Abstract
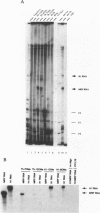
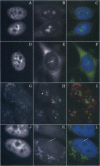
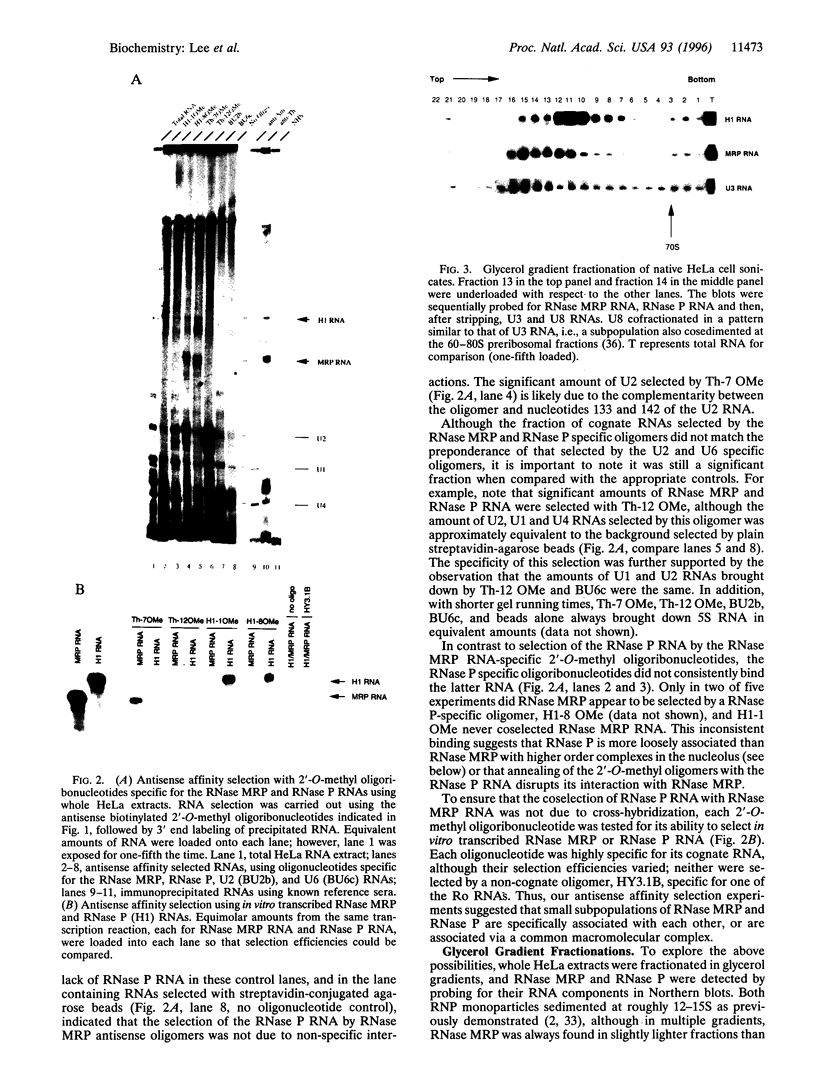
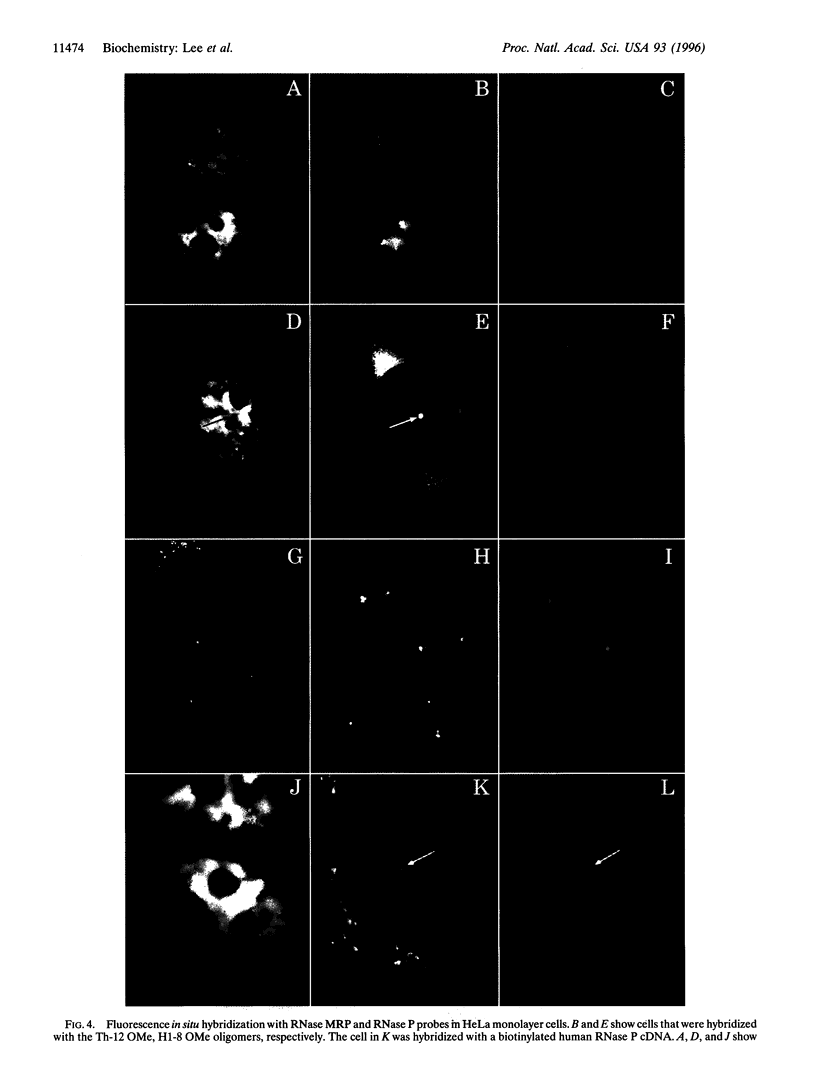
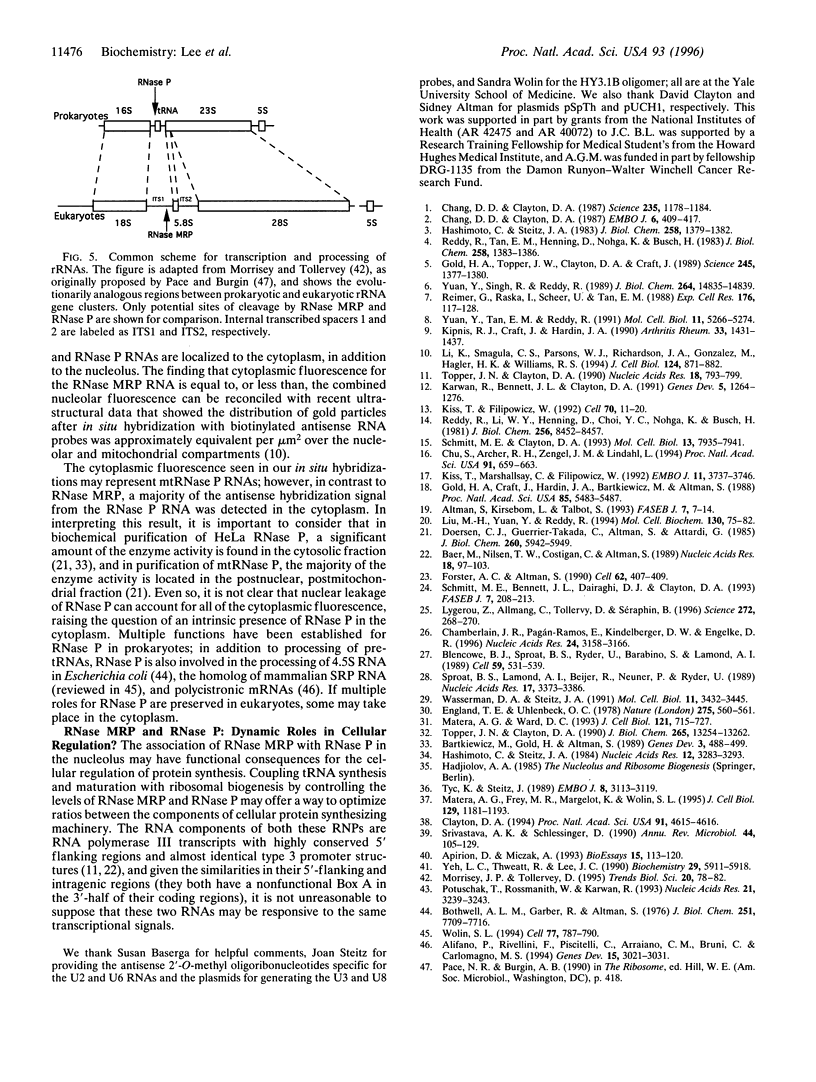
RNase mitochondrial RNA processing enzyme (MRP) is a nucleolar ribonucleoprotein particle that participates in 5.8S ribosomal RNA maturation in eukaryotes. This enzyme shares a polypeptide and an RNA structural motif with ribonuclease P (RNase P), a nuclear endoribonuclease originally described in the nucleus that processes RNA transcripts to generate their mature 5' termini. Both enzymes are also located in mitochondria. This report further characterizes the relationship between RNase MRP and RNase P. Antisense affinity selection with biotinylated 2'-O-methyl oligoribonucleotides and glycerol gradient fractionation experiments demonstrated that small subpopulations of RNase MRP and RNase P associate with each other in vivo in macromolecular complex, possibly 60-80S preribosomes. This latter notion was supported by fluorescence in situ hybridization experiments with antisense oligonucleotides that localized that RNA components of RNase MRP and RNase P to the nucleolus and to discrete cytoplasmic structures. These findings suggest that small subpopulations of RNase MRP and RNase P are physically associated, and that both may function in ribosomal RNA maturation or ribosome assembly.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alifano P., Rivellini F., Piscitelli C., Arraiano C. M., Bruni C. B., Carlomagno M. S. Ribonuclease E provides substrates for ribonuclease P-dependent processing of a polycistronic mRNA. Genes Dev. 1994 Dec 15;8(24):3021–3031. doi: 10.1101/gad.8.24.3021. [DOI] [PubMed] [Google Scholar]
- Altman S., Kirsebom L., Talbot S. Recent studies of ribonuclease P. FASEB J. 1993 Jan;7(1):7–14. doi: 10.1096/fasebj.7.1.7916700. [DOI] [PubMed] [Google Scholar]
- Apirion D., Miczak A. RNA processing in prokaryotic cells. Bioessays. 1993 Feb;15(2):113–120. doi: 10.1002/bies.950150207. [DOI] [PubMed] [Google Scholar]
- Baer M., Nilsen T. W., Costigan C., Altman S. Structure and transcription of a human gene for H1 RNA, the RNA component of human RNase P. Nucleic Acids Res. 1990 Jan 11;18(1):97–103. doi: 10.1093/nar/18.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkiewicz M., Gold H., Altman S. Identification and characterization of an RNA molecule that copurifies with RNase P activity from HeLa cells. Genes Dev. 1989 Apr;3(4):488–499. doi: 10.1101/gad.3.4.488. [DOI] [PubMed] [Google Scholar]
- Blencowe B. J., Sproat B. S., Ryder U., Barabino S., Lamond A. I. Antisense probing of the human U4/U6 snRNP with biotinylated 2'-OMe RNA oligonucleotides. Cell. 1989 Nov 3;59(3):531–539. doi: 10.1016/0092-8674(89)90036-6. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Garber R. L., Altman S. Nucleotide sequence and in vitro processing of a precursor molecule to Escherichia coli 4.5 S RNA. J Biol Chem. 1976 Dec 10;251(23):7709–7716. [PubMed] [Google Scholar]
- Chamberlain J. R., Pagán-Ramos, Kindelberger D. W., Engelke D. R. An RNase P RNA subunit mutation affects ribosomal RNA processing. Nucleic Acids Res. 1996 Aug 15;24(16):3158–3166. doi: 10.1093/nar/24.16.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. A mammalian mitochondrial RNA processing activity contains nucleus-encoded RNA. Science. 1987 Mar 6;235(4793):1178–1184. doi: 10.1126/science.2434997. [DOI] [PubMed] [Google Scholar]
- Chang D. D., Clayton D. A. A novel endoribonuclease cleaves at a priming site of mouse mitochondrial DNA replication. EMBO J. 1987 Feb;6(2):409–417. doi: 10.1002/j.1460-2075.1987.tb04770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S., Archer R. H., Zengel J. M., Lindahl L. The RNA of RNase MRP is required for normal processing of ribosomal RNA. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):659–663. doi: 10.1073/pnas.91.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A. A nuclear function for RNase MRP. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4615–4617. doi: 10.1073/pnas.91.11.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doersen C. J., Guerrier-Takada C., Altman S., Attardi G. Characterization of an RNase P activity from HeLa cell mitochondria. Comparison with the cytosol RNase P activity. J Biol Chem. 1985 May 25;260(10):5942–5949. [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
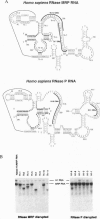
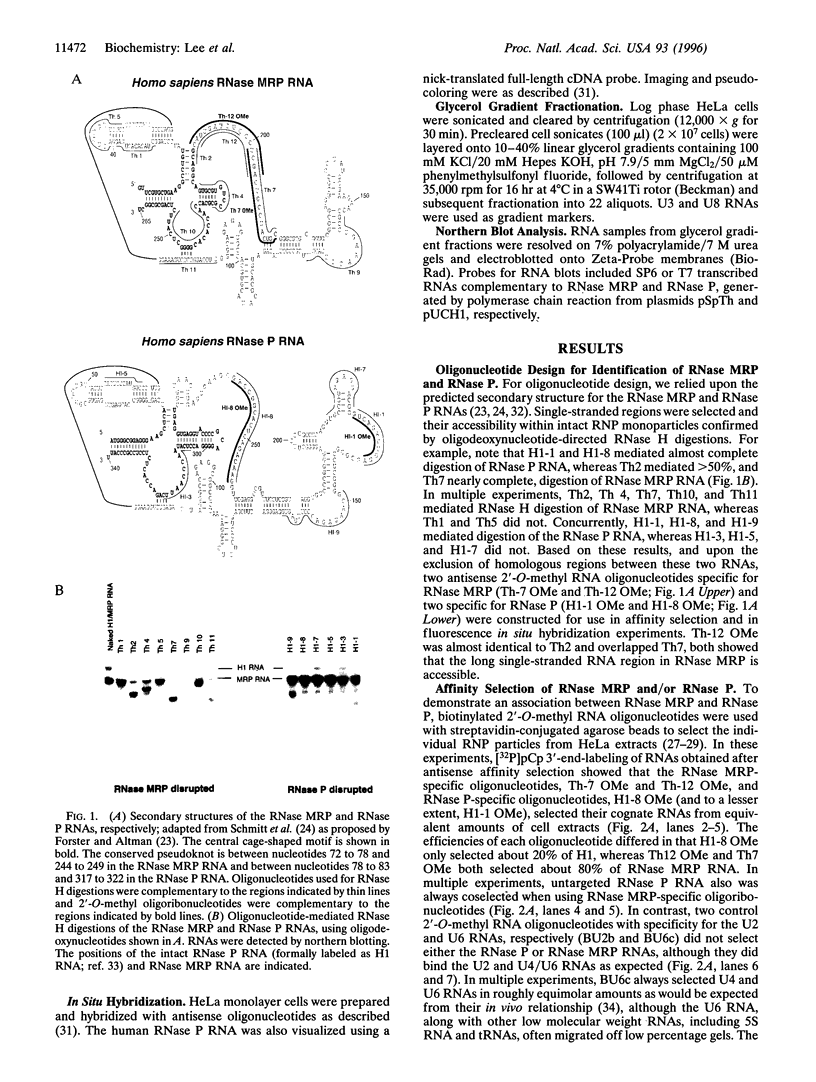
- Forster A. C., Altman S. Similar cage-shaped structures for the RNA components of all ribonuclease P and ribonuclease MRP enzymes. Cell. 1990 Aug 10;62(3):407–409. doi: 10.1016/0092-8674(90)90003-w. [DOI] [PubMed] [Google Scholar]
- Gold H. A., Craft J., Hardin J. A., Bartkiewicz M., Altman S. Antibodies in human serum that precipitate ribonuclease P. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5483–5487. doi: 10.1073/pnas.85.15.5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold H. A., Topper J. N., Clayton D. A., Craft J. The RNA processing enzyme RNase MRP is identical to the Th RNP and related to RNase P. Science. 1989 Sep 22;245(4924):1377–1380. doi: 10.1126/science.2476849. [DOI] [PubMed] [Google Scholar]
- Hashimoto C., Steitz J. A. Sequential association of nucleolar 7-2 RNA with two different autoantigens. J Biol Chem. 1983 Feb 10;258(3):1379–1382. [PubMed] [Google Scholar]
- Hashimoto C., Steitz J. A. U4 and U6 RNAs coexist in a single small nuclear ribonucleoprotein particle. Nucleic Acids Res. 1984 Apr 11;12(7):3283–3293. doi: 10.1093/nar/12.7.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karwan R., Bennett J. L., Clayton D. A. Nuclear RNase MRP processes RNA at multiple discrete sites: interaction with an upstream G box is required for subsequent downstream cleavages. Genes Dev. 1991 Jul;5(7):1264–1276. doi: 10.1101/gad.5.7.1264. [DOI] [PubMed] [Google Scholar]
- Kipnis R. J., Craft J., Hardin J. A. The analysis of antinuclear and antinucleolar autoantibodies of scleroderma by radioimmunoprecipitation assays. Arthritis Rheum. 1990 Sep;33(9):1431–1437. doi: 10.1002/art.1780330917. [DOI] [PubMed] [Google Scholar]
- Kiss T., Filipowicz W. Evidence against a mitochondrial location of the 7-2/MRP RNA in mammalian cells. Cell. 1992 Jul 10;70(1):11–16. doi: 10.1016/0092-8674(92)90528-k. [DOI] [PubMed] [Google Scholar]
- Kiss T., Marshallsay C., Filipowicz W. 7-2/MRP RNAs in plant and mammalian cells: association with higher order structures in the nucleolus. EMBO J. 1992 Oct;11(10):3737–3746. doi: 10.1002/j.1460-2075.1992.tb05459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Smagula C. S., Parsons W. J., Richardson J. A., Gonzalez M., Hagler H. K., Williams R. S. Subcellular partitioning of MRP RNA assessed by ultrastructural and biochemical analysis. J Cell Biol. 1994 Mar;124(6):871–882. doi: 10.1083/jcb.124.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. H., Yuan Y., Reddy R. Human RNaseP RNA and nucleolar 7-2 RNA share conserved 'To' antigen-binding domains. Mol Cell Biochem. 1994 Jan 12;130(1):75–82. doi: 10.1007/BF01084270. [DOI] [PubMed] [Google Scholar]
- Lygerou Z., Allmang C., Tollervey D., Séraphin B. Accurate processing of a eukaryotic precursor ribosomal RNA by ribonuclease MRP in vitro. Science. 1996 Apr 12;272(5259):268–270. doi: 10.1126/science.272.5259.268. [DOI] [PubMed] [Google Scholar]
- Matera A. G., Frey M. R., Margelot K., Wolin S. L. A perinucleolar compartment contains several RNA polymerase III transcripts as well as the polypyrimidine tract-binding protein, hnRNP I. J Cell Biol. 1995 Jun;129(5):1181–1193. doi: 10.1083/jcb.129.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matera A. G., Ward D. C. Nucleoplasmic organization of small nuclear ribonucleoproteins in cultured human cells. J Cell Biol. 1993 May;121(4):715–727. doi: 10.1083/jcb.121.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. P., Tollervey D. Birth of the snoRNPs: the evolution of RNase MRP and the eukaryotic pre-rRNA-processing system. Trends Biochem Sci. 1995 Feb;20(2):78–82. doi: 10.1016/s0968-0004(00)88962-8. [DOI] [PubMed] [Google Scholar]
- Potuschak T., Rossmanith W., Karwan R. RNase MRP and RNase P share a common substrate. Nucleic Acids Res. 1993 Jul 11;21(14):3239–3243. doi: 10.1093/nar/21.14.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R., Li W. Y., Henning D., Choi Y. C., Nohga K., Busch H. Characterization and subcellular localization of 7-8 S RNAs of Novikoff hepatoma. J Biol Chem. 1981 Aug 25;256(16):8452–8457. [PubMed] [Google Scholar]
- Reddy R., Tan E. M., Henning D., Nohga K., Busch H. Detection of a nucleolar 7-2 ribonucleoprotein and a cytoplasmic 8-2 ribonucleoprotein with autoantibodies from patients with scleroderma. J Biol Chem. 1983 Feb 10;258(3):1383–1386. [PubMed] [Google Scholar]
- Reimer G., Raska I., Scheer U., Tan E. M. Immunolocalization of 7-2-ribonucleoprotein in the granular component of the nucleolus. Exp Cell Res. 1988 May;176(1):117–128. doi: 10.1016/0014-4827(88)90126-7. [DOI] [PubMed] [Google Scholar]
- Schmitt M. E., Bennett J. L., Dairaghi D. J., Clayton D. A. Secondary structure of RNase MRP RNA as predicted by phylogenetic comparison. FASEB J. 1993 Jan;7(1):208–213. doi: 10.1096/fasebj.7.1.7678563. [DOI] [PubMed] [Google Scholar]
- Schmitt M. E., Clayton D. A. Nuclear RNase MRP is required for correct processing of pre-5.8S rRNA in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Dec;13(12):7935–7941. doi: 10.1128/mcb.13.12.7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproat B. S., Lamond A. I., Beijer B., Neuner P., Ryder U. Highly efficient chemical synthesis of 2'-O-methyloligoribonucleotides and tetrabiotinylated derivatives; novel probes that are resistant to degradation by RNA or DNA specific nucleases. Nucleic Acids Res. 1989 May 11;17(9):3373–3386. doi: 10.1093/nar/17.9.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A. K., Schlessinger D. Mechanism and regulation of bacterial ribosomal RNA processing. Annu Rev Microbiol. 1990;44:105–129. doi: 10.1146/annurev.mi.44.100190.000541. [DOI] [PubMed] [Google Scholar]
- Topper J. N., Clayton D. A. Characterization of human MRP/Th RNA and its nuclear gene: full length MRP/Th RNA is an active endoribonuclease when assembled as an RNP. Nucleic Acids Res. 1990 Feb 25;18(4):793–799. doi: 10.1093/nar/18.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper J. N., Clayton D. A. Secondary structure of the RNA component of a nuclear/mitochondrial ribonucleoprotein. J Biol Chem. 1990 Aug 5;265(22):13254–13262. [PubMed] [Google Scholar]
- Tyc K., Steitz J. A. U3, U8 and U13 comprise a new class of mammalian snRNPs localized in the cell nucleolus. EMBO J. 1989 Oct;8(10):3113–3119. doi: 10.1002/j.1460-2075.1989.tb08463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman D. A., Steitz J. A. Structural analyses of the 7SK ribonucleoprotein (RNP), the most abundant human small RNP of unknown function. Mol Cell Biol. 1991 Jul;11(7):3432–3445. doi: 10.1128/mcb.11.7.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin S. L. From the elephant to E. coli: SRP-dependent protein targeting. Cell. 1994 Jun 17;77(6):787–790. doi: 10.1016/0092-8674(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Yeh L. C., Thweatt R., Lee J. C. Internal transcribed spacer 1 of the yeast precursor ribosomal RNA. Higher order structure and common structural motifs. Biochemistry. 1990 Jun 26;29(25):5911–5918. doi: 10.1021/bi00477a005. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Singh R., Reddy R. Rat nucleolar 7-2 RNA is homologous to mouse mitochondrial RNase mitochondrial RNA-processing RNA. J Biol Chem. 1989 Sep 5;264(25):14835–14839. [PubMed] [Google Scholar]
- Yuan Y., Tan E., Reddy R. The 40-kilodalton to autoantigen associates with nucleotides 21 to 64 of human mitochondrial RNA processing/7-2 RNA in vitro. Mol Cell Biol. 1991 Oct;11(10):5266–5274. doi: 10.1128/mcb.11.10.5266. [DOI] [PMC free article] [PubMed] [Google Scholar]