Abstract
Background
Type I glycogen storage disease (GSD) is caused by a deficiency of glucose-6-phosphatase resulting in severe fasting hypoglycemia.
Objective
We compared the efficacy of a new modified starch with the currently used cornstarch therapy in patients with type Ia and Ib GSD.
Design
This was a randomized, 2-d, double-blinded, crossover pilot study comparing the commonly used uncooked cornstarch with the experimental starch in 12 subjects (6 GSDIa, 6 GSDIb) aged ≥13 y. At 2200, the subjects were given 100 g of digestible starch, and glucose and lactate were measured hourly until the subject's plasma glucose concentration reached 60 mg/dL or until the subject had fasted for 10 h. The order in which the products were tested was randomized in a blinded fashion.
Results
The matched-pair Gehan rank test for censored survival was used to compare the therapies. The experimental starch maintained blood glucose concentrations significantly longer than did the traditional therapy (P = 0.013) in the 2-sided analysis. Most of the benefit was found to be after glucose concentrations fell below 70 mg/dL. The currently used cornstarch resulted in higher peak glucose concentrations and a more rapid rate of fall than did the new starch.
Conclusions
The experimental starch was superior to standard therapy in preventing hypoglycemia (≤60 mg/dL). This therapy may allow patients with GSD to sleep through the night without awakening for therapy while enhancing safety. Additional studies are warranted to determine whether alternative dosing will further improve control in the therapeutic blood glucose range.
Introduction
Glycogen storage disease (GSD) type I is a rare autosomal recessive disorder of glycogen metabolism that affects ≈1 in 100 000 live births (1). Mutations in the genes that encode glucose-6-phosphatase (2) and glucose-6-phosphate translocase (3) cause type Ia and type Ib GSD, respectively. Because glucose-6-phosphatase catalyzes the final step of both glycogenolysis and gluconeogenesis, abnormal glucose-6-phosphatase activity results in impaired endogenous glucose production. Severe hypoglycemia consequently occurs within 3-4 h of a meal. During infancy, children exhibit episodes of hypoglycemia, developmental delay, hepatomegaly, and poor growth. A series of metabolic derangements also result from the enzymatic defect, because the liver remains responsive to the normal neuroendocrine responses to impending hypoglycemia. Concentrations of glucose-6-phosphate increase from glycogenolysis, and shunting into alternative pathways results in accumulation of lactic acid, triglycerides, cholesterol, and uric acid (1, 2, 4-6).
The primary goal of treatment is to maintain normal blood glucose concentrations (>70 mg/dL) throughout the day and night, which will prevent activation of counterregulatory responses. In newborns and infants, frequent feeds during the day combined with overnight infusion of dextrose or a glucose-containing formula maintain normoglycemia (7-9). In 1984, uncooked cornstarch was found to be the most effective therapy for maintaining blood glucose concentrations in the desirable range (10). Although cornstarch has dramatically improved the quality of life for patients with GSD type I, it has a limited duration of action. A previous study suggested that cornstarch therapy only prevents hypoglycemia for a median time of 4.25 h in children (11). All children must awaken in the middle of the night for therapy, and delayed administration of the therapy can be associated with the development of hypoglycemia, seizures, and neurologic injury. Even as adults, almost all patients still require therapy every 4-6 h, and overnight therapy is required in >90% of patients to achieve optimal metabolic control (19, 10-13).
The constant anxiety about avoiding hypoglycemia and the necessity to interrupt sleep to receive therapy 1 to 3 times per night is deeply detrimental for these patients and their families. The effort is exhausting, and the resulting fatigue affects the work and quality of life of the patients and their families. In addition, exhaustion from waking up every night eventually leads to delayed administration of a cornstarch dose, which puts children with GSD Ia at extreme risk from hypoglycemia.
An ideal food in GSD type I would be able to provide an 8-h duration of reliable blood glucose concentrations and have a low glycemic index, ie, it would avoid the rapid development of high glucose concentrations and subsequent secretion of insulin. The low glycemic index is a necessary feature of an ideal food because a high insulin concentration would not only increase the risk of hypoglycemia but would also prevent the accumulation of alternative fuels for the brain (lactate and ketones) if hypoglycemia were to occur (14–22). In addition, this food must not result in the delivery of too much undigested material to the colon, thus causing abdominal cramping and flatulence (23). The volume of food must not be too large to administer as a meal and the preparation time, storage, and costs are all important considerations.
Recently, scientists at the starch research laboratory based at Glasgow Caledonian University (Glycologic Ltd, Glasgow, UK; international patent WO2005044284) developed a controlled heat-moisture processing of a high-amylopectin-containing cornstarch (24) that is more amenable to digestion by intestinal enzymes than other forms of starch. Initial investigations performed in England showed that this experimental cornstarch improved maintenance of glucose concentrations in 20 patients with GSD tested during the day. The study was limited, however, by several confounding variables including duration of fasting, activity, and variable treatment strategies that are inherent during the day (24). The aim of this double-blind, crossover study was to determine whether the new modified cornstarch could maintain normoglycemia longer than the standard cornstarch in a cohort with type I GSD during an overnight fast.
Subjects and Methods
Subjects
All subjects followed by the University of Florida Glycogen Storage Disease Program with a proven diagnosis of type I GSD established either biochemically or by liver biopsy were eligible to participate, and the diagnosis of type Ia or Ib was proven by mutation analysis. These studies were approved by the Institutional Review Board of the University of Florida, and informed consent (and assent from the child when under the age of 18 y) was obtained before enrollment in this study. A total of 12 participants over 13 y of age were enrolled (6 subjects with GSDIa and 6 subjects with GSDIb). All subjects were treated with uncooked cornstarch therapy around the clock at baseline.
Test products
For these investigations, the experimental heat-moisture processed cornstarch Glycosade (Vitaflo International Ltd, Liverpool, United Kingdom) was compared with uncooked Argo brand cornstarch, the standard starch preparation used in the United States (ACH Food Companies Inc, Memphis, TN). The characteristics of these products are summarized in Table 1. All starch products were delivered to the institutional metabolic kitchen where the products were measured and distributed. The investigators, subjects, and research team were blinded with regard to the starch preparation. All starch doses were administered as a 100 g of digestible starch dose in 177 mL (6 ounces) of an artificially sweetened, carbohydrate-free, lemon-raspberry drink (Crystal-Light; Kraft Foods Inc, Northfield, IL) to mask the taste. The dose was taken orally or through a gastrostomy tube if requested by the participant.
Table 1. Characteristics of the conventional and experimental starches examined in the study1.
| Conventional starch | Experimental starch | |
|---|---|---|
| Moisture content (%) | 10.9 | 11.9 |
| Amylopectin content (%) | 72.8 | 99.5 |
| Total carbohydrate, wet base (%) | 87.5 | 82.5 |
| Resistant starch (%) | 60.5 | 67.7 |
| Glycemic index (GI units) | 70 | 30 |
Conventional starch was Argo (ACH Food Companies Inc, Memphis, TN); experimental starch was Glycosade (Vitaflo International Ltd, Liverpool, United Kingdom).
Study design
The study was a double-blinded, randomized, 2-d, crossover pilot study comparing the standard cornstarch product with the experimental starch preparation. The study was a consecutive, 2-night, in-patient stay at the General Clinical Research Unit at the University of Florida. At 2200, the subjects were given 100 g of carbohydrate starch (either the conventional or experimental preparation), and glucose and lactate were measured hourly through an in-dwelling intravenous line until the subject's plasma glucose concentration reached 60 mg/dL or the subject had fasted for 10 h. All glucose and lactate determinations were assayed in a CLIA-approved laboratory housed on our research unit by using a YSI Analyzer (YSI Incorporated, Yellow Springs, OH). Venous pH was measured every other hour in the CLIA-approved hospital laboratory. A venous pH <7.25 or lactate concentrations >10 mmol/L were established criteria for stopping the study, but these abnormalities never developed in any of the subjects. The alternate starch was administered on the second night of the study. The participants were asked to be sedentary for 4 h before administration of the cornstarch, and the same meal was served for dinner both nights of the study to minimize possible confounders.
Statistical analysis
The primary outcome analysis of the trial was the time to reach a plasma glucose concentration of 60 mg/dL. This was analyzed in an actuarial manner, because not all subjects were able to reach this level during their time on the trial, making them right censored. Because the trial was a crossover protocol, we used the Gehan (25) ranks in a match pair set-up. This is a close analogue of the Wilcoxon's sign rank test (26). Qualitatively, we used Kaplan-Meier curves to plot the time-to-event outcome data (27). The numerous unknown factors prevented a prospective power analysis. Key unknown ingredients included degree of correlation between the paired observations and the degree of censoring. The data from this pilot study can now provide estimates of these unknown factors for planning future studies.
Results
The clinical characteristics of the subjects are summarized in Table 2. There was a sex distribution of 5 females and 7 males, and the subjects ranged in age from 14 to 34 y with a mean age of 22.8 y. All patients were in fair to good metabolic control at the time of the studies (Table 3).
Table 2. Clinical characteristics of the subjects enrolled in the study1.
| Subject | Age | Sex | GSD | Mutation | UCCS | UCCS daily | BMI |
|---|---|---|---|---|---|---|---|
| y | doses/24 h | g·kg−1·24 h−1 | kg/m2 | ||||
| 01 | 32 | M | Ia | R83C/R83C | 6 | 5.6 | 21.3 |
| 02 | 15 | M | Ia | F117C/Q347X | 8 | 3.8 | 26.1 |
| 03 | 24 | M | Ia | R83C/R83C | 6 | 8.5 | 24.3 |
| 04 | 25 | M | Ia | R83C/R83C | 8 | 2.8 | 32.5 |
| 05 | 15 | F | Ia | R83C/R83C | 6 | 4.9 | 22.1 |
| 06 | 34 | F | Ia | R83C/R83C | 6 | 4.6 | 29.8 |
| 07 | 30 | F | Ib | R28C/G339C | 6 | 6.3 | 31.2 |
| 08 | 19 | M | Ib | G339C/1042delCT | 5 | 3.9 | 33.5 |
| 09 | 24 | F | Ib | G339C/1042delCT | 5 | 4.6 | 23.3 |
| 10 | 23 | M | Ib | G68K/1103ins12 | 6 | 6.1 | 22.1 |
| 11 | 19 | F | Ib | G+1toT (550 + 1)/C183R | 4 | 3.9 | 20.7 |
| 12 | 14 | M | Ib | G+1toT (550 + 1)/C183R | 4 | 5.7 | 18.4 |
| Mean | 23 | — | — | — | 6 | 5 | 25 |
| SD | 7 | — | — | — | 1 | 2 | 5 |
GSD, glycogen storage disease; UCCS, uncooked cornstarch.
Table 3. Biochemical characteristics of the subjects enrolled in the study1.
| Subject | AST | ALT | Triglyceride | Cholesterol | Uric acid | ESR |
|---|---|---|---|---|---|---|
| U/L | U/L | mg/dL | mg/dL | mg/dL | mm/h | |
| 01 | 39 | 23 | 316 | 161 | 3.8 | 29 |
| 02 | 22 | 20 | 321 | 144 | 6.9 | 4 |
| 03 | 29 | 23 | 770 | 319 | 5.7 | 14 |
| 04 | 44 | 48 | 425 | 256 | 7.8 | 15 |
| 05 | 26 | 25 | 286 | 182 | 9.9 | 19 |
| 06 | 49 | 37 | 324 | 155 | 7.3 | 32 |
| 07 | 20 | 10 | 250 | 161 | 5.2 | 44 |
| 08 | 29 | 21 | 84 | 93 | 7.5 | 31 |
| 09 | 41 | 31 | 90 | 79 | 9.9 | 76 |
| 10 | 68 | 61 | 551 | 176 | 8.5 | 120 |
| 11 | 17 | 11 | 75 | 88 | 7.2 | 66 |
| 12 | 26 | 19 | Not reported | Not reported | 7.8 | 27 |
| Mean | 34 | 27 | 317 | 165 | 7 | 40 |
| SD | 15 | 15 | 210 | 72 | 2 | 33 |
Baseline laboratory results were obtained at the time of enrollment in the study with intravenous line placement. AST, aspartate aminotransferase; ALT, alanine aminotransferase; ESR, erythrocyte sedimentation rate.
The baseline mean blood glucose measurement obtained immediately before the 2200 dose of the test starch was consistent from day 1 to day 2 and for both the experimental and the control arms of the study. The mean (±SD) blood glucose concentration across all subjects at time zero was 77 ± 14 mg/dL. All subjects stopped the daily protocol because of reaching the stop criteria of hypoglycemia (blood glucose < 60 mg/dL) or because 10 h had elapsed from the time of the starch dose. None of the subjects became acidotic during the protocol, and the stop criteria of a lactate concentration equal to 10 mmol/L was not reached.
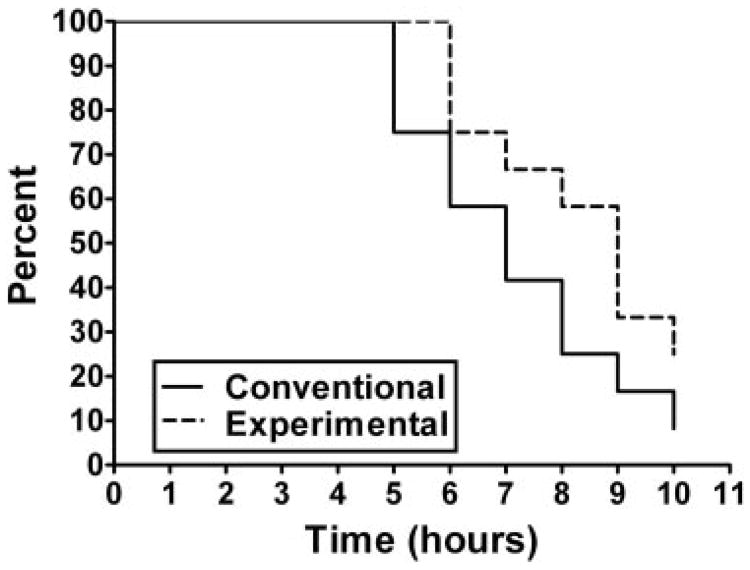
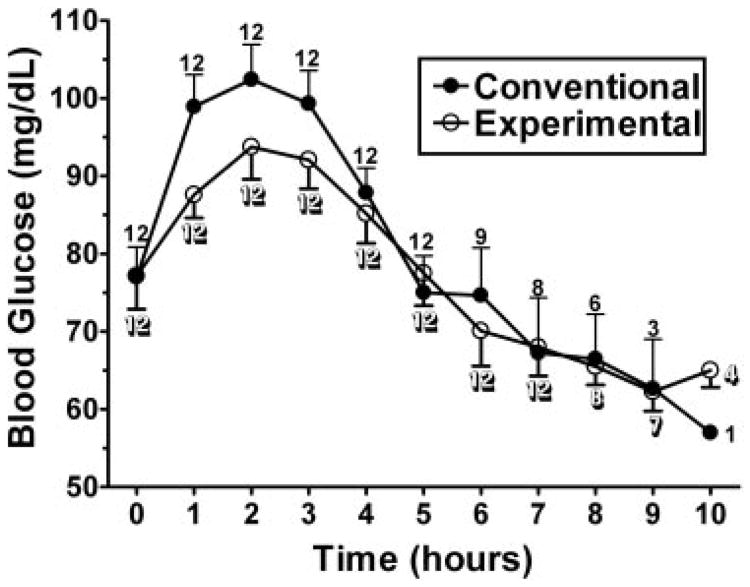
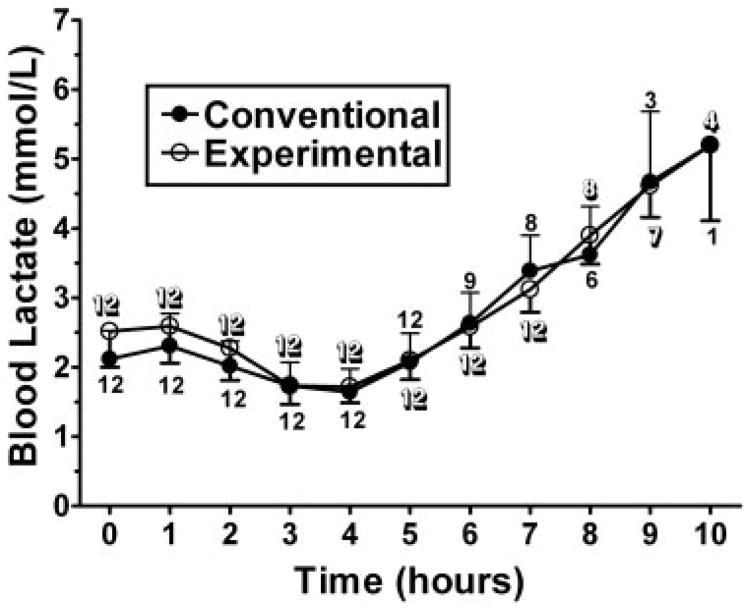
In the 2-sided analysis, the experimental cornstarch preparation was found to maintain blood glucose concentrations significantly longer than the currently used cornstarch preparation (P = 0.013). The Kaplan-Meier curve for duration above 60 mg/dL is depicted in Figure 1. Although the experimental starch maintained blood glucose concentrations significantly longer than the standard starch, most of the benefit was after glucose concentrations fell below 70 mg/dL. The standard cornstarch was found to have a higher peak glucose concentration and a more rapid rate of fall when compared with the experimental starch (Figure 2). No significant difference was found in the time maintained above 70 mg/dL or for lactate concentrations (Figure 3).
Figure 1.
Kaplan-Meier curves depicting subject survival for maintenance of glucose concentrations >60 mg/dL for the experimental (Glycosade; Vitaflo International Ltd, Liverpool, United Kingdom) and the conventional (Argo; ACH Food Companies Inc, Memphis, TN) starches (n = 12). The experimental starch maintained blood glucose concentrations significantly longer than did the conventional cornstarch (P = 0.013, Gehan ranks test in a match pair set-up).
Figure 2.
Mean (±SD) glucose concentrations after administration of 100 g of the conventional (Argo; ACH Food Companies Inc, Memphis, TN) or experimental (Glycosade; Vitaflo International Ltd, Liverpool, United Kingdom) starch. The numbers located at the data points represent the n for that product at the given time point. The conventional starch had a higher peak glucose concentration and a more rapid rate of fall than did the experimental starch.
Figure 3.
Mean (±SD) lactate concentrations after administration of 100 g of the conventional (Argo; ACH Food Companies Inc, Memphis, TN) or experimental (Glycosade; Vitaflo International Ltd, Liverpool, United Kingdom) starch. The numbers located at the data points represents the n for that product at the given time point. No significant difference was found in lactate concentrations when comparing the 2 starches.
Discussion
Although current cornstarch therapy has eased the daily management of GSD and has markedly improved these patients' quality of life for the past 25 y, the limited duration of action of the currently used cornstarch preparation necessitates finding an improved substance that will allow for fewer doses throughout the day and night. In this study, we showed that the experimental starch was superior to the standard therapy at preventing hypoglycemia during the overnight period.
A previous study similarly evaluated this starch against standard therapy during the day (24). Although that study also suggested that the modified experimental starch was superior to conventional therapy, the benefit was limited to the rate of glucose fall. This apparent difference can be explained by changes in the study design. Our study evaluated subjects with type I GSD, who have a more consistent biochemical fasting profile than do a combination of type I and III subjects (4). Fasting times for the 2 different forms of GSD are not comparable. Performing the study at night also allowed standardization of activity, replicable meals, and a consistent fasting time before commencement of the study. Removing the aforementioned confounders allowed for more consistent data to be collected and likely contributed to the improved duration of action shown with the experimental product.
Our study showed improved prevention of hypoglycemia, a slowed rate of increase in blood sugar, and a slowed rate of blood glucose fall with the experimental cornstarch compared with the currently used product. Although the therapeutic range for glucose is > 70 mg/dL, the new product may add safety by slowing the drop of glucose once 70 mg/dL is reached. This added protection will lessen the impact of a missed cornstarch dose because the slower rate of fall of glucose allows lactate concentrations to increase. Lactate can be used directly by the brain as a fuel in the setting of hypoglycemia (15, 28), and the experimental product should decrease the risk of seizures and neurologic injury in this population. Of note, it has been reported that hypoglycemia lessens with age because of the production of glucose-6-phosphatase beta (29). This was not found in the present study, and all participants developed hypoglycemia during the overnight fast with traditional therapy.
Although the new product appears encouraging regarding safety and glucose control, no significant difference was found in lactate concentrations between the cornstarch preparations. Lactate increases in GSD with counterregulation, and it will be critical to maintain glucose concentrations above the 70-mg/dL threshold for this product to be used to extend the duration between doses. Lactate concentrations have been associated with long-term complications in type I GSD, and near normal lactate concentrations are achieved with optimal therapy (11). In this study, only one dose of the experimental product was studied. Future studies are warranted to determine whether alternative dosing will improve control in the therapeutic range.
In conclusion, the newly created modified cornstarch, Glycosade, is a promising alternative to traditional cornstarch preparations and may improve quality of life and safety in this population. This therapy, in particular, offers the potential for patients with GSD to sleep through the night without awakening for therapy. Future studies are needed to learn the appropriate dosing of this promising product and to determine whether the product will release glucose at a rate sufficient to meet daytime needs.
Acknowledgments
We thank the nursing staff and technologists of the General Clinical Research Center for their contributions to the study and to the subjects who participated in the study.
The authors' responsibilities were as follows—CEC: contributed to study conception and design, conducted the study, prepared data, and drafted the manuscript; KB: contributed to conception and study design; PJL: contributed to study conception and design; JJS: analyzed and interpreted data; DWT: analyzed and interpreted data; MNS: contributed to execution of protocol; GPAS: involved in study conception and design; DAW: contributed to study conception and design, conducted study, drafted the manuscript, and secured funding for the study. All authors participated fully in critically revising the manuscript and approved the final version of the manuscript to be published. None of the authors had a personal or financial conflict of interest.
Footnotes
Supported in part by Mentored Career Award K23 RR 017560 (DAW). Grant funding for this project was provided by the Children's Fund for GSD Research, and support was received from NIH General Clinical Research Center grant M01 RR 00082.
References
- 1.Chen YT, Burchell A. Glycogen storage diseases. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic basis of inherited disease. 8th. New York, NY: McGraw-Hill; 2001. [Google Scholar]
- 2.Lei KJ, Shelly LL, Pan CJ, et al. Mutations in the glucose-6-phosphatase gene that cause glycogen storage disease type Ia. Science. 1993;262:580–3. doi: 10.1126/science.8211187. [DOI] [PubMed] [Google Scholar]
- 3.Lei KJ, Shelly LL, Lin B, et al. Mutations in the glucose-6-phosphatase gene are associated with glycogen storage disease types Ia and IaSP but not 1b and 1c. J Clin Invest. 1995;95:234–40. doi: 10.1172/JCI117645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfsdorf JI, Holm IA, Weinstein DA. Glycogen storage diseases Phenotypic, genetic, and biochemical characteristics, and therapy. Endocrinol Metab Clin North Am. 1999;28:801–23. doi: 10.1016/s0889-8529(05)70103-1. [DOI] [PubMed] [Google Scholar]
- 5.Hagen T, Korson MS, Wolfsdorf JI. Urinary lactate excretion to monitor the efficacy of treatment of type I glycogen storage disease. Mol Genet Metab. 2000;70:189–95. doi: 10.1006/mgme.2000.3013. [DOI] [PubMed] [Google Scholar]
- 6.Wolfsdorf JI, Weinstein DA. Glycogen storage diseases. Rev Endocr Metab Disord. 2003;4:95–102. doi: 10.1023/a:1021831621210. [DOI] [PubMed] [Google Scholar]
- 7.Stanley CA, Mills JL, Baker L. Intragastric feeding in type I glycogen storage disease: factors affecting the control of lactic acidemia. Pediatr Res. 1981;15:1504–8. doi: 10.1203/00006450-198112000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Yudkoff M, Nissim I, Stanley C, Baker L, Segal S. Glycogen storage disease: effects of glucose infusions on [15N] glycine kinetics and nitrogen metabolism. J Pediatr Gastroenterol Nutr. 1984;3:81–8. [PubMed] [Google Scholar]
- 9.Wolfsdorf JI, Keller RJ, Landy H, Crigler JF., Jr Glucose therapy for glycogenosis type 1 in infants: comparison of intermittent uncooked cornstarch and continuous overnight glucose feedings. J Pediatr. 1990;117:384–91. doi: 10.1016/s0022-3476(05)81077-7. [DOI] [PubMed] [Google Scholar]
- 10.Chen YT, Cornblath M, Sidbury JB. Cornstarch therapy in type I glycogen-storage disease. N Engl J Med. 1984;310:171–5. doi: 10.1056/NEJM198401193100306. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein DA, Wolfsdorf JI. Effect of continuous glucose therapy with uncooked cornstarch on the long-term clinical course of type 1a glycogen storage disease. Eur J Pediatr. 2002;161(suppl):S35–9. doi: 10.1007/s00431-002-1000-2. [DOI] [PubMed] [Google Scholar]
- 12.Wolfsdorf JI, Crigler JF., Jr Cornstarch regimens for nocturnal treatment of young adults with type I glycogen storage disease. Am J Clin Nutr. 1997;65:1507–11. doi: 10.1093/ajcn/65.5.1507. [DOI] [PubMed] [Google Scholar]
- 13.Wolfsdorf JI, Ehrlich S, Landy HS, Crigler JF., Jr Optimal daytime feeding regimen to prevent postprandial hypoglycemia in type 1 glycogen storage disease. Am J Clin Nutr. 1992;56:587–92. doi: 10.1093/ajcn/56.3.587. [DOI] [PubMed] [Google Scholar]
- 14.Wolfsdorf JI, Plotkin RA, Laffel LM, Crigler JF., Jr Continuous glucose for treatment of patients with type 1 glycogen-storage disease: comparison of the effects of dextrose and uncooked cornstarch on biochemical variables. Am J Clin Nutr. 1990;52:1043–50. doi: 10.1093/ajcn/52.6.1043. [DOI] [PubMed] [Google Scholar]
- 15.Evans M, Amiel SA. Carbohydrates as a cerebral metabolic fuel. J Pediatr Endocrinol Metab. 1998;11:99–102. doi: 10.1515/jpem.1998.11.s1.99. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins DJ, Vuksan V, Kendall CW, et al. Physiological effects of resistant starches on fecal bulk, short chain fatty acids, blood lipids and glycemic index. J Am Coll Nutr. 1998;17:609–16. doi: 10.1080/07315724.1998.10718810. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins DJ, Wolever TM, Jenkins AL. Starchy foods and glycemic index. Diabetes Care. 1988;11:149–59. doi: 10.2337/diacare.11.2.149. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins DJ, Wolever TM, Jenkins AL, Josse RG, Wong GS. The glycaemic response to carbohydrate foods. Lancet. 1984;2:388–91. doi: 10.1016/s0140-6736(84)90554-3. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins DJ, Thorne MJ, Taylor RH, et al. Slowly digested carbohydrate food improves impaired carbohydrate tolerance in patients with cirrhosis. Clin Sci (Lond) 1984;66:649–57. doi: 10.1042/cs0660649. [DOI] [PubMed] [Google Scholar]
- 20.Crapo PA, Insel J, Sperling M, Kolterman OG. Comparison of serum glucose, insulin, and glucagon responses to different types of complex carbohydrate in noninsulin-dependent diabetic patients. Am J Clin Nutr. 1981;34:184–90. doi: 10.1093/ajcn/34.2.184. [DOI] [PubMed] [Google Scholar]
- 21.Crapo PA, Kolterman OG, Waldeck N, Reaven GM, Olefsky JM. Postprandial hormonal responses to different types of complex carbohydrate in individuals with impaired glucose tolerance. Am J Clin Nutr. 1980;33:1723–8. doi: 10.1093/ajcn/33.8.1723. [DOI] [PubMed] [Google Scholar]
- 22.Crapo PA, Reaven G, Olefsky J. Postprandial plasma-glucose and -insulin responses to different complex carbohydrates. Diabetes. 1977;26:1178–83. doi: 10.2337/diab.26.12.1178. [DOI] [PubMed] [Google Scholar]
- 23.Smit GP, Ververs MT, Belderok B, Van Rijn M, Berger R, Fernandes J. Complex carbohydrates in the dietary management of patients with glycogenosis caused by glucose-6-phosphatase deficiency. Am J Clin Nutr. 1988;48:95–7. doi: 10.1093/ajcn/48.1.95. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharya K, Orton RC, Qi H, et al. A novel starch for the treatment of glycogen storage diseases. J Inherit Metab Dis. 2007;30:350–7. doi: 10.1007/s10545-007-0479-0. [DOI] [PubMed] [Google Scholar]
- 25.Gehan EA. A generalized Wilcoxon test for comparing arbitrarily singly censored samples. Biometrika. 1965;52:203–23. [PubMed] [Google Scholar]
- 26.Lehmann EL. Nonparametrics: statistical methods based on ranks. San Francisco, CA: Holden-Day; 1975. [Google Scholar]
- 27.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;55:447–81. [Google Scholar]
- 28.Smith D, Pernet A, Hallett WA, Bingham E, Marsden PK, Amiel SA. Lactate: a preferred fuel for human brain metabolism in vivo. J Cereb Blood Flow Metab. 2003;6:658–64. doi: 10.1097/01.WCB.0000063991.19746.11. [DOI] [PubMed] [Google Scholar]
- 29.Shieh JJ, Pan CJ, Mansfield BC, Chou JY. A glucose-6-phosphate hydrolase, widely expressed outside the liver, can explain age-dependent resolution of hypoglycemia in glycogen storage disease type Ia. J Biol Chem. 2003;47:98–103. doi: 10.1074/jbc.M309472200. [DOI] [PubMed] [Google Scholar]