Abstract
Background
A marked decrease of Aβ42 in the cerebrospinal fluid (CSF) of patients with incipient Alzheimer's Disease (AD) has been well documented. However, contradictory results have been reported from studies on plasma Aβ levels as diagnostic markers for AD.
Objective
To investigate dynamic changes in human plasma Aβ levels, evaluate the effects of aging and amyloidosis on these dynamics, and determine their correlation with CSF Aβ levels.
Design, Settings, and Participants
This was a repeated plasma and CSF sampling study conducted at the Washington University School of Medicine in St. Louis. Older adults with amyloid deposition (Amyloid +), age-matched controls without amyloid deposition (Amyloid −), and younger normal controls (YNC) were enrolled for the study.
Main Outcome Measures
Hourly measurements of plasma Aβ were compared between groups by age and amyloidosis. Plasma Aβ and CSF Aβ levels were compared for correlation, linear increase, and circadian patterns.
Results
Circadian patterns were observed in plasma Aβ, with diminished amplitudes with aging. Linear increase of Aβ was only observed for CSF Aβ in YNC and Amyloid − groups, but not in the Amyloid + group. No linear increase was observed for plasma Aβ. No significant correlations were found between plasma and CSF Aβ levels.
Conclusions
Plasma Aβ, like CSF, demonstrates a circadian pattern which is reduced in amplitude with increasing age but is unaffected by amyloid deposition. However, we found no evidence that plasma and CSF Aβ levels were related on an hourly or individual basis.
INTRODUCTION
Dementia is one of the most prevalent diseases among older populations.1 As people continue to live longer and the aging population increases, so will the number of individuals with Alzheimer's disease (AD), the most common form of dementia.2 Amyloid-beta (Aβ) plaques are a hallmark of AD pathology. The amyloid hypothesis suggests that an imbalance between Aβ production and clearance is responsible for the amyloid plaque buildup found in brains of AD patients.3
Prior studies have shown that cerebrospinal fluid (CSF) concentrations of Aβ42, the most amyloidgenic species of Aβ, were decreased by approximately half in individuals with AD compared with age-matched controls.4-7 Our recent study on the dynamics of CSF Aβ levels further elucidated that CSF Aβ levels were not static, but demonstrated an intrinsic circadian rhythm that diminished with aging, and a pattern of linear increase over time during the study which was lost in individuals with AD.8 Disrupted circadian rhythms in sleep and activities have also been associated with AD in prior studies,9 and certain circadian characteristics were correlated with cognitive performance in an older population.10 These findings highlight the importance of both concentrations and circadian patterns of CSF Aβ as diagnostic and predictive markers for AD and amyloidosis.
Plasma is a more accessible and less invasive source than CSF for estimating Aβ levels in circulation. However, results from different studies using plasma Aβ as diagnostic markers are conflicting.11-13 Various factors, including differences in study designs and choices of laboratory assays, may have contributed to the inconsistency in these reports. Our recent study on CSF Aβ dynamics also underlined the importance of sampling time in determining Aβ levels, since Aβ levels fluctuated by up to 40% from peak to trough, and this fluctuation followed a circadian pattern.8 However, it is unclear whether plasma Aβ levels also have dynamic patterns and whether plasma Aβ levels correlate with CSF levels.
In this study, plasma and CSF Aβ40 and Aβ42 levels over time were measured in study participants using a highly sensitive and standardized Luminex xMAP bead-based method. The aims of our study were to explore how plasma Aβ levels change over time, whether Aβ dynamics differs with aging and amyloidosis, and if there is a correlation between plasma and CSF Aβ levels and dynamics.
METHODS
STUDY DESIGN
This was a repeated plasma and CSF sampling study conducted at the Washington University School of Medicine in St. Louis. Participants of this study were from two age groups: a group of older participants (N=20) consisting of both AD patients and cognitively normal older controls, and a younger normal control group (YNC, N=10). The group of older participants were drawn from individuals age 60 years and older who were enrolled in longitudinal studies of aging and dementia conducted by the Knight Alzheimer Disease Research Center (ADRC) at Washington University. Participants in this group were analyzed for ApoE genotypes, and assessed clinically for cognitive performance. Individuals were deemed to be cognitively normal with a Clinical Dementia Rating (CDR) of 0, or to have very mild dementia with CDR of 0.5, or mild dementia with CDR of 1.Younger controls were recruited from the community and were between the ages of 18 and 60 years. All participants were in good general health and had no neurological illnesses other than dementia for AD patients. Individuals with active infections, bleeding disorders, or those who were treated with anti-coagulants were excluded from this study. None of the participants were involved in anti-Aβ trials at the time when they participated in our study. All human study protocols were approved by the Washington University Human Studies Committee and the General Clinical Research Center Advisory Committee. Informed consent was obtained from all participants.
ESTIMATING STATUS OF AMYLOID DEPOSITION IN OLDER PARTICIPANTS
For older participants (N=20), we determined the status of brain amyloid deposition either by Pittsburgh compound B Positron Emission Tomography (PIB PET)scan14, or by CSF Aβ42 to Aβ40 ratios based on the high inverse correlation between CSF Aβ42 values and PIB PET values15-17. PIB binds to fibrillar amyloid cerebral deposits, and the binding potentials of the prefrontal, precuneus, lateral temporal, and gyrus rectus were averaged to yield the mean cortical binding potential (MCBP) for each participant. A MCBP of 0.18or greater was considered amyloid plaque positive (Amyloid +), and a MCBP of less than 0.18 was considered amyloid plaque negative (Amyloid−)14. PIB PET scans were obtained for twelve participants to determine the presence of amyloid plaques in the brain. For the remaining eight older participants without PIB PET scan data, we utilized the CSF Aβ42 to Aβ40 ratio to predict their amyloid deposition status. A separate data set of combined PIB PET and CSF Aβ42 to Aβ40 ratio was modeled to determine the optimal cutoff value of the CSF Aβ42 to Aβ40 ratio for prediction of amyloidosis. This additional second data set was composed of twenty-six participants with both PIB PET measures and CSF Aβ levels as measured by the ELISA, and the eight older participants who only had ELISA data for CSF Aβ levels. Data of the 26 participants with both PIB and ELISA measurements was used as the training set to estimate the sensitivity and specificity of the CSF Aβ42 to Aβ40 ratio as compared to the gold standard of PIB PET scan in determining amyloid deposition status. Receiver Operating Characteristic (ROC) was constructed with an Area Under the Curve (AUC) of 0.95 (eFigure 1). Based on the ROC analysis, a CSF Aβ42 to Aβ40 ratio of less than 7% was chosen as the cut-off value for positive amyloid deposition, with a sensitivity of 100% and specificity of 72%. The status of amyloid deposition was assigned for the eight participants who did not have PIB PET scan, based on this cut-off value and their respective CSF ELISA measurements.
SAMPLE COLLECTION
An intrathecal lumbar catheter and an intravenous catheter were placed between 7:30 AM and 9:00 AM and sample collection started between 8:00 AM and 9:30 AM in all participants. Six milliliters of CSF were obtained each hour for 36 hours; likewise 6 milliliters of blood plasma were obtained hourly for the first 15 hours, decreasing to only odd hours for the remainder of the study. CSF and plasma aliquots were frozen at −80C immediately after collection in 1.7ml Axygen maximum-recovery polypropylene tubes. Participants were encouraged to stay in bed and were allowed free choice of when to sleep, read, watch television, use their laptops or talk throughout the study. Participants had meals served at 9:00 AM, 1:00 PM, and 6:00 PM, and snacks at 11:00 AM, 3:00 PM and 8:00PM.
CSF AND PLASMA ANALYSIS
CSF and plasma were thawed and analyzed for levels of Aβ40 and Aβ42 using Luminex xMAP bead-based methods (INNO-BIA plasma Aβ forms). Each sample was assessed in duplicate. All samples from each participant were measured together on the same XMAP plate to avoid inter-plate variation. The means of the intra-sample coefficient of variation for duplicates was 5.8% for Aβ40 and 3.8% for Aβ42.
COSINOR ANALYSIS
Cosinor analysis was performed as previously described in Huang et al 8. Briefly, single cosinor analysis was used to analyze the patterns of 36-hour CSF and plasma Aβ40 and Aβ42 levels in each participant. A cosine transformation was applied to the time variable using 24 hours as the default circadian cycle, and the PROC NLIN procedure in Statistical Analysis Software (SAS) was used to estimate the parameters of the circadian patterns for Aβ fluctuations. Mesor (midline of the Aβ oscillation), amplitude (distance between the peak and mesor) and acrophase (the time corresponding to the peak of the curve) were calculated for each participant and averaged within each group. For the group-averaged data, linear increase of Aβ levels over time was estimated using the PROC REG procedure in SAS. Cosinor analysis was applied to data after linear trend subtraction from the raw Aβ values.
STATISTICAL ANALYSIS
All analyses were performed using SAS version 9.2 (Statistical Analysis Software, North Carolina). Graphs were plotted in GraphPad Prism version 4.03 for Windows (GraphPad Software, San Diego California USA). The relationship of CSF to plasma was explored in this study. Circadian rhythms of Aβ fluctuation over time were examined in this study as previously described.8
RESULTS
DEMOGRAPHICS OF STUDY PARTICIPANTS
The study population consisted of 60% women and 40% men. The demographic summary of participants is summarized in Table 1. There was no statistical difference in age between the Amyloid− and Amyloid + groups (p=0.61). There was a statistical difference in genotype distribution between the Amyloid− and Amyloid + group (p<0.05).
Table 1.
Characteristics of study participants.
| YNC (N=10) | Amyloid – (N=8) | Amyloid + (N=12) | |
|---|---|---|---|
| Age | 35.6±12.9 | 72.0±6.9 | 73.6±6.9* |
| CDR rating | |||
| 0 | NA | 6 | 3 |
| 0.5 | NA | 1 | 8 |
| 1 | NA | 1 | 1 |
| ApoE genotype | |||
| E3/E3 | NA | 6 | 7** |
| E2/E3 | NA | 2 | 0 |
| E3/E4 | NA | 0 | 5 |
No statistical difference in age between Amyloid – and Amyloid + groups, p=0.61
Statistically different distribution of ApoE genotypes between Amyloid – and Amyloid + groups, p<0.05.
INCREASED PLASMA Aβ40 AND Aβ42 WITH AGING
Plasma levels of Aβ40 and Aβ42 were significantly higher for both older groups compared to YNC group (over 30% higher, p<0.01, Table 2), and were not different between Amyloid + and Amyloid− groups (p>0.05), suggesting plasma Aβ levels increase with aging. The correlation coefficients were 0.71 between age and plasma Aβ40 (p<0.01, N=30), and 0.40 between age and plasma Aβ42 (p=0.03).
Table 2.
36-hr mean and standard deviation (SD) for plasma Aβ (Aβ42 and Aβ40) in three groups. YNC group had lower levels of plasma Aβ42 and Aβ40 compared to the other two groups.
| Group | Plasma Aβ42 | Plasma Aβ40 | ||
|---|---|---|---|---|
| 36-hr mean | 36-hr SD | 36-hr mean | 36-hr SD | |
| YNC (N=10) | 6.9±1.8 pM | 1.0±0.7 pM | 39.1±8.7 pM | 4.7±2.7 pM |
| Amyloid − (N=8) | 8.8±2.4 pM** | 0.6±0.3 pM | 55.0±10.2 pM** | 4.0±1.4 pM |
| Amyloid + (N=12) | 9.9±2.6 pM** | 0.7±0.3 pM | 55.5±9.0 pM** | 3.8±1.3 pM |
Values presented are mean±SD. YNC group was set as the reference group for statistical comparisons. Statistically significant differences were marked as
(p<0.01)
*(p<0.05).
Variability of plasma Aβ in each participant was calculated as the standard deviation of serial Aβ measurements over time, and the 36-hr mean Aβ concentration was averaged by group (YNC, Amyloid −, Amyloid +) as shown in Table 2. Despite increased plasma Aβ levels in the two older groups, Aβ variability over time decreased by about 30% for Aβ42 (p=0.06) and 20% for Aβ40 (p=0.09) compared to YNC group. Taken together, plasma Aβ levels increased, but Aβ variability decreased with age. Concentrations and variability were also investigated for CSF Aβ peptides (Table 3). CSF Aβ42 levels decreased by 17% in Amyloid − group (p=0.08), and by 44% in Amyloid + group (p<0.01) compared to YNC group. CSF Aβ42 variability decreased by 36% in Amyloid − group (p=0.07), and by 65% in Amyloid + group (p<0.01) when compared to YNC group. Changes in individual CSF and plasma Aβ levels are presented in eFigure 2.
Table 3.
36-hr mean and standard deviation (SD) for CSF Aβ (Aβ42 and Aβ40) in three groups. Amyloid + group had lower levels of CSF Aβ42 compared to the other two groups.
| Group | CSF Aβ42 | CSF Aβ40 | ||
|---|---|---|---|---|
| 36-hr mean | 36-hr SD | 36-hr mean | 36-hr SD | |
| YNC (N=10) | 374.1±148.7 pM | 75.9±36.0 pM | 2365.0±566.7 pM | 487.4±176.7 pM |
| Amyloid − (N=8) | 311.6±153.3 pM | 48.4±26.8 pM | 2984.6±1686.5 pM | 635.5±490.7 pM |
| Amyloid + (N=12) | 208.0±97.7 pM** | 26.7±17.6 pM** | 2484.7±813.2 pM | 414.9±174.7 pM |
Values presented are mean±SD. YNC group was set as the reference group for statistical comparisons. Statistically significant differences were marked as
(p<0.01)
*(p<0.05).
These results suggest that CSF Aβ42 levels and variability declined with amyloidosis, and to a lesser extent, with age. No difference was found in mean Aβ40 or mean Aβ40 variability (p>0.05) between YNC, Amyloid − and Amyloid + groups.
LINEAR INCREASE IN CSF BUT NOT IN PLASMA Aβ
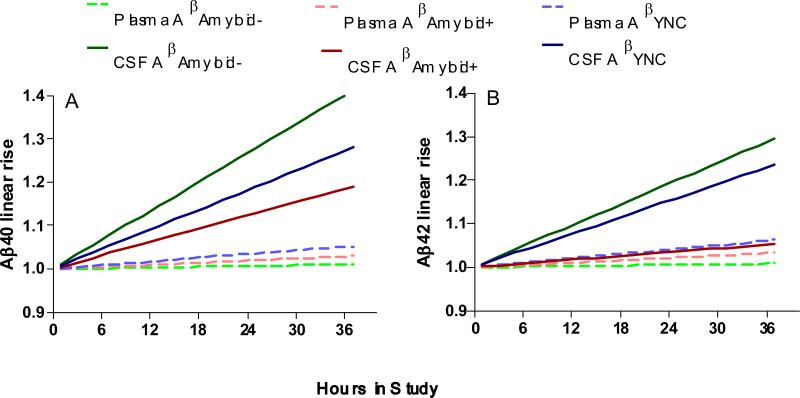
Linear increase of CSF Aβ40 (Figure 1A) was observed in both YNC (18%/24hr) and Amyloid − group (27%/24hr ), but diminished in Amyloid + group (12%/24hr).Similarly, linear increase of CSF Aβ42 (Figure 1B) was observed in both YNC (15%/24hr) and Amyloid − group (19%/24hr ), but dramatically reduced in Amyloid + group (3%/24hr). When examining plasma Aβ40 and Aβ42 levels, no linear increase was observed in any of the three groups (all <4%/24hr, Figure 1A and 1B)
Figure 1.
CSF Aβ40 (A) and Aβ42 (B) concentrations linearly increase in YNC and Amyloid − groups. However, in the Amyloid + group, the linear increase of CSF Aβ42 was absent and the linear increase of CSF Aβ40 was diminished. In contrast, plasma Aβ40 (A) and Aβ42 (B) did not have a linear rise in any of the three groups.
DECREASED PLASMA Aβ CIRCADIAN AMPLITUDES WITH AGING
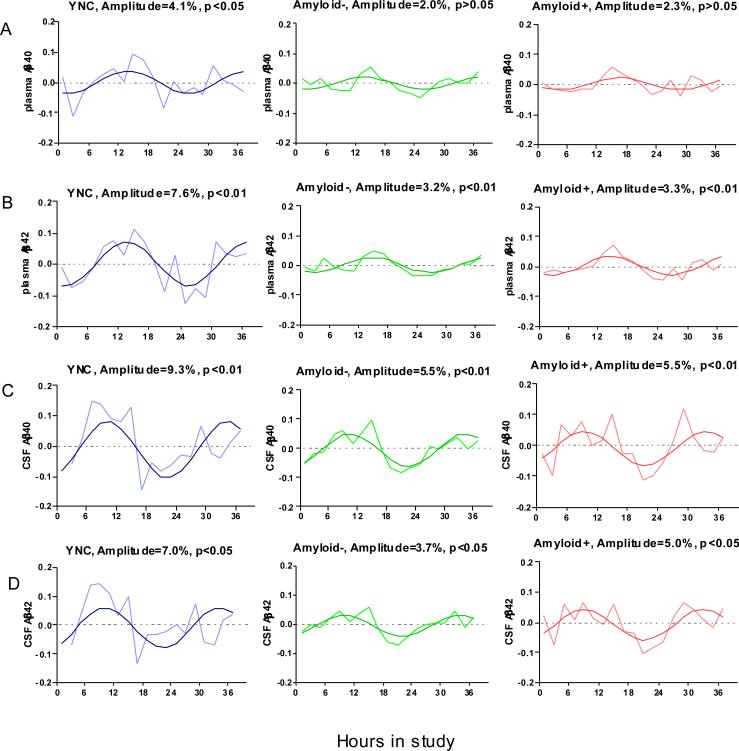
Cosinor analysis was used to assess the circadian patterns of plasma Aβ40 (Figure 2A) and Aβ42 (Figure 2B) dynamics in Amyloid +, Amyloid −, and YNC group using the mean-adjusted group average. Amplitudes of diurnal pattern were increased 2 fold in YNC group compared to the two older groups.
Figure 2.
Aβ diurnal patterns diminish with increased age. The diurnal amplitude of Plasma Aβ40 (A), Plasma Aβ42 (B), CSF Aβ40 (C), and CSF Aβ42(D) were decreased by approximately half in the older groups (Amyloid − and Amyloid +) compared to the younger group, regardless of amyloid deposition. Data are group averaged mean-adjusted Aβ levels over time.
Cosinor analysis was also used to assess the circadian patterns of CSF Aβ dynamics in Amyloid +, Amyloid − and YNC group using the mean-adjusted group average data after linear trend was subtracted (Figure 2C and 2D). Similar to the plasma data, amplitudes of diurnal CSF Aβ fluctuation in the YNC group were 40% to 70% higher than the two older groups. Goodness of fit of these cosinor models (R squared) are between 0.29 and 0.64. (eTable 1).
Cosinor analysis was used to assess the circadian patterns of CSF and plasma Aβ dynamics in each individual participant. Cosinor parameters, including mesor, amplitude and amplitude to mesor ratio, were calculated for each participant and averaged for the three groups (eTable 2 and eTable 3). The cosinor goodness of fit for each participant was highly correlated for plasma Aβ40 and Aβ42 (r=0.92, p<0.0001) in YNC, and less so for Amyloid− (r=0.6, p=0.04), and for Amyloid+ groups (r=0.7, p=0.05) (eTable 4).
Correlations between age and cosinor amplitudes for mean-adjusted CSF and plasma Aβ peptides were calculated (eFigure 4). Significant negative correlations with age were found for CSF Aβ40, CSF Aβ42, and plasma Aβ40 (p<0.05). Negative but non-significant correlation was found between age and plasma Aβ42 (p=0.16).
In summary, diurnal patterns were observed in both CSF and plasma Aβ levels, with the highest amplitudes in both fluids occurring in the YNC group. For the group average data, the acrophases of the cosinar curves differed between CSF and plasma Aβ levels. The peaks for CSF Aβ levels occurred around 9 hours after the start of the study (corresponding to 6PM), and the peaks for plasma Aβ levels occurred around 15 hours after the start of the study (corresponding to 12 midnight), thus approximately 6h time difference between the plasma and CSF peaks for Aβ levels. However, individual cosinor models were highly variable in terms of their acrophase (plasma Aβ40 acrophase 2.3±6.5 hours, Aβ42 acrophase 0.05±7.8 hours) and a similar 6-hour lag in plasma Aβ was not observed.
LACK OF CORRELATION BETWEEN CSF AND PLASMA Aβ LEVELS
Average CSF and plasma Aβ levels of each participant over time were calculated (36-hr mean) and correlations between these two sets of values were estimated. Correlation coefficients between average CSF and plasma Aβ42 levels were −0.14 (p=0.46) for all participants, 0.52 (p=0.16) for Amyloid −, −0.27 (p=0.42) for Amyloid + and −0.01 (p=0.97) for YNC. Correlation coefficients between average CSF and plasma Aβ40 levels were −0.19 for all participants, −0.48 (p=0.18) for Amyloid −, −0.51 (p=0.11) for Amyloid +, and −0.01 (p=0.97) for YNC.
Furthermore, to test CSF and plasma Aβ association on individual levels, hourly CSF Aβ40 and Aβ42 measurements were correlated with plasma Aβ levels for each participant. The average correlation coefficient for Aβ40 was 0.03 (SD: 0.32, Min: −0.62, Max: 0.48), and for Aβ42 was 0.08 (SD: 0.38, Min: −0.57, Max: 0.73). Since changes in plasma Aβ may lag those in CSF, the correlations between CSF Aβ levels at a specific time and plasma Aβ levels at 6 hours later were also investigated. The average correlation coefficient for Aβ40 was 0.03 (SD: −0.20, Min: −0.92, Max: 0.50), and for Aβ42 was 0.08 (SD: −0.13, Min: −0.90, Max: 0.51) after the time effect was taken into consideration.
CORRELATION BETWEEN PLASMA Aβ40 AND Aβ42
For each study participant, hourly plasma Aβ40 and Aβ42 levels were highly correlated (average r=0.79, eTable 5). No statistical differences in the mean correlation coefficients were found among the Amyloid+, Amyloid −, and YNC group (p>0.05). In addition to the strong hourly Aβ40 and Aβ42 correlation in each individual, statistically significant correlations were observed between the 36-hr average Aβ40 and Aβ42 combining the three groups (p<0.01, eFigure 3).
COMMENT
We found a significant linear rise in CSF Aβ for YNC and Amyloid – groups with decreased Aβ40 and absent Aβ42 linear rise in the Amyloid + group. We found no evidence for a linear rise in plasma Aβ for any group. Together, these findings suggest that the Aβ linear rise in CSF is a specific central effect of increasing CSF Aβ concentrations in the lumbar space. We postulate that the procedure of repeated sampling of lumbar CSF may cause increased subarachnoid CSF closer to the brain to be sampled in the lumbar space. If correct, an Aβ rostral-caudal gradient may exist in healthy controls, but not in the presence of amyloidosis. Alternative explanations include increased central Aβ production due to decreased contiguous sleep18 or increased stress19 or decreased clearance20 from the central compartment. To our knowledge, this is the first report of a plasma Aβ circadian pattern over time. The plasma Aβ circadian amplitudes decrease with aging independent of amyloidosis, similar to findings in the CSF. The amplitude of the plasma Aβ42 circadian pattern is similar to CSF, while the relative amplitude of plasma Aβ40 is half that of CSF. The average acrophase of plasma Aβ is 6 hours later compared to the CSF Aβ40 acrophase. Although a 6 hour delay in plasma acrophase could be due to the time it takes for lumbar CSF to be resorbed into the blood stream, this is not supported by the lack of correlation between hourly CSF and plasma Aβ values as CSF Aβ concentrations are highly dynamic hour to hour,21, 22 while plasma changes relatively little over a few hours. This suggests that other factors contribute to dynamic changes in CSF Aβ outside of circadian regulation. These other factors may include neuronal activity,23 secretase activity, APP production or transport and clearance mechanisms.
Further, the age-associated increase in plasma Aβ was not mirrored by a similar increase in CSF and several other studies have shown no difference in plasma Aβ levels between controls and AD, despite the marked reduction in CSF Aβ42 levels in AD. Therefore, plasma Aβ may be largely influenced by peripheral mechanisms of production (e.g. platelets or other cells) and clearance (e.g., within the liver) that are different from CSF Aβ. Thus, circadian processes may influence both the peripheral and central production and clearance of Aβ.
These findings provide useful results for both plasma and CSF Aβ diagnostic and therapeutic biomarker studies. For example, time of collection for both blood and CSF should be controlled. Further, this study suggests Aβ peptides in both central and peripheral compartments are regulated in a circadian pattern that is part of the normal dynamic physiologic control of Aβ concentrations. This study also indicates that peripheral Aβ concentrations are not useful as direct surrogates for central Aβ concentrations.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants from AstraZeneca (RJB), the US National Institutes of Health (NIH) grants K08 AG027091-01, K23 AG 03094601, R-01-NS065667 (RJB), P50 AG05681-22, P01 AG03991-22, WU CTSA award (UL1 RR024992), and also grants from an Anonymous Foundation (RJB), and the Knight Initiative for Alzheimer Research. We are grateful to the Clinical Core of the Knight Alzheimer's Disease Research Center for characterization of the older participants and to the participants for their time and effort.
REFERENCES
- 1.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005 Dec 17;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronson MK, Ooi WL, Geva DL, Masur D, Blau A, Frishman W. Dementia. Age-dependent incidence, prevalence, and mortality in the old old. Arch Intern Med. 1991 May;151(5):989–992. doi: 10.1001/archinte.151.5.989. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002 Jul 19;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.Motter R, Vigo-Pelfrey C, Kholodenko D, et al. Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol. 1995 Oct;38(4):643–648. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- 5.Galasko D, Chang L, Motter R, et al. High cerebrospinal fluid tau and low amyloid beta42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Arch Neurol. 1998 Jul;55(7):937–945. doi: 10.1001/archneur.55.7.937. [DOI] [PubMed] [Google Scholar]
- 6.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid A beta(42) in humans. Annals of Neurology. 2006 Mar;59(3):512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 7.Andreasen N, Hesse C, Davidsson P, et al. Cerebrospinal fluid beta-amyloid(1-42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol. 1999 Jun;56(6):673–680. doi: 10.1001/archneur.56.6.673. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Potter R, Sigurdson W, et al. Effects of Age and Amyloid Deposition on A{beta} Dynamics in the Human Central Nervous System. Arch Neurol. 2011 Sep 12; doi: 10.1001/archneurol.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weldemichael DA, Grossberg GT. Circadian rhythm disturbances in patients with Alzheimer's disease: a review. Int J Alzheimers Dis. 2010;2010 doi: 10.4061/2010/716453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011 Nov;70(5):722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaffe K, Weston A, Graff-Radford NR, et al. Association of plasma beta-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA. 2011 Jan 19;305(3):261–266. doi: 10.1001/jama.2010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009 Apr;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukumoto H, Tennis M, Locascio JJ, Hyman BT, Growdon JH, Irizarry MC. Age but not diagnosis is the main predictor of plasma amyloid beta-protein levels. Arch Neurol. 2003 Jul;60(7):958–964. doi: 10.1001/archneur.60.7.958. [DOI] [PubMed] [Google Scholar]
- 14.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006 Aug 8;67(3):446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 15.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006 Mar;59(3):512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 16.Fagan AM, Mintun MA, Shah AR, et al. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer's disease. EMBO Mol Med. 2009 Nov;1(8-9):371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weigand SD, Vemuri P, Wiste HJ, et al. Transforming cerebrospinal fluid Abeta42 measures into calculated Pittsburgh Compound B units of brain Abeta amyloid. Alzheimers Dement. 2011 Mar;7(2):133–141. doi: 10.1016/j.jalz.2010.08.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-{beta} Dynamics Are Regulated by Orexin and the Sleep-Wake Cycle. Science. 2009 Sep 24; doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang J-E, Cirrito JR, Dong H, Csernansky JG, Holtzman DM. Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(25):10673–10678. doi: 10.1073/pnas.0700148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010 Dec 24;330(6012):1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bateman RJ, Wen G, Morris JC, Holtzman DM. Fluctuations of CSF amyloid-beta levels: implications for a diagnostic and therapeutic biomarker. Neurology. 2007 Feb 27;68(9):666–669. doi: 10.1212/01.wnl.0000256043.50901.e3. [DOI] [PubMed] [Google Scholar]
- 22.Slats D, Claassen JAHR, Spies PE, et al. Hourly variability of cerebrospinal fluid biomarkers in Alzheimer's disease subjects and healthy older volunteers. Neurobiology of Aging. (0) doi: 10.1016/j.neurobiolaging.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Cirrito JR, Yamada KA, Finn MB, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48(6):913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.