Hodgkin lymphoma is considered highly curable with approximately 70% of patients achieving long term disease control using ABVD induction chemotherapy [1]. The standard management for relapsed/refractory patients is salvage chemotherapy followed by autologous stem cell transplantation [2]. Unfortunately, this approach is only effective for about 50% of the patients [3,4]. For patients who relapse after autologous stem cell transplantation, the prognosis is poor and treatment options remain largely palliative. Allogeneic stem cell transplantation is an option for a subset of patients but its use is limited by transplant associated morbidities/mortality and lack of long term disease control [5-7].
Brentuximab vedotin, or SGN-35, is an antibody-drug conjugate that selectively delivers monomethyl auristatin E (MMAE), an antimicrotubule agent, into a CD30-expressing cell. CD30 is expressed on the surface of Reed-Sternberg cells in Hodgkin lymphoma [8,9]. Chen et al. reported a 75% objective response rate in a multi-center phase II study involving patients with relapsed/refractory Hodgkin lymphoma [10]. Brentuximab vedotin recently received FDA approval for the treatment of patients with Hodgkin lymphoma after failure of autologous stem cell transplant (ASCT) or failure of at least two prior multi-agent chemotherapy regimens in patients who are not ASCT candidates.
Since brentuximab vedotin utilizes an antibody to CD30 to target delivery of the MMAE, loss of CD30 is a possible mechanism of acquired drug resistance. We report on two patients with relapsed Hodgkin lymphoma that initially achieved partial remissions with brentuximab vedotin and then developed progressive disease, whose relapsed tumors were found to retain expression of CD30.
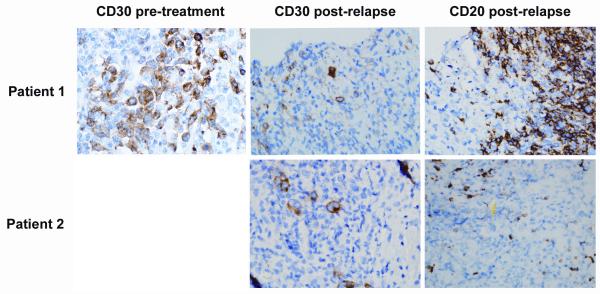
The first case, a 27 year old woman, presented with cough and mediastinal lymphadenopathy. She underwent a mediastinascopy and was found to have nodular sclerosing Hodgkin lymphoma. Her disease was staged at IVA due to liver involvement. She was treated with 6 cycles of induction chemotherapy including adriamycin, bleomycin, vinblastine and dacarbazine (ABVD) and achieved a complete remission. Her remission lasted for about a year and then she relapsed with nodal disease. She was then treated with two cycles of salvage chemotherapy with ifosfamide, carboplatin and etoposide (ICE), and underwent autologous stem cell transplantation using a total body irradiation based preparative regimen (1200 cGy). She achieved a complete remission again after her stem cell transplantation. However, nine years later, she relapsed in the hilar lymph nodes and lung. Consequently, she was treated on the pivotal study of brentuximab vedotin for treatment of patients with relapsed or refractory Hodgkin lymphoma for 8 months. Her best response was a partial remission after two cycles. A CT scan after 8 cycles revealed a new area of consolidation in the lung. It was unclear whether this represented infectious pneumonia or progressive Hodgkin lymphoma. She underwent a CT guided lung biopsy and it revealed a lymphoid infiltrate comprised of a mixture of T cells (CD3 positive) and B cells (CD20 and PAX-5 positive) with focal clusters of large atypical cells. Immunohistochemical staining shows that these large atypical cells were CD30 positive (Figure 1), CD 15 and PAX 5 negative (not shown), and CD 20 negative (Figure 1). The pre-treatment CD30 staining pattern from her previous biopsy was similar to that seen after relapse (Figure 1). The overall findings were consistent with a recurrent Hodgkin lymphoma.
Figure 1.
Immunohistochemical staining showed that in Patients 1 (3 top panels) and Patient 2 (2 bottom panels), the large atypcial cells were CD30-positive (1 left panel, and 2 middle panels) and CD20-negative (2 right panels). For patient 1, we have both pre-treatment and post-treatment samples with immunohistochemical staining. For CD30 immunostainining, we used the monoclonal mouse anti-human CD30, clone Ber-H2, code No M 0751 and visualized with the DAKO Envision/HRP kit, (Dakocytomation). For CD20 immunostaining, we used anti -Human CD20, clone L26 code no M 0755 and visualized with DAKO Envision/HRP kits (Dakocytomation).
The second case was a 19 year old man presented with supraclavicular lymphadenopathy. He underwent an excisional biopsy and was found to have Hodgkin lymphoma. He was staged at IIIA due to diffuse lymphadenopathy and received ABVD as induction chemotherapy. He had persistent disease at the end of therapy and went on to receive salvage ICE chemotherapy followed by autologous stem cell transplantation with involved field radiation. He had residual disease prior to the autologous stem cell transplant. Subsequently he relapsed and then underwent double umbilical cord blood transplantation. Unfortunately his disease recurred and additional treatments included rituximab, gemcitabine, vinorelbine, liposomal doxorubicin, MOPP, and palliative radiation. He was then enrolled on the SGN-35-07 study: “An intensive QT-QTc study to investigate the effects of brentuximab vedotin on cardiac ventricular repolarization in patients with CD30-positive malignancies” for 6 months. His best response was partial remission after 4 cycles. FDG-PET scan after the 10th cycle showed a new liver lesion. It was unclear whether the liver lesion represented infection or progressive lymphoma. A CT-guided biopsy of the liver mass revealed nodules with fibrosis and lymphoid aggregates composed of scattered large atypical cells admixed with eosinophils and neutrophils. These large atypical cells were positive for CD15 and CD30 (Figure 1), weakly positive for PAX-5 (not shown), and negative for CD20 (Figure 1) and CD45, consistent with recurrent Hodgkin lymphoma.
Both of our patients achieved significant tumor reduction following treatment with brentuximab vedotin, even though they were heavily pretreated. Although the responses in these two patients were not prolonged, the updated pivotal phase II trial results reported by Chen et al. showed that a third of the patients achieved complete remission (CR), and the median duration of CR was 20.5 months12 [11]. In Figure 1, the top panels show that the tumor masses in both patients had persistent CD30 expression. One patient received 8 cycles of brentuximab vedotin and another patient received 10 cycles. The persistence of CD30 expression at the time of progressive disease demonstrates that loss of CD30 does not appear to be the mechanism of resistance to brentuximab vedotin in these two patients. Of 3 total patients that we have re-biopsied following relapse post-brentuximab vedotin, all 3 have remained CD30 positive: the two patients described in this letter and a patient with anaplastic large cell lymphoma. Other possible mechanisms of resistance to brentuximab vedotin include decreased drug exposure, increased transporter proteins that efflux drug from the cell, and diminished binding of auristatin to tubulin isotypes [12-14].
Footnotes
Disclosures
R. Chen has consultant and research funding from Seattle Genetics and E. Sievers is an employee of Seattle Genetics.
References
- 1.Johnson PW, Radford JA, Cullen MH, et al. Comparison of ABVD and alternating or hybrid multidrug regimens for the treatment of advanced Hodgkin’s lymphoma: results of the United Kingdom Lymphoma Group LY09 Trial (ISRCTN97144519) J Clin Oncol. 2005;23:9208–18. doi: 10.1200/JCO.2005.03.2151. [DOI] [PubMed] [Google Scholar]
- 2.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97:616–23. doi: 10.1182/blood.v97.3.616. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359:2065–71. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 4.Yuen AR, Rosenberg SA, Hoppe RT, Halpern JD, Horning SJ. Comparison between conventional salvage therapy and high-dose therapy with autografting for recurrent or refractory Hodgkin’s disease. Blood. 1997;89:814–22. [PubMed] [Google Scholar]
- 5.Sureda A, Robinson S, Canals C, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26:455–62. doi: 10.1200/JCO.2007.13.2415. [DOI] [PubMed] [Google Scholar]
- 6.Anderlini P, Saliba R, Acholonu S, et al. Fludarabine-melphalan as a preparative regimen for reduced-intensity conditioning allogeneic stem cell transplantation in relapsed and refractory Hodgkin’s lymphoma: the updated M.D. Anderson Cancer Center experience. Haematologica. 2008;93:257–64. doi: 10.3324/haematol.11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen R, Palmer JM, Popplewell L, et al. Reduced intensity allogeneic hematopoietic cell transplantation can induce durable remission in heavily pretreated relapsed Hodgkin lymphoma. Ann Hematol. 2011;90:803–8. doi: 10.1007/s00277-010-1146-3. [DOI] [PubMed] [Google Scholar]
- 8.Falini B, Pileri S, Pizzolo G, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995;85:1–14. [PubMed] [Google Scholar]
- 9.Re D, Thomas RK, Behringer K, Diehl V. From Hodgkin disease to Hodgkin lymphoma: biologic insights and therapeutic potential. Blood. 2005;105:4553–60. doi: 10.1182/blood-2004-12-4750. [DOI] [PubMed] [Google Scholar]
- 10.Chen R, Gopal AK, Smith SE, et al. Results of a Pivotal Phase 2 Study of Brentuximab Vedotin (SGN-35) in Patients with Relapsed or Refractory Hodgkin Lymphoma. ASH Annual Meeting Abstracts. 2010;116:283. [Google Scholar]
- 11.Chen RW, Gopal AK, Smith SE, et al. Results from a pivotal phase II study of brentuximab vedotin (SGN-35) in patients with relapsed or refractory Hodgkin lymphoma (HL) ASCO Meeting Abstracts. 2011;29:8031. [Google Scholar]
- 12.Ghetie MA, Ghetie V, Vitetta ES. Anti-CD19 antibodies inhibit the function of the P-gp pump in multidrug-resistant B lymphoma cells. Clin Cancer Res. 1999;5:3920–7. [PubMed] [Google Scholar]
- 13.Gan PP, McCarroll JA, Po’uha ST, et al. Microtubule dynamics, mitotic arrest, and apoptosis: drug-induced differential effects of betaIII-tubulin. Mol Cancer Ther. 2010;9:1339–48. doi: 10.1158/1535-7163.MCT-09-0679. [DOI] [PubMed] [Google Scholar]
- 14.Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer. 2010;10:194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]