above studies are relevant for MHC-matched donor-recipient pairs of mice, crossing the MHC barrier using PT/Cy represented a substantial challenge. The “cells followed by Cy” approach can induce tolerance to MHC-incompatible cardiac allografts31 but not skin grafts,32 and cannot induce hematopoietic chimerism in MHC-mismatched donor-recipient combinations. Mayumi and Good achieved durable chimerism in several mismatched strain combinations using intravenous or intraperitoneal administration of 50-100 micrograms of anti-Thy-1.2 mAb on day -1, intravenous injection of 90 million allogeneic spleen cells mixed with 30 million allogeneic bone marrow cells from the same donor on day 0, and intraperitoneal injection of 200 mg/kg CP on day 2.33 Thus, some form of pre-transplantation conditioning, likely to decrease the number of MHC-alloreactive T cells, is required to achieve hematopoietic chimerism across the MHC barrier. To better understand how to employ PT/Cy in the clinic, particularly its potentials and limitations, and how to integrate with pre-transplant conditioning across MHC-mismatched barriers it is important to review the knowledge and emerging studies on mechanistic studies of Cy-induced tolerance.
Primer on mechanistic studies of Cy-induced tolerance
It is likely that a number of mechanisms contribute to the establishment of bi-directional tolerance by PT/Cy, and that this multistep process proceeds through several distinct and sequential phases, as illustrated in Figure 1. The first step includes selective killing of proliferating alloantigen-stimulated T cells. This mechanism of Cy action on alloaggressive T cells should be termed “clonal destruction” to distinguish it from the intrathymic clonal T cell deletion discussed below-namely, shortly after allografting, alloreactive T cells are primed in the secondary lymphoid organs during the process of bi-directional in vivo “allogenic mixed lymphocyte reaction.” Given that T cells undergoing replicative DNA synthesis are uniquely sensitive to Cy, both anti-host and anti-donor T cells are selectively destroyed (Fig. 1A). This was originally revealed by analyses of T cell receptor (TCR) Vβ subunits that recognize endogenous superantigens in disparate murine allo-combinations.34 Further analysis using specific Vβ TCR probes have shown that the clonal destruction of alloaggressive T cells occurs within the first few days after Cy administration, suggesting that this approach clearly differs from other strategies used for tolerance induction where the targeted Vβ deletion occurs more gradually.35 Thus, in the setting of the large burden of alloreactive T cells, such as seen when HLA barriers are transgressed, the cytotoxic effect of Cy may be advantageous over other noncytotoxic approaches in promoting tolerance induction. Given that Cy also induces apoptosis of resting T cells,36 this “induction phase” of Cy-induced tolerance is characterized by both selective allodepletion and non-specific T cell killing. The overall level of T cell depletion after PT/Cy administration is unknown and likely influenced by extrinsic variables such as individual variation of the drug metabolism and intrinsic T cell characteristics. While there is no data on the relation between the pharmacokinetics of Cy and T cell depletion, several lines of evidence support the differential sensitivity of naïve T cells (Tn) versus effector (Teffs)/memory T cells to Cy-mediated killing. We have found that Tn while abundant in donor grafts are nearly absent early after alloBMT with PT/Cy. (L. Luznik and E. Fuchs, unpublished observations) The relative resistance of donor Teffs/memory T cells to PT/Cy has been demonstrated in mouse models where donor pre-immunization against a recipient’s alloantigens abrogates the protection from GHVD otherwise afforded by PT/Cy. From a clinical perspective, this is relevant since the persistence of donor non-alloreactive T cells can provide the transplant recipient with donor-derived immunity to fight infections. These cells can also contribute to the overall reconstitution of peripheral T cell pools and immune competence in the long term, a process that is important given the slow recovery of thymic and T cell function post-transplant.
Figure 1. Proposed mechanism and stages for the induction of transplantation tolerance by high-dose cyclophosphamide.
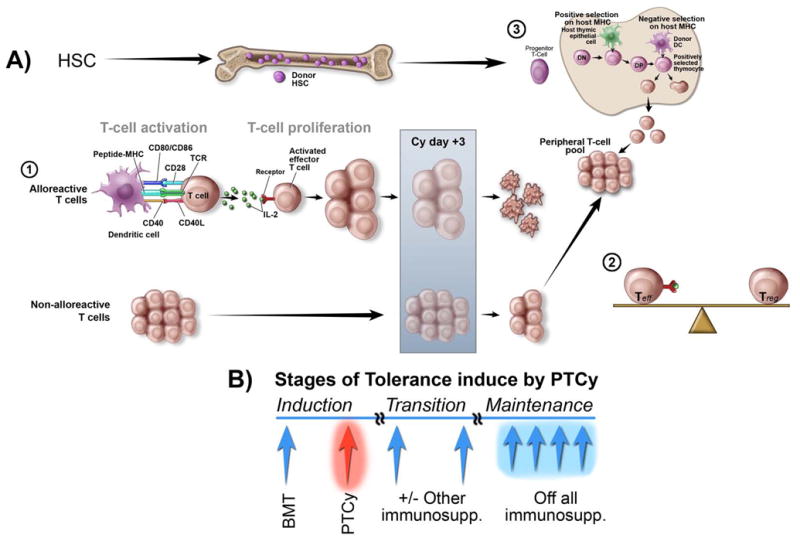
A) Mechanisms of tolerance induction with PT/Cy. The promotion of tolerance through induction of donor hematopoietic chimerism with PT/Cy can be viewed as a three-step process. The first step occurs after alloreactive T cells undergo proliferation. The replicative DNA synthesis renders alloreactive proliferating T cells uniquely sensitive to Cy. Both anti-host and anti-donor T cells are selectively destroyed with PT/Cy administered on day +3. The persisting donor T cells contribute to a peripheral T cell pool whose composition of effector and regulatory T cells determines the post-transplant clinical outcomes. After transplantation, the graft goes through a transition during which the alloreactive and regulatory forces come into balance. The third step is characterized by intrathymic clonal deletion of donor-HSC-derived, anti-host T cells in the thymus. The de novo thymically-derived T cells also contribute to the peripheral T cell pool and influence the clinical outcomes, although their contribution may be delayed for months to years after allografting depending on the species and age of the host. B) Sequential stages of tolerance induced by PT/Cy. The “induction phase” refers to the period characterized by the PT/Cy-induced clonal destruction of alloreactive T cells. Given that the degree of clonal destruction with Cy is dependent on the size of the alloreactive T cell burden, and likely incomplete especially in an HLA-mismatched setting, there is a transition phase and a need for constraining the persistent non-deleted alloagressive T cell clones. We hypothesize that the metastable phase during which the alloreactive and regulatory forces come into balance and central tolerance becomes operational precedes development of the full tolerance. In the clinic, this is a period dominated by relevant clinical outcomes such as delayed rejection or GVHD, and these responses are controlled with the use of other immunosuppressive drugs after PT/Cy. How these drugs influence immunoregulatory mechanisms and whether rejection or GVHD represent the failure to establish the balance between alloagressive effector T cells and Tregs needs to be determined. The ultimate development of tolerance and the establishment of mechanisms that maintain this process results in the withdrawal of all immunosuppressants. HSC (hematopoietic stem cells). Teff (Effector T cells). Treg (Regulatory T cells).
The development of peripheral tolerance represents the second step in the evolution of Cy-induced tolerance. Several mechanisms ranging from clonal deletion, and anergy, to regulatory T cell (Treg)-mediated suppression and infectious tolerance had been implicated in the establishment of peripheral tolerance.37 Clonal destruction of alloreactive Teffs discussed above is likely the dominant mechanism of peripheral tolerance induced by PT/Cy; however, given that clonal deletion is likely not complete and that CD4+Tregs have an important role in tolerogenesis induced by a variety of strategies,38 it is likely that suppressive/regulatory mechanisms contribute to the Cy-induced tolerance as well. The need for actively constraining the persistent non-deleted alloagressive T cell clones may be especially relevant in the MHC-mismatched setting since the size of the alloreactive T cell burden is at least 10–100 fold higher. The relevance of Tregs after PT/Cy was examined in several experimental models by Hisanori Mayumi, Kikuo Nomoto, and colleagues.33,39,40 In the MHC-mismatched setting Mayumi and Good examined the role of Tregs using a model that relies on the permanent acceptance of donor skin grafts in animals treated with PT/Cy. Using the adoptive transfer, they first examined for the presence of regulatory mechanisms early after allografting (2 weeks) and showed that administration of naïve recipient splenocytes leads to the rapid rejection of donor skin grafts in chimeras tolerized with PT/Cy.33 In the same model, adoptive transfer of splenocytes from 2-week old “tolerant” chimeras to naïve recipients did not influence the rejection of donor skin allografts. These experiments suggest that Cy-tolerance in early phases can be readily abrogated by naïve donor T cells and that it is not transferable. They concluded that the main mechanism early after PT/Cy is the reduction of effector T cells rather than any form of suppression. However, subsequent studies by the same group demonstrated that adoptive transfer of spleen cells from 10–week old chimeras to naïve recipients results in permanent acceptance of skin allografts, an effect that can be abrogated by the concomitant CD4+ T cell depletion, indicating that CD4+Tregs were responsible for the effect.39 From these studies, it can be concluded that despite the dominance of cytotoxic depletion of alloreactive Teffs there is a role for Tregs but their contribution to tolerance induction with PT/Cy needs to be further defined. Preliminary studies with transgenic mice in whom Foxp3+ Tregs can be selectively depleted suggest that their absence abrogates GVHD protection provided by posttransplant Cy.41 This conclusion is consistent with recent observations that Foxp3 Tregs are critical for tolerance induction in MHC-matched and mismatched models using anti-T cell antibodies and co-stimulatory blockade.42
The third step in the process of Cy-induced tolerance includes central deletion of donor-HSC-derived anti-host T cells in the thymus. This mechanism whose advantage is that it can’t be broken with TLR-ligation and/or infections is essential for maintaining lifelong tolerance after allografting. The existence of intrathymic clonal deletion after PT/Cy was also confirmed by using superantigen-disparate murine allo-combinations,43 a well-studied system to explain self-tolerance. As depicted in Figure 1, stable donor HSC engraftment in the recipient leads to the establishment of intrathymic chimerism through repopulation of the thymus with donor HSC-derived DCs.Their presence is crucial for the T cell education where they promote intrathymic clonal deletion of any newly developing donor HSC-derived T cells that recognize donor antigens. Thus, consistent with other strategies, PT/Cy following tolerization of the peripheral T cell repertoire promotes the establishment of long-term tolerance that is maintained by intrathymic deletion. The T cells emerging from the thymus, together with those initially transferred with a graft, also contribute to the peripheral T cell pools and through overall Treg/Teff balance, shape clinical outcomes posttransplant (Figure 1).
Schema depicted on Figure 1B summarizes sequential processes and tolerogenic mechanisms induced by PT/Cy and challenges of the approach for the clinical translation. Since the degree of clonal destruction with Cy in “induction phase” is likely incomplete in an MHC-mismatched setting, there is a need for constraining the persistent non-deleted alloagressive HVG and GVH T cell clones. We hypothesize that the “transition phase” during which the alloreactive and regulatory forces come into balance and central tolerance becomes operational precedes development of the full tolerance. These responses may be controlled by use of other immunosuppressive drugs after PT/Cy. The ultimate development of tolerance and the establishment of mechanisms that maintain this process should result in the withdrawal of all immunosuppressants.
Translational studies of Cy-induced tolerance
Crossing the MHC barrier and integrating PT/Cy in clinically relevant models of alloSCT represented a substantial challenge. As discussed above, the “cells followed by Cy” approach can induce tolerance to MHC-incompatible cardiac allografts but not skin grafts, and cannot induce hematopoietic chimerism in MHC-mismatched donor-recipient combinations without pre-transplantation conditioning.31-33 However, PT/Cy is still useful as it reduces the intensity of conditioning required for induction of tolerance and chimerism. For example, PT/Cy lowers the dose of total body irradiation required for sustained donor hematopoietic chimerism from 500 cGy to 100 cGy in MHC-matched donor-recipient pairs,44 and from 700 cGy to 500 cGy in MHC-mismatched donor-recipient pairs, respectively.45 The amount of TBI required for durable chimerism with MHC-mismatched cells can be reduced even further by adding an immunosuppressive agent to the conditioning regimen as shown by Mayumi and Good and discussed above.33 Although this protocol eliminated the requirement for any TBI, the cell dose requirement, roughly 6 × 109 cells/kg in humans, is not clinically feasible. Ildstad and colleagues were able to lower the dose of TBI from 500 cGy to 300 cGy by adding anti-lymphocyte globulin to conditioning prior to transplantation of 10 million marrow cells and PT/Cy.46 Our group was able to achieve tolerance and multi-lineage mixed hematopoietic chimerism across MHC-barriers in mice conditioned with fludarabine, 200 cGy TBI, transplanted with 10 million marrow cells, and given Cy 200 mg/kg IP on day 2. This regimen was truly nonmyeloablative as autologous hematopoiesis recovered in mice that were conditioned but did not receive an infusion of marrow.47 In addition to suppressing graft rejection in sublethally conditioned mice, PT/Cy also inhibited GVHD in lethally irradiated mice given MHC-mismatched bone marrow plus a high dose of donor T cells. The ability to suppress both graft rejection and GVHD makes PT/Cy an attractive addition to standard pharmacologic immunosuppression after HLA-haploidentical BMT, with the proviso that to avoid blocking Cy-induced tolerance, therapy with a calcineurin inhibitor should be delayed until the completion of PT/Cy.30
Cy-induced tolerance: Clinical studies
Based on our promising pre-clinical results, a Phase I/II clinical trial of haploidentical BMT to treat high-risk hematologic malignancies was initiated in 1999. Conditioning was based on the nonablative regimen of fludarabine (Flu) and low-dose TBI developed by Storb and co-workers in Seattle.48 GvHD prophylaxis consisted of cyclophosphamide (Cy) given on day +3 post-transplant, a calcineurin-inhibitor, tacrolimus, and mycophenolic acid mofetil (MMF). We were concerned that inadequate immunosuppressive activity of the conditioning regimen could potentially increase the risk of graft rejection so our strategy was to increase the dose of Flu from 90 mg/m2 to 150 mg/m2 and to introduce Cy pre-transplant, if needed, in a dose-escalating manner using a Bayesian continual reassessment model. Two of the first three patients transplanted after conditioning without Cy rejected their grafts. Thus, Cy was added to the conditioning regimen according to the Bayesian model at a total dose of 29 mg/kg given on days -5 and -6. Engraftment was achieved in 8 of the next 10 patients so the Cy dose was adopted for further accrual on the Phase II portion of the trial. Autologous hematopoietic recovery occurred in 3 of 4 patients who rejected their grafts. Acute GvHD occurred in 6 of 8 engrafted patients on the Phase II portion of the trial and responded to therapy in 5 of the patients. Outcomes of the first 13 patients were published as a proof-of-principle in 2002.49
Over the next several years, Phase II trials of haploidentical BMT using post-transplant Cy were continued at our transplant centers in Baltimore and Seattle. Outcomes of the first 68 patients were published jointly in 2008.50 In the interim, a modification was made to the Baltimore regimen by increasing the total dose of post-transplant Cy to 100 mg/kg given over days +3 and +4 with the intention of decreasing the incidence of GvHD. Hematopoietic recovery occurred with median times to neutrophil (>500/mcL) and platelet recovery (>20,000/mcL) of 15 and 24 days, respectively. Primary graft failure occurred in 13% of patients, and was fatal due to infection in one in which autologous hematopoiesis failed to occur. In general, complete T-cell engraftment was observed by day +28 or the grafts were rejected. Cumulative incidences of grades II-IV and grades III-IV acute GVHD by day 200 were 34% and 6%, respectively. There was a trend toward a lower incidence of extensive chronic GVHD among recipients of two versus one dose of post-transplantation Cy (5% versus 25%; p=.05). There was no difference in the incidence of severe acute GvHD between one or two doses of post-transplant Cy. The cumulative incidences of non-relapse mortality and relapse at 1 year were 15% and 51%, respectively. Actuarial overall and event-free survivals (EFS) at two years after transplantation were 36% and 26%, respectively. Patients with lymphoid malignancies appeared to have an improved EFS compared to those with myeloid malignancies (p=.02).
Following these encouraging results, the Bone Marrow Transplant Clinical Trials Network (BMT CTN) sponsored a multi-center Phase II trial of haploidentical BMT (CTN 0603) for high-risk hematologic malignancies after reduced intensity conditioning (RIC) which was run in parallel with a Phase II trial (CTN 0604) of RIC and transplantation of two units of unrelated umbilical cord blood (dUCB) as the donor source. RIC consisted of Flu, Cy and 2 Gy TBI although the dosing of Flu and Cy differed slightly between the trials. Eligibility criteria were the same. The endpoint for both trials was overall survival at 6 months which was compared to historical survival data of 60% after RIC and transplantation using HLA-matched, unrelated donors reported in a retrospective CIBMTR study.51 Accrual was rapid and the outcomes of the two trials were published jointly in 2011.52 In each trial, survival at 6 months exceeded the 60% reported for unrelated donors. The 1-year probabilities of overall and progression-free survival were 54% and 46% after dUCB transplantation and 62% and 48% after haploidentical bone marrow. The day +56 cumulative incidence of neutrophil recovery was 94% after dUCB and 96% after Haplo-marrow. The 100-day cumulative incidence of grade II-IV acute GVHD was 40% after dUCB and 32% after haploidentical bone marrow. The 1-year cumulative incidences of non-relapse mortality and relapse after dUCB transplantation were 24% and 31%, respectively with corresponding results of 7% and 45% after haploidentical bone marrow. These multi-center studies confirmed the utility of dUCB and haploidentical bone marrow as alternative donor sources and established equipoise for an ongoing multi-center randomized clinical trial to assess the relative efficacy of these two transplant strategies (CTN 1101).
Post-transplant Cy as GvHD prophylaxis was developed initially for haploidentical bone marrow transplants after RIC but more recently several small studies have extended the approach to myeloablative conditioning and to peripheral blood stem cells (PBSC) as the graft source. Most of these studies have been preliminary and reported in abstract form at national meetings but the results have been very promising. One study of myeloablative haploidentical transplants was published recently by the group in Philadelphia which was a novel adaptation of Cy-induced tolerance.53 Pre-transplant conditioning consisted of 12 Gy of fractionated TBI administered on days -9 to -6 followed sequentially by infusion of a fixed dose of haploidentical donor lymphocytes (2 × 108 CD3/kg) that had not been mobilized by G-CSF on day -6 after which a total dose of 120 mg/kg Cy was given on days -3 and -2. On day 0, patients underwent transplantation of a PBSC allograft that had undergone immunomagnetic selection for CD34 and against CD3 using an Isolex 300i system. The goals of their approach in the setting of haploidentical transplantation were (i) myeloablative conditioning, (ii) circumvent the theoretical problem of exposing stem cells to the alkylating agent Cy post-transplant in the regimen as originally developed, (iii) use of PBSC instead of BM and (iv) use a fixed dose of T-cells that was not skewed to a TH-2 phenotype. Outcomes reported for 27 patients were impressive. Engraftment kinetics of neutrophils and platelets were typical for after transplantation of PBSCT (median of 12 d) and slightly delayed for platelets (median of 21 d). There were only two primary graft failures in patients who were found to be alloimmunized against donor HLA antigens, now recognized as a major risk factor for rejection in haploidentical transplants.54,55 At one year post-transplant, cumulative incidences of NRM (22%), grades II-IV (59%) and grades III/IV (7%) acute GvHD were quite acceptable and similar to outcomes after myeloablative transplants from matched donors. Chronic GvHD reportedly was infrequent. About half of the patients had chemotherapy-resistant disease. Survival of these patients was only 27% at 3 years but survival of patients without disease at the time of transplant was 75%. Importantly, deaths from infection were low despite a high incidence of CMV reactivation.
Using the approach of post-transplant Cy for haploidentical transplantation, two studies of myeloablative conditioning were reported at the ASH meeting in 2011. In the study by Symons et al.56, 27 patients received bone marrow transplants after conditioning with conventional BuCy the majority of which were not in remission at the time of transplant. Not unexpectedly, relapse at 1 year was 66% (EFS of 23.5%) but NRM was low (12%). Incidences of severe acute GvHD (7%) and chronic GvHD (13%) were also remarkably low. Sizemore et al.57 studied 20 better risk patients using the conditioning regimen of BuFlu and PBSC as the graft source instead of BM. Higher rates of severe acute GvHD (20%) and chronic GvHD (42%) were observed but NRM at 1 year was low (10%). EFS at 1 year was 51% for all patients and 76% for low risk patients which was encouraging.
A different approach to myeloablative bone marrow transplantation from haploidentical donors with post-transplant Cy was taken by the Seattle group in which an anti-CD45 antibody radiolabeled with 131I was added to the conditioning regimen of FluCy 2 Gy TBI for very high-risk patients with relapsed or refractory leukemia. Following a dosimetry dose to determine biodistribution, a treatment dose of radiolabled antibody was administered on day -14. The median dose of radiolabeled antibody to bone marrow was 9 Gy rendering the conditioning regimen myeloablative. Preliminary results of 7 patients including 2 patients with blast phase CML were reported recently.58 All patients were fully engrafted with donor-derived T-cells and granulocytes by day +28. Three patients were alive at a median follow-up of one year including two patients with blast phase CML. One of the surviving CML patients with no prior history of acute or chronic GvHD relapsed in myeloid blast phase about one year post-transplant. At the time of relapse, HLA-typing of the leukemia showed that the leukemia had undergone mutation to uniparental disomy of the HLA region presumably rendering the leukemia resistant to any GvL effect mediated by T-cells of the haploidentical donor, an observation reported by other groups studying haploidentical HCT.59,60
To date, haploidentical nonablative transplantation with post-transplant Cy has used bone marrow as the graft source. Use of PBSC instead of marrow for haploidentical transplantation may allow wider applicability of this approach but there has been concern about risk of acute and chronic GvHD due to the 5-10-fold higher number of T-cells in the allograft. Recently, groups in Houston and Seattle/London have reported small studies in which PBSC were substituted for bone marrow.61,62 Conditioning used by the Houston or Seattle/London groups was FluMelTT or FluCy 2 GyTBI, respectively. In both studies, the incidences of severe acute GvHD (11% vs. 6%), chronic GvHD (8% vs. not observed) and NRM at 1 year (16% for both) were low suggesting that PBSC can be substituted for marrow as the donor source in haploidentical transplantation with outcomes that are similar to those observed after transplantation of haploidentical bone marrow.
Given the success of Cy-induced tolerance in the setting of haploidentical transplantation, Luznik et al.63 reported a large study of patients who underwent allogeneic BMT from HLA-matched donors after myeloablative conditioning using BuCy and post-transplant Cy as a single agent for prophylaxis of GvHD. A total of 117 patients with high-risk hematologic malignancies were transplanted from 78 related or 39 unrelated donors. Half of the patients were not in remission at the time of transplant. The incidence of GvHD was remarkably low (40% grades II-IV acute, 10% grades III/IV acute, 10% chronic) showing the effectiveness of the approach. There was no difference in incidence of acute or chronic GvHD between related or unrelated donors. Almost two-thirds of the patients did not require any additional immunosuppressive therapy after post-transplant Cy. Patients who did develop GvHD responded to steroids alone in 20% of cases or steroids plus a second agent (calcineurin or non-calcineurin) in 75% of cases. The graft-versus-tumor effect of the allogeneic transplant was not impaired by post-transplant Cy. EFS at 2 years was 54% for patients in remission and 29% for patients with active disease. This study showed that post-transplant Cy was effective as a single agent to prevent GvHD in the majority of patients undergoing myeloablative transplantation from matched donors. Only 3% of deaths were due to infection suggesting that immune reconstitution is robust in such patients. Obviating the need for ongoing immunosuppression post-transplant provides an optimal platform for cellular therapy to prevent or treat relapsed disease. A second trial of myeloablative conditioning with FluBu, BMT from HLA-matched related or unrelated donors and post-transplant Cy as a single prophylactic agent has been completed with accrual of over 90 patients. Outcomes will be reported as the data mature [Luznik, O’Donnell and de Lima, unpublished]. This study will also include a kinetic analysis of immune reconstitution post-transplant.
A number of ancillary studies accompanying clinical trials of haploidentical BMT with post-transplant Cy have been performed. A retrospective study by Burroughs, et al.,64 compared the outcomes of patients with relapsed Hodgkin lymphoma (92% of whom had failed autologous transplants) who had undergone nonablative conditioning and transplantation with either haploidentical donors or HLA-matched related or unrelated donors in Baltimore and Seattle. GvHD prophylaxis after transplantation with matched donors was a calcineurin inhibitor plus MMF. Although the number of patients in each group was relatively small, it appeared that outcomes after haploidentical transplantation were no worse and possibly better than those after transplantation from matched donors. Two year NRM was similar for haploidentical and unrelated donors (9% and 8%, respectively) compared to 21% for related donors. EFS at 2 years was 51% for haploidentical donors compared to 23% and 29% for related and unrelated donors, respectively. The finding of similar outcomes after haploidentical, matched related or unrelated donors for a variety of hematoloigic malignancies was also reported recently by Bashey, et al.65 in abstract form. Kasamon et al.66 looked at the effect of HLA disparity on transplant outcomes in a retrospective analysis of 185 patients transplanted in Baltimore. No association was found between the degree of mismatching at 5 HLA loci and the risk of acute GvHD or NRM. There was a trend toward improved EFS with increasing HLA disparity which has been confirmed in an analysis of larger numbers of patients (E. Fuchs, unpublished). Symons, et al. examined the potential role of KIR genes in graft-versus-tumor activity in a retrospective study of 86 patients who had undergone haploidentical BMT following RIC.67 EFS and OS were significantly better in the group of patients who were homozygous for the KIR “A” haplotype which encodes only one activating KIR gene and their donor who expressed at least one KIR “B” haplotype which encodes several activating KIR genes. Models that incorporated information from recipient HLA typing, with or without donor HLA typing, were not predictive of outcome in this cohort of patients. Neither the degree of HLA mismatching nor KIR genotype has yet factored into a model for optimal selection of haploidentical donors. In this regard, although potentially relevant in the selection of haploidentical donors, there have been no studies thus far of the effect of non-inherited maternal antigens on outcomes after haploidentical transplants.
Collectively, these clinical data suggest that PT/Cy has been successfully translated from basic and preclinical models into clinical practice. The data to date have been sufficiently encouraging to warrant further exploration and development of this approach in successor trials.
Acknowledgments
Grant support: This work was supported by National Institutes of Health grant P01 CA015396
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beatty PG, Clift RA, Mickelson EM, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985;313:765–71. doi: 10.1056/NEJM198509263131301. [DOI] [PubMed] [Google Scholar]
- 2.Buckner CD, Epstein RB, Rudolph RH, Clift RA, Storb R, Thomas ED. Allogeneic marrow engraftment following whole body irradiation in a patient with leukemia. Blood. 1970;35:741–50. [PubMed] [Google Scholar]
- 3.Clift RA, Hansen JA, Thomas ED, et al. Marrow transplantation from donors other than HLA-identical siblings. Transplantation. 1979;28:235–42. doi: 10.1097/00007890-197909000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Powles RL, Morgenstern GR, Kay HE, et al. Mismatched family donors for bone-marrow transplantation as treatment for acute leukaemia. Lancet. 1983;1:612–5. doi: 10.1016/s0140-6736(83)91793-2. [DOI] [PubMed] [Google Scholar]
- 5.Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:893–8. doi: 10.1038/sj.bmt.1703015. [DOI] [PubMed] [Google Scholar]
- 6.Szydlo R, Goldman JM, Klein JP, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15:1767–77. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]
- 7.Reisner Y, Kapoor N, Kirkpatrick D, et al. Transplantation for acute leukaemia with HLA-A and B nonidentical parental marrow cells fractionated with soybean agglutinin and sheep red blood cells. Lancet. 1981;2:327–31. doi: 10.1016/s0140-6736(81)90647-4. [DOI] [PubMed] [Google Scholar]
- 8.Ash RC, Horowitz MM, Gale RP, et al. Bone marrow transplantation from related donors other than HLA-identical siblings: effect of T cell depletion. Bone Marrow Transplant. 1991;7:443–52. [PubMed] [Google Scholar]
- 9.Aversa F, Tabilio A, Velardi A, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339:1186–93. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 10.Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447–54. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 11.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 12.Mehta J, Singhal S, Gee AP, et al. Bone marrow transplantation from partially HLA-mismatched family donors for acute leukemia: single-center experience of 201 patients. Bone Marrow Transplant. 2004;33:389–96. doi: 10.1038/sj.bmt.1704391. [DOI] [PubMed] [Google Scholar]
- 13.Leen AM, Christin A, Myers GD, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283–92. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amrolia PJ, Muccioli-Casadei G, Huls H, et al. Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood. 2006;108:1797–808. doi: 10.1182/blood-2006-02-001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921–8. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 16.Perruccio K, Topini F, Tosti A, et al. Photodynamic purging of alloreactive T cells for adoptive immunotherapy after haploidentical stem cell transplantation. Blood Cells Mol Dis. 2008;40:76–83. doi: 10.1016/j.bcmd.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 17.Guinan EC, Boussiotis VA, Neuberg D, et al. Transplantation of anergic histoincompatible bone marrow allografts. N Engl J Med. 1999;340:1704–14. doi: 10.1056/NEJM199906033402202. [DOI] [PubMed] [Google Scholar]
- 18.Reisner Y, Hagin D, Martelli MF. Haploidentical hematopoietic transplantation: current status and future perspectives. Blood. 2011;118:6006–17. doi: 10.1182/blood-2011-07-338822. [DOI] [PubMed] [Google Scholar]
- 19.Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res. 47:65–77. doi: 10.1007/s12026-009-8139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz R, Dameshek W. Drug-induced immunological tolerance. Nature. 1959;183:1682–3. doi: 10.1038/1831682a0. [DOI] [PubMed] [Google Scholar]
- 21.Berenbaum MC, Brown IN. Prolongation of Homograft Survival in Mice with Single Doses of Cyclophosphamide. Nature. 1963;200:84. doi: 10.1038/200084a0. [DOI] [PubMed] [Google Scholar]
- 22.Berenbaum MC, Brown IN. Dose-Response Relationships for Agents Inhibiting the Immune Response. Immunology. 1964;7:65–71. [PMC free article] [PubMed] [Google Scholar]
- 23.Aisenberg AC, Wilkes B. Immunological tolerance induced by cyclophosphamide assayed by plaque spleen cell method. Nature. 1967;213:498–9. doi: 10.1038/213498a0. [DOI] [PubMed] [Google Scholar]
- 24.Nirmul G, Severin C, Taub RN. Cyclophosphamide-induced immunologic tolerance to skin homografts. Surg Forum. 1971;22:287–8. [PubMed] [Google Scholar]
- 25.Nirmul G, Severin C, Taub RN. Mechanisms and kinetics of cyclophosphamide-induced specific tolerance to skin allografts in mice. Transplant Proc. 1973;5:675–8. [PubMed] [Google Scholar]
- 26.Owen RD. Immunogenetic Consequences of Vascular Anastomoses between Bovine Twins. Science. 1945;102:400–1. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 27.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–6. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 28.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayumi H, Umesue M, Nomoto K. Cyclophosphamide-induced immunological tolerance: an overview. Immunobiology. 1996;195:129–39. doi: 10.1016/S0171-2985(96)80033-7. [DOI] [PubMed] [Google Scholar]
- 30.Nomoto K, Eto M, Yanaga K, Nishimura Y, Maeda T. Interference with cyclophosphamide-induced skin allograft tolerance by cyclosporin A. J Immunol. 1992;149:2668–74. [PubMed] [Google Scholar]
- 31.Matsuura A, Katsuno M, Suzuki Y, et al. Cyclophosphamide-induced tolerance in fully allogeneic heart transplantation in mice. Cell Immunol. 1994;155:501–7. doi: 10.1006/cimm.1994.1142. [DOI] [PubMed] [Google Scholar]
- 32.Shin T, Mayumi H, Himeno K, Sanui H, Nomoto K. Drug-induced tolerance to allografts in mice. I. Difference between tumor and skin grafts. Transplantation. 1984;37:580–4. doi: 10.1097/00007890-198406000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Mayumi H, Good RA. Long-lasting skin allograft tolerance in adult mice induced across fully allogeneic (multimajor H-2 plus multiminor histocompatibility) antigen barriers by a tolerance-inducing method using cyclophosphamide. J Exp Med. 1989;169:213–38. doi: 10.1084/jem.169.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eto M, Mayumi H, Tomita Y, et al. Specific destruction of host-reactive mature T cells of donor origin prevents graft-versus-host disease in cyclophosphamide-induced tolerant mice. J Immunol. 1991;146:1402–9. [PubMed] [Google Scholar]
- 35.Gibbons C, Sykes M. Manipulating the immune system for anti-tumor responses and transplant tolerance via mixed hematopoietic chimerism. Immunol Rev. 2008;223:334–60. doi: 10.1111/j.1600-065X.2008.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strauss G, Osen W, Debatin KM. Induction of apoptosis and modulation of activation and effector function in T cells by immunosuppressive drugs. Clin Exp Immunol. 2002;128:255–66. doi: 10.1046/j.1365-2249.2002.01777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waldmann H. Tolerance: an overview and perspectives. Nat Rev Nephrol. 2010;6:569–76. doi: 10.1038/nrneph.2010.108. [DOI] [PubMed] [Google Scholar]
- 38.Li XC, Turka LA. An update on regulatory T cells in transplant tolerance and rejection. Nat Rev Nephrol. 2010;6:577–83. doi: 10.1038/nrneph.2010.101. [DOI] [PubMed] [Google Scholar]
- 39.Kong YY, Eto M, Omoto K, Umesue M, Hashimoto A, Nomoto K. Regulatory T cells in maintenance and reversal of peripheral tolerance in vivo. J Immunol. 1996;157:5284–9. [PubMed] [Google Scholar]
- 40.Tomita Y, Mayumi H, Eto M, Nomoto K. Importance of suppressor T cells in cyclophosphamide-induced tolerance to the non-H-2-encoded alloantigens. Is mixed chimerism really required in maintaining a skin allograft tolerance? J Immunol. 1990;144:463–73. [PubMed] [Google Scholar]
- 41.Ganguly S, Fuchs EJ, Radojcic V, Luznik L. Critical Role of CD4+Foxp3+ T cells in GVHD Prevention with High-dose Posttransplantation Cyclophosphamide (Cy) Blood. 2010;116 [Google Scholar]
- 42.Kendal AR, Chen Y, Regateiro FS, et al. Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J Exp Med. 2011;208:2043–53. doi: 10.1084/jem.20110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eto M, Mayumi H, Tomita Y, Yoshikai Y, Nomoto K. Intrathymic clonal deletion of V beta 6+ T cells in cyclophosphamide-induced tolerance to H-2-compatible, Mls- disparate antigens. J Exp Med. 1990;171:97–113. doi: 10.1084/jem.171.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luznik L, Engstrom LW, Iannone R, Fuchs EJ. Posttransplantation cyclophosphamide facilitates engraftment of major histocompatibility complex-identical allogeneic marrow in mice conditioned with low-dose total body irradiation. Biol Blood Marrow Transplant. 2002;8:131–8. doi: 10.1053/bbmt.2002.v8.pm11939602. [DOI] [PubMed] [Google Scholar]
- 45.Colson YL, Wren SM, Schuchert MJ, et al. A nonlethal conditioning approach to achieve durable multilineage mixed chimerism and tolerance across major, minor, and hematopoietic histocompatibility barriers. J Immunol. 1995;155:4179–88. [PubMed] [Google Scholar]
- 46.Colson YL, Li H, Boggs SS, Patrene KD, Johnson PC, Ildstad ST. Durable mixed allogeneic chimerism and tolerance by a nonlethal radiation-based cytoreductive approach. J Immunol. 1996;157:2820–9. [PubMed] [Google Scholar]
- 47.Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98:3456–64. doi: 10.1182/blood.v98.12.3456. [DOI] [PubMed] [Google Scholar]
- 48.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 49.O'Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8:377–86. doi: 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- 50.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–50. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giralt S, Logan B, Rizzo D, et al. Reduced-intensity conditioning for unrelated donor progenitor cell transplantation: long-term follow-up of the first 285 reported to the national marrow donor program. Biol Blood Marrow Transplant. 2007;13:844–52. doi: 10.1016/j.bbmt.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 52.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA- mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 118:282–8. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grosso D, Carabasi M, Filicko-O'Hara J, et al. A 2-step approach to myeloablative haploidentical stem cell transplantation: a phase 1/2 trial performed with optimized T-cell dosing. Blood. 2011;118:4732–9. doi: 10.1182/blood-2011-07-365338. [DOI] [PubMed] [Google Scholar]
- 54.Anasetti C, Amos D, Beatty PG, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320:197–204. doi: 10.1056/NEJM198901263200401. [DOI] [PubMed] [Google Scholar]
- 55.Ciurea SO, Thall PF, Wang X, et al. Donor-specific anti-HLA Abs and graft failure in matched unrelated donor hematopoietic stem cell transplantation. Blood. 2011;118:5957–64. doi: 10.1182/blood-2011-06-362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Symons H, Chen AR, Luznik L, Kasamon YL, Meade JB, Jones RJ, et al. Myeloablative Haploidentical Bone Marrow Transplantation Using T-cell Replete Peripheral Blood Stem cells and Nyeloablative Conditioning in Patients with High Risk Hematologic Malignancies who Lack Conventional Donors is Welll Tolerated and Produces Excellent Relapse-Free Survival: Results of a Prospective Phase II Trial. Blood. 2011;118:4151. doi: 10.1016/j.bbmt.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 57.Sizemore CABA, Sanacore M, Manion K, Holland HK, Morris LE, et al. Haploidentical Transplantation Using T-Cell Replete Peripheral Blood Stem Cells and Myeloablative Conditioning in Patients with High-Risk Hematologic Malignancies Who Lack Conventional Donors Is Well Tolerated and Produces Excellent Relapse-Free Survival: Results of A Prospective Phase II Trial. Blood. 2011;118:889. doi: 10.1016/j.bbmt.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 58.O'Donnell PVHE, Gooley TA, Luznik L, Fuchs EJ. Cyclophosphamide-induced tolerance following bone marrow transplantation from haploidentical donors. Haematologica Edicion Espanola. 2010;95(Extra 1):266–71. [Google Scholar]
- 59.Vago L, Perna SK, Zanussi M, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361:478–88. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 60.Villalobos IB, Takahashi Y, Akatsuka Y, et al. Relapse of leukemia with loss of mismatched HLA resulting from uniparental disomy after haploidentical hematopoietic stem cell transplantation. Blood. 2010;115:3158–61. doi: 10.1182/blood-2009-11-254284. [DOI] [PubMed] [Google Scholar]
- 61.Raj KSM, Kazmi M, Harringon E, Getzendaner LC, Lee SJ, et al. Peripheral Blood Progenitor Cell (PBPC) Transplantation from Haploidentical Donors Following Reduced Intensity Conditioning (RIC) for High-Risk Hematologic Malignancies. Biology of blood and marrow transplantation. Biology of Blood and Marrow Transplantation. 2012;S331 [Google Scholar]
- 62.Ciurea SOSR, Bayraktar UD, Xie S, Rondon G, Parmar S, et al. Improved Early Outcomes with T-Cell Replete (TCR) Compared with T-Cell Depleted (TCD) Haploidentical Stem Cell Transplantation (HaploSCT) Blood. 2011;118:320. [Google Scholar]
- 63.Luznik L, Bolanos-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 115:3224–30. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burroughs LM, O'Donnell PV, Sandmaier BM, et al. Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14:1279–87. doi: 10.1016/j.bbmt.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bashey AZX, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-Cell Replete Haploidentical Transplantation Using Post-Transplant Cyclophosphamide Results in Equivalent Non-Relapse Mortality and Disease-Free Survival Compared to Transplantation From HLA-Identical Sibling and Matched Unrelated Donors: A Stratified Cox Model Analysis of Two Hundred and Sixty Contemporaneous Allogeneic Transplants From a Single Center. Blood. 2011;118:833. [Google Scholar]
- 66.Kasamon YL, Luznik L, Leffell MS, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16:482–9. doi: 10.1016/j.bbmt.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Symons HJ, Leffell MS, Rossiter ND, Zahurak M, Jones RJ, Fuchs EJ. Improved survival with inhibitory killer immunoglobulin receptor (KIR) gene mismatches and KIR haplotype B donors after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant. 2010;16:533–42. doi: 10.1016/j.bbmt.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


