Abstract
Extracts from the immature fruit of Citrus aurantium are often used for weight loss but are reported to produce adverse cardiovascular effects. Root extracts of Rhodiola rosea have notable antistress properties. The hypothesis of these studies was that C aurantium (6% synephrine) and R rosea (3% rosavins, 1% salidroside) in combination would improve diet-induced obesity alterations in adult male Sprague-Dawley rats. In normal-weight animals fed standard chow, acute administration of C aurantium (1-10 mg/kg) or R rosea (2-20 mg/kg) alone did not reduce deprivation-induced food intake, but C aurantium (5.6 mg/kg) + R rosea (20 mg/kg) produced a 10.5% feeding suppression. Animals maintained (13 weeks) on a high-fat diet (60% fat) were exposed to 10-day treatments of C aurantium (5.6 mg/kg) or R rosea (20 mg/kg) alone or in combination. Additional groups received vehicle (2% ethanol) or were pair fed to the C aurantium + R rosea group. Although high-fat diet intake and weight loss were not influenced, C aurantium + R rosea had a 30% decrease in visceral fat weight compared with the other treatments. Only the C aurantium group had an increased heart rate (+7%) compared with vehicle. In addition, C aurantium + R rosea administration resulted in an elevation (+15%) in hypothalamic norepinephrine and an elevation (+150%) in frontal cortex dopamine compared with the pair-fed group. These initial findings suggest that treatments of C aurantium + R rosea have actions on central monoamine pathways and have the potential to be beneficial for the treatment of obesity.
Keywords: Bitter orange, Golden root, Leptin, Diet supplement, Kaolin, Dopamine
1. Introduction
Obesity is a metabolic disorder that is associated with physiological alterations that promote increased adiposity. This not only poses a health risk but also makes treatment strategies difficult to implement. The rapid rise in obesity rates has outpaced the approval rate by regulatory agencies for novel therapies, which has resulted in a surge in the use of botanical supplements for weight loss by consumers [1-3]. Regardless of their efficacy, weight loss supplements can have detrimental influences on health. Ephedra (Ephedra sinica), for example, produces moderate weight loss but has an increased risk of severe cardiovascular and cerebrovascular events [4]. A potentially “safe” surrogate for ephedra is an extract from the immature fruit of Citrus aurantium (also known as “bitter orange”), which is present in about one-third of all marketed supplements [1]. The active alkaloids of C aurantium are synephrine and octopamine (and, to a lesser extent, tyramine), which are structurally similar to epinephrine (E) and norepinephrine (NE) and have sympathomimetic effects [5,6]. Synephrine is the most abundant alkaloid and makes up approximately 6% of commercially available C aurantium products [7]. Synephrine has different pharmacologic actions based on whether the hydroxyl group is in the para- (p-synephrine) or meta- (m-synephrine) position on the phenyl ring [8,9]. C aurantium predominantly contains p-synephrine, which is a β-3 adrenergic receptor agonist with actions to increase thermogenesis and lipolysis [8]. Several studies have focused on C aurantium and p-synephrine in rodents and humans [10-12]. However, it is unclear whether doses of C aurantium extract that reduce food intake and promote weight loss can impact hemodynamic measures in a diet-induced model of obesity.
Another botanical extract, Rhodiola rosea (also known as “golden root”), is categorized as an “adaptogen” because it is beneficial for maintaining appropriate or enhanced adaptive responses to challenging environments or stressors [13-15]. The adaptogenic mechanisms of R rosea also impart cardioprotective effects mediated by its putative actions on monoamines such as NE and serotonin (5-HT) [13]. Although R rosea has not been shown to produce sustained body weight reductions, R rosea does impact food intake [16,17]. Given the cardioprotective effects of R rosea and its ability to influence feeding behavior, the hypothesis of these studies was that C aurantium and R rosea in combination would be effective for the treatment of obesity. The first objective was to determine the effective dose of C aurantium and R rosea alone or in combination that acutely suppressed food intake. The second objective was to determine the subchronic effects of C aurantium + R rosea in combination on brain and plasma monoamine levels, hemodynamic measurements, adipose tissue weight, and related hormone levels in a model of diet-induced obesity. Because these experiments would be the first to examine the potential interaction between C aurantium and R rosea on obesity outcomes and because long-term treatment with C aurantium has adverse effects on cardiovascular parameters [18], a 10-day subchronic treatment period was used to examine the initial hormonal and metabolic alterations. A 10-day treatment period has been used by others to examine novel therapeutics on obesity outcomes [19].
2. Methods and materials
2.1. Botanical extracts
Both C aurantium (6% synephrine; batch no. 20111208) and R rosea (3% rosavins, 1% salidroside; batch no. 12807050) were acquired from Unichem Inc (Newark, NJ, USA). Unichem is an National Sanitation Foundation-certified Good Manufacturing Practice facility and provides a Certificate of Analysis with each raw material supplied. In addition, the Botanical Core at Rutgers University verified the high-performance liquid chromatography (HPLC) analysis of C aurantium and R rosea. It was also determined that these materials did not contain pesticides or microbial contaminants and were acceptable in terms of the botanical integrity guidelines set forth by the National Institutes of Health.
2.2. Animals
Adult male Sprague-Dawley rats (weighing approximately 300 g) acquired from Harlan Laboratories (Frederick, MD, USA) were individually housed and placed on a 12/12-hour light/dark schedule (lights off at 1700 hours). Rats were fed standard chow [Purina Rat Diet 5012 (St. Louis, MO, USA) 13% fat, 27% protein, 3.1 kcal/g], unless otherwise noted, and water was available at all times during the experiments. All procedures were approved by the Institutional Animal Care and Use Committee of Rutgers University and were in accordance with National Institutes of Health guidelines.
2.3. Dose response of C aurantium and R rosea alone or in combination on acute food intake in 24-hour food-deprived rats
The purpose of this experiment was to establish a combinational dose that would effectively suppress the rebound hyperphagia that accompanies refeeding. Each botanical or botanical combination was tested in a within-subject design. That is, one group (n = 10) received C aurantium (saline: 1, 3.2, 5.6, or 10 mg/kg), another group (n = 8) received R rosea (2% ethanol: 2, 6.4, or 20 mg/kg), and a third group (n = 10) received C aurantium (1, 3.2, 5.6, or 10 mg/kg) + R rosea (2, 6.4, or 20 mg/kg). For C aurantium + R rosea dosing, the vehicle was 2% ethanol. In a separate pilot study, it was determined that solubility and feeding effects of C aurantium were identical whether the vehicle was saline or 2% ethanol. For all testing, animals were deprived of food for 24 hours (beginning at 1100 hours) before each treatment. One hour before refeeding, animals received an oral gavage of the respective botanical or combination of botanicals. Total volume gavaged was 2.5 mL, and in the case of the combined treatment, animals received a mixture of both botanicals to ensure that they were gavaged only once per day at the same time as the other groups. Each oral gavage was randomized and separated by a 1-week washout period for animals within the groups. The 1-week washout period was chosen to make certain that the animals had normal eating patterns and body weight before receiving the subsequent dosing. Animals were deprived of chow at 1100 hours in the light phase. Refeeding was performed in the light phase (at 1100 hours) with the first 4 feeding time points occurring before lights out. Food intake and spillage were measured to the nearest 0.1 g. Food intake measurements were recorded at 0.5, 1, 2, 4, and 24 hours postrefeeding.
2.4. Dose combination effects on visceral illness by measuring kaolin intake
To determine whether the suppression of food intake by C aurantium (5.6 mg/kg) + R rosea (20 mg/kg) in combination was caused by visceral illness, pica behavior was measured after administration. One week after the dose series described in Section 2.3 was completed, the animals were randomly assigned into a group (n = 10) and placed on ad libitum chow with continuous access to nonnutritive dry kaolin pellets (kaolin with 1% acacia gum; K5001; Research Diets, New Brunswick, NJ, USA) for 7 days. After a 24-hour food deprivation, animals received an oral gavage of either 2% ethanol (vehicle) or C aurantium (5.6 mg/kg) + R rosea (20 mg/kg) in a randomized fashion. Dosing was performed 1 hour before refeeding. Food and kaolin intake measurements were recorded at 0.5, 1, 2, 4, and 24 hours postrefeeding. Food intake and spillage were measured to the nearest 0.1 g.
2.5. Effects of botanical treatment conditions on food intake and body weight during the 10-day subchronic treatment schedule in a model of diet-induced obesity
To determine the effects of the botanicals alone or in combination on neural, metabolic, and endocrine markers of diet-induced obesity, a total of 91 adult male Sprague-Dawley rats were exposed to a high-fat diet (Research Diets, D12492, 60% fat, 20% protein) for 13 weeks and remained on high-fat diet throughout. Animals were gavaged for 10 consecutive days with either C aurantium (5.6 mg/kg; Ca; n = 20), R rosea (20 mg/kg; Rr; n = 17), C aurantium (5.6 mg/kg) + R rosea (20 mg/kg; CR; n = 20), or vehicle (2% ethanol; V; n = 17). A group of pair-fed animals (PF; n = 17), which were not gavaged, received the equivalent average caloric intake of the CR group. Body weights and food intake were recorded daily following the first day of dosing. Food intake and spillage were measured to the nearest 0.1 g.
2.6. Fat pad weight and hormonal measurements after the 10-day subchronic treatment schedule
After the completion of the 10-day treatments, the Ca (n = 10), Rr (n = 9), CR (n = 10), V (n = 9), and PF (n = 9) groups were decapitated after a 24-hour food deprivation. A 24-hour food deprivation was used to eliminate confounding effects of a difference in food intake and normalize the metabolic state of the animals. Thus, animals were decapitated 48 hours after the final botanical dose at 1100 hours in the light phase. Animals from each group were decapitated in a counter-balanced fashion, and decapitations were performed in a separate room to minimize stress. Brain tissue was immediately sectioned using a brain matrix to standardize the sections for the frontal cortex, striatum, and medial hypothalamus regions based on anatomical markers for each area [20]. Tissue was weighed and stored at −80°C until assayed. Approximately 4 mL of trunk blood from each rat was collected into an EDTA-containing vacutainer tube, and approximately 20 μL was removed for blood glucose measurement (Alphatrak glucometer; Abbott Laboratories, Abbott Park, IL, USA). Blood samples were kept on ice until centrifugation at 3000 rpm for 10 minutes at 4°C, and plasma was removed and stored at −80°C until assayed. To quantify body fat, a midabdominal incision was performed on each carcass to identify and remove subcutaneous, retroperitoneal, and epididymial fat. These fat pads were weighed to the nearest 0.01 g. Plasma leptin and corticosterone levels were analyzed by radioimmunoassay (leptin sensitivity, 0.5 ng/mL [Millipore Corp, Billerica, MA, USA]; corticosterone sensitivity, 25 ng/mL [MP Biomedicals LLC, Santa Anna, CA, USA]).
2.7. Plasma and regional brain monoamine levels after botanical treatments and feeding conditions
Blood plasma samples and brain sections were analyzed by reverse-phase HPLC (Dionex Ultimate 3000, Thermofisher, Sunnyvale, CA, USA) with electrochemical detection (Coulochem III, Thermofisher, Sunnyvale, CA, USA). An acetonitrile-based phosphate buffer mobile phase (MD-TM; Dionex, Thermofisher, Sunnnyvale, CA, USA) was used for all experiments. The internal standard, 3,4-dihydroxybenzylamine, was added to all samples before extraction. Blood plasma was prepared for HPLC analysis using the plasma catecholamine extraction kit (Thermofisher, Sunnyvale, CA, USA) to measure levels of NE, dopamine (DA), and E. Individual brain sections for each region were homogenized in 550 μL of 0.1 N perchloric acid solution. Homogenates were then centrifuged at 12 000 rpm for 5 minutes. The supernatant was filtered using a 0.2-μm nylon disposable syringe filter before being analyzed. This extraction procedure permitted measurements of NE, DA, 5-HT, and the catecholamine metabolite homovanillic acid (HVA) by HPLC. Peak analysis was performed with Chromeleon 7.1 software (Dionex). Values for each monoamine and HVA were expressed as nanograms divided by the wet tissue weight (in milligrams) of each sample.
2.8. Hemodynamic measurements pretreatment and posttreatment in the botanical and control groups
For 3 consecutive days before beginning their respective 10-day treatments, animals were habituated to the noninvasive blood pressure (BP) CODA (Kent Scientific, Torrington, CT, USA) system. This computerized system measures systolic and diastolic BP, mean BP, and heart rate via tail volume pressure recordings. Animals were acclimated to the CODA system 5 minutes before the recording trials. Measurements for each time were determined by averaging the values for 3 successful recording trials. Animals were given 2 acclimation days followed by a baseline test day on the third day. After the completion of the 10-day treatments, the remaining animals in each group (Ca: n = 10, Rr: n = 8, CR: n = 10, V: n = 8, and PF: n = 8) were tested in the CODA system to determine their posttreatment values. For this, all groups were tested 24 hours after the final dose of their respective treatment.
2.9. Statistical analyses
Power analysis was performed to determine adequate sample sizes for each group based on the expected variance for each end point. The data are presented as the means ± SE. Acute effects of the botanicals on food intake and kaolin intake were measured by 2-way repeated-measures analysis of variance ANOVA. Acute food intakes for each botanical treatment (C aurantium, R rosea, and C aurantium + R rosea) were analyzed separately to account for the differences in treatment length. Kaolin intake and acute chow intake data were analyzed separately with a dependent t test. For the 10-day treatments, body weight, cumulative food intake, and hemodynamic measurements were analyzed using a 2-way ANOVA with repeated measures. Body fat and hormone assays were analyzed with a 1-way ANOVA, and quantification of catecholamine concentrations was analyzed with a 1-way ANOVA for each region. Post hoc comparisons were made when appropriate with least significant difference (LSD) tests. All statistical analyses and power analyses were performed using Statistica 7.1 software (StatSoft Inc, Tulsa, OK, USA), and significance was set at α = .05.
3. Results
3.1. Dose response of C aurantium and R rosea alone or in combination on acute food intake in 24-hour food-deprived rats
The individual doses of C aurantium (1-10 mg/kg) and R rosea (2-20 mg/kg) did not suppress the refeeding response compared with vehicle at all time points measured (see Fig. 1A and B for 24-hour intake). When the botanicals were administered in combination, however, there were significant differences among doses (F12,108 = 3.7, P < .005) at the 24 hour time point only. Post hoc LSD tests indicated that there was a significant difference between vehicle and the C aurantium (3.2 mg/kg) + R rosea (20 mg/kg) (P < .01) and C aurantium (5.6 mg/kg) + R rosea (20 mg/kg) combination (P < .005; see Fig. 1C).
Fig. 1.
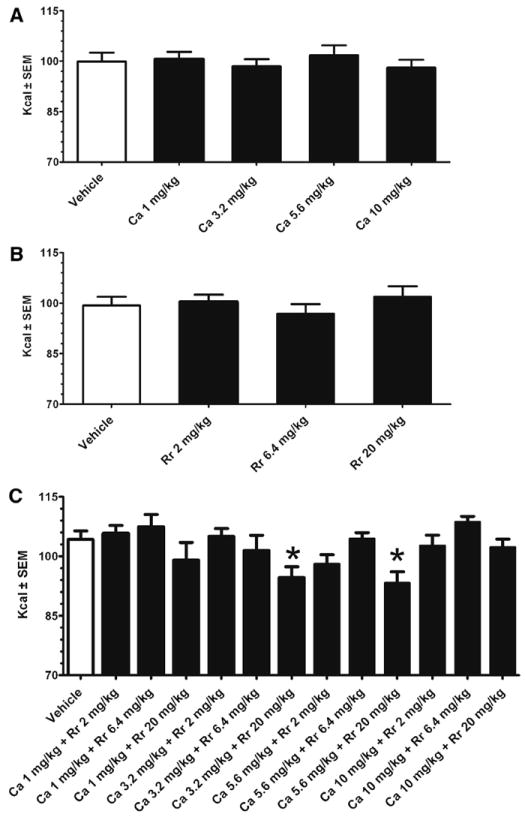
Food-deprived (24 hours) refeeding response of standard chow at the 24 hour time point after dosing of C aurantium, R rosea, and C aurantium + R rosea. Intakes (in kilocalories) are mean ± SE. Data represent intake from separate groups of adult male Sprague-Dawley rats (approximately 300 g) for each botanical. In this within-subject design, botanicals or vehicle were randomly administered by oral gavage 1-week apart. Food intake and spillage were measured to the nearest 0.1 g and converted to kcal. Data were analyzed by a 2-way repeated-measures ANOVA followed by post hoc LSD test. (A) C aurantium (n = 10) did not significantly influence food intake compared with vehicle (saline). (B) R rosea (n = 8) did not significantly influence food intake compared with vehicle (2% ethanol). (C) C aurantium + R rosea (n = 10) significantly influenced food intake (P < .005). There was a significant difference from vehicle (2% ethanol) at the dose indicated by an asterisk.
3.2. Dose combination effects on visceral illness by measuring kaolin intake
Before beginning the 10-day dosing experiments, the combinational dose was tested to determine whether it reduced acute food by malaise or nausea by measuring pica behavior. In a separate groups of animals (n = 10), kaolin intake and chow intake were measured following the dose of either vehicle (2% ethanol) or C aurantium (5.6 mg/kg) + R rosea (20 mg/kg) separated by a 1-week washout period. As illustrated in Fig. 2A, there was no significant difference in kaolin intake between combinational doses and vehicle. There was, however, a decrease in standard chow intake (P < .05; see Fig. 2B).
Fig. 2.
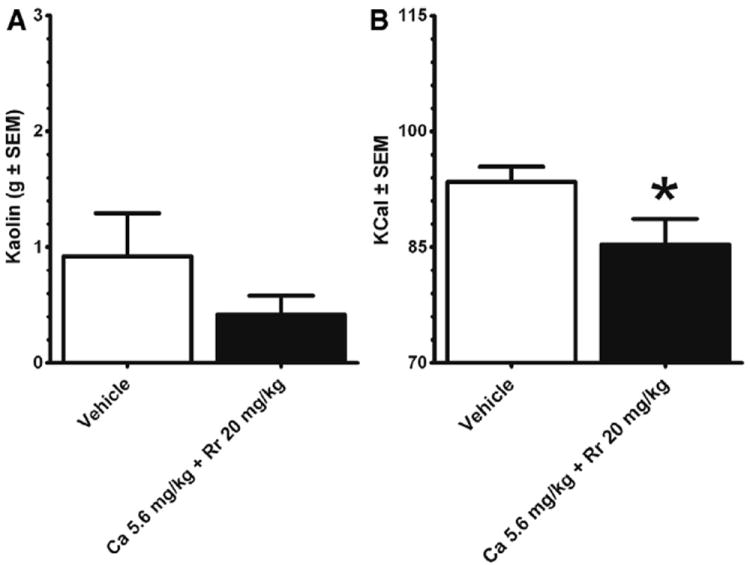
Food-deprived (24 hours) refeeding response of nonnutritive kaolin at the 24 hour time point after dosing of C aurantium (5.6 mg/kg) + R rosea (20 mg/kg). Kaolin and chow were presented after the 24-hour food deprivation. (A) Kaolin intakes (in grams) are mean ± SE. (B) Standard chow intakes are expressed as kcal ± SEM. Adult male Sprague-Dawley rats (n = 10) used in this experiment were randomly assigned after completion of the experiments illustrated in Fig 1. The C aurantium + R rosea dose compared with vehicle (2% ethanol) administered 1 week apart did not significantly influence kaolin intake but decreased chow intake (P < .05). Data were analyzed by a dependent t test. Significance is designated by an asterisk.
3.3. Effects of botanical treatment conditions on food intake and body weight during the 10-day subchronic treatment schedule in a model of diet-induced obesity
The pretreatment body weights for each group were 473 ± 8.9 g for C aurantium, 473 ± 7.9 g for R rosea, 475 ± 8.2 g for C aurantium + R rosea, 478 ± 7.7 g for vehicle, and 473 ± 6.7 g for pair fed. For the 10-day cumulative food intake, there was not an overall difference, but a planned comparison between C aurantiun + R rosea and vehicle group approached significance (P = .09; see Fig. 3A). Overall, the body weights were reduced over the 10-day dosing, with an effect over time (F9,74 = 3.1, P < .01) and a group × time effect (F36,774 = 2.1, P < .001). However, post hoc testing did not reveal any group differences (see Fig. 3B).
Fig. 3.
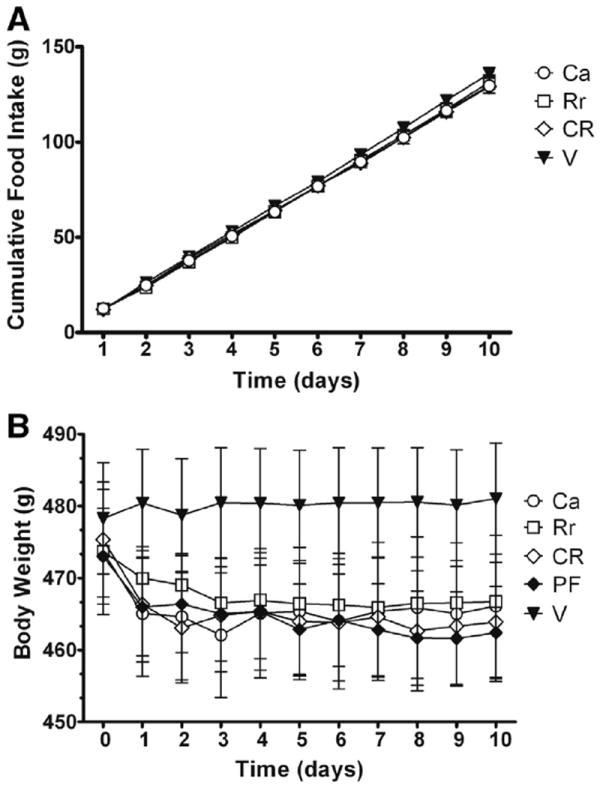
Cumulative food intake and mean body weights of animals over the 10-day treatment period. Adult male Sprague-Dawley rats exposed to high-fat diet for 13 weeks received a 10-day treatment of Ca (5.6 mg/kg; n = 20), Rr (20 mg/kg; n = 17), CR (5.6 mg/kg + 20 mg/kg; n = 20), or V (2% ethanol; n = 17) or were PF (n = 17) to the CR group. Day 0 is the pretreatment body weight for each group. Data were analyzed by 2-way repeated-measures ANOVA followed by post hoc LSD test. (A) Mean (± SEM; in grams) cumulative high-fat diet intake over the 10-day treatment period. (B) Mean body weights over the 10-day treatment period. There was a significant difference overall group × time effect (F36,774 = 2.1, P < .001), and post hoc testing failed to reveal any group differences.
3.4. Fat pad weight and hormonal measurements after the 10-day subchronic treatment schedule
Fat was expressed as fat weight in grams divided by 100 grams of body weight. Despite no statistical group differences in body weight, there were group differences in visceral fat, but not subcutaneous fat (see Fig. 4A). For the retroperitoneal fat, planned comparisons revealed a reduction in the C aurantium + R rosea group compared with the C aurantium treatment group (P < .05; see Fig. 4B). For epididymal fat, there was a difference among groups (F4,42 = 3.9, P < .01). Post hoc testing revealed that there was a decrease in the C aurantium + R rosea combination group compared with the C aurantium (P < .05), R rosea, and pair-fed groups (P < .05; see Fig. 4C). When epididymal fat + retroperitoneal fat values were combined, as to represent visceral fat, there was a difference among groups (F4,42 = 2.7, P < .05; see Fig. 4D). Post hoc testing revealed reduced visceral fat in the C aurantium + R rosea group compared with the C aurantium, R rosea, and pair-fed groups (P < .05, for all). In addition, the combined visceral fat showed a reduction between the C aurantium + R rosea and the vehicle-treated group; this reduction approached significance (P = .09). Trunk blood was examined for blood glucose levels, corticosterone, and leptin. There were no differences in blood glucose levels among groups. Although the C aurantium + R rosea combination group had the lowest plasma leptin levels, there was no statistical difference among groups. Plasma corticosterone was significantly different among groups (F4,76 = 2.5, P < .05). A post hoc test further revealed that the C aurantium group had a significantly lower level of corticosterone when compared with the pair-fed and vehicle groups (P < .05 for both; see Table 1).
Fig. 4.
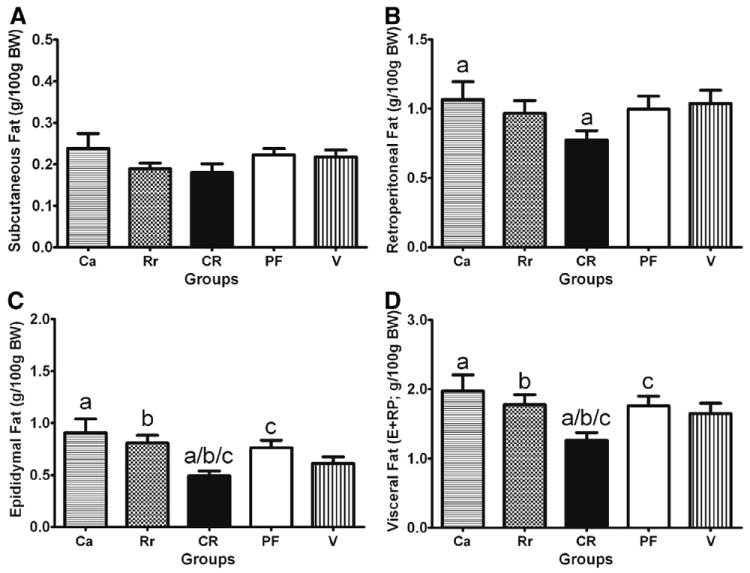
Mean white adipose tissue among groups after the 10-day treatment period. Adult male Sprague-Dawley rats exposed to high-fat diet for 13 weeks received a 10-day treatment of Ca (5.6 mg/kg; n = 10), Rr (20 mg/kg; n = 9), CR (5.6 mg/kg + 20 mg/kg; n = 10), or V (2% ethanol; n = 9) or were PF (n = 9) to the CR group. (A) Subcutaneous fat pad weights (g/100 g body weight). (B) Retroperitoneal fat pad weights (g/100 g body weight). (C) Epididymal fat pad weights (g/100 g body weight). (D) Visceral fat pad weight, which is epididymal (E) + retroperitoneal (RP) fat pad weights (g/100 g body weight). Comparisons were made using a 1-way ANOVA with post hoc LSD tests. Similar letters indicate a significant difference (P < .05) between groups.
Table 1.
Blood parameters and plasma hormone levels after botanical treatments
| Ca | Rr | CR | V | PF | |
|---|---|---|---|---|---|
| Glucose (mg/dL) | 110 ± 1.87 | 113 ± 1.97 | 115 ± 1.87 | 112 ± 1.97 | 111 ± 1.97 |
| Leptin (ng/mL) | 3.7 ± 0.44 | 3.94 ± 0.47 | 3.0 ± 0.44 | 3.55 ± 0.47 | 3.23 ± 0.47 |
| Corticosterone (ng/mL) | 80.7 ± 31.6 *,a | 107.8 ± 33.3 | 111.8 ± 31.6 | 132.5 ± 33.3 | 123.2 ± 33.3a |
Adult male Sprague-Dawley rats fed a high-fat diet for 13 weeks received a 10-day treatment of Ca (5.6 mg/kg; n = 10), Rr (20 mg/kg; n = 9), CR (5.6 mg/kg + 20 mg/kg; n = 10), or V (n = 9) or were PF (n = 9) to the CR group. Values are means ± SE. Data were analyzed with 1-way ANOVA with post hoc LSD tests. Similar letters denote a significant difference (P < .05) between groups.
Significant difference (P < .05) from vehicle.
3.5. Plasma and regional brain monoamine levels after botanical treatments and feeding conditions
Overall, there was a difference among groups in plasma NE levels (F4,38 = 2.6, P < .05). Post hoc testing revealed that the C aurantium + R rosea combination group had reduced NE levels compared with the vehicle and pair-fed groups (P < .05). Groups receiving C aurantium or R rosea alone had significantly decreased plasma NE levels compared with the vehicle group (P < .05). No differences were observed in plasma E levels between groups (see Table 2). Brain regional dissection revealed differences in monoamine levels. Medial hypothalamic NE was also influenced by botanical treatment (F4,38 = 2.7, P < .05). Post hoc testing revealed an elevation in the C aurantium + R rosea group compared with all other groups (P < .05 for all). There also was a difference among groups for frontal cortex DA (F4,38 = 2.5, P = .05). Planned comparisons revealed that the C aurantium + R rosea group had elevated DA levels compared with the pair-fed and C aurantium groups (P < .05 for both; see Table 3).
Table 2.
Plasma catecholamine concentrations (ng/mL) after botanical treatments
| Ca | Rr | CR | V | PF | |
|---|---|---|---|---|---|
| NE | 13.9 ± 2.2 * | 14 ± 2.3 * | 12.4 ± 2.1 *,a | 20.7 ± 2.3 | 19.1 ± 2.3a |
| E | 18.9 ± 2.8 | 18.6 ± 2.9 | 20.4 ± 2.6 | 21.9 ± 2.9 | 23.4 ± 2.9 |
Adult male Sprague-Dawley rats fed a high-fat diet for 13 weeks received a 10-day treatment of Ca (5.6 mg/kg; n = 10), Rr (20 mg/kg; n = 9), CR (5.6 mg/kg + 20 mg/kg; n = 10), or V (n = 9) or were PF (n = 9) to the CR group. Values are means ± SE. Data were analyzed with 1-way ANOVA with post hoc LSD tests. Similar letters denote a significant difference (P < .05) between groups.
Significant differences (P < .05) from vehicle.
Table 3.
Regional monoamine concentrations (ng/mg of tissue) after botanical treatments
| Ca | Rr | CR | V | PF | |
|---|---|---|---|---|---|
| Medial hypothalamus | |||||
| NE | 1.55 ± 0.07 | 1.58 ± 0.08 | 1.8 ± 0.07 * | 1.59 ± 0.08 | 1.52 ± 0.08 |
| DA | 1.09 ± 0.23 | 1.11 ± 0.26 | 1.75 ± 0.23 | 1.67 ± 0.28 | 1.38 ± 0.26 |
| 5-HT | 1.87 ± 0.25 | 1.78 ± 0.3 | 2.14 ± 0.27 | 1.63 ± 0.3 | 1.82 ± 0.28 |
| HVA | 0.55 ± 0.13 | 0.48 ± 0.14 | 0.83 ± 0.13 | 0.74 ± 0.14 | 0.8 ± 0.14 |
| Striatum | |||||
| DA | 6.04 ± 0.73 | 8.03 ± 0.82 | 6.96 ± 0.73 | 6.64 ± 0.87 | 7.22 ± 0.82 |
| HVA | 2.77 ± 0.34 | 3.78 ± 0.38 | 3.25 ± 0.34 | 2.95 ± 0.41 | 3.93 ± 0.38 |
| Frontal cortex | |||||
| NE | 1.2 ± 0.07 | 1.12 ± 0.08 | 1.15 ± 0.08 | 1.21 ± 0.08 | 1.02 ± 0.08 |
| DA | 0.37 ± 0.15a | 1.13 ± 0.28 | 1.27 ± 0.23a,b | 1.05 ± 0.21 | 0.41 ± 0.28b |
| 5-HT | 3.54 ± 0.23 | 3.28 ± 0.26 | 3.52 ± 0.23 | 3.21 ± 0.28 | 2.99 ± 0.26 |
| HVA | 0.97 ± 0.26 | 0.85 ± 0.24 | 1.1 ± 0.22 | 0.86 ± 0.28 | 0.7 ± 0.24 |
Adult male Sprague-Dawley rats fed a high-fat diet for 13 weeks received a 10-day treatment of Ca (5.6 mg/kg; n = 10), Rr (20 mg/kg; n = 9), CR (5.6 mg/kg + 20 mg/kg; n = 10), or V (n = 9) or were PF (n = 9) to the CR group. Values are means ± SE. Data were analyzed with 1-way ANOVA with post hoc LSD tests. Dopamine in the frontal cortex was analyzed using planned comparisons. Similar letters denote a significant difference (P < .05) between groups.
Significant elevation (P < .05) compared with all groups.
3.6. Hemodynamic measurements pretreatment and posttreatment in the botanical and control groups
Cardiovascular measurements of heart rate, diastolic BP, systolic BP, and mean BP by using noninvasive volume pressure recording were performed after the 10-day treatment. Measurements indicated that there was an effect on heart rate (F4,39 = 2.6, P < .05). Post hoc LSD testing revealed that the C aurantium dose increased heart rate compared with vehicle and pair-fed animals (P < .05; see Table 4). There was also a significant difference in heart rate between the animals receiving the C aurantium + R rosea and the pair-fed animals (P < .05; see Table 4).
Table 4.
Hemodynamic measurements before and after botanical treatment schedule
| Parameter | Baseline | Ca | Rr | CR | V | PF |
|---|---|---|---|---|---|---|
| SBP (mm Hg) | 167 ± 2.2 | 167 ± 4.9 | 172 ± 5.1 | 170 ± 5.1 | 172 ± 4.8 | 179 ± 4.9 |
| DBP (mm Hg) | 120 ± 2.0 | 119 ± 4.4 | 124 ± 4.8 | 124 ± 4.0 | 128 ± 5.6 | 132 ± 5.3 |
| MBP (mm Hg) | 135 ± 2.0 | 135 ± 4.3 | 139 ± 4.8 | 139 ± 4.1 | 142 ± 5.2 | 148 ± 5.1 |
| Heart rate (beats/min) | 448 ± 4.1 | 468 ± 11.7 *,a | 452 ± 4.4 | 460 ± 9.4b | 437 ± 9.9 | 431 ± 10.7a,b |
Adult male Sprague-Dawley rats fed a high-fat diet for 13 weeks received a 10-day treatment of Ca (5.6 mg/kg; n = 10), Rr (20 mg/kg; n = 8), CR (5.6 mg/kg + 20 mg/kg; n = 10), or V (n = 8) or were PF (n = 8) to the CR group. Values are means ± SE. Baseline values are the mean values of all animals before treatments. Data were analyzed with 1-way ANOVA with post hoc LSD tests. Similar letters denote a significant difference (P < .05) between groups. SBP, systolic BP; DBP, diastolic BP; MBP, mean BP.
Significant differences (P < .05) from vehicle.
4. Discussion
This study was designed to determine the effects of the botanical extracts C aurantium, R rosea, and their combination on food intake and body weight regulation in diet-induced obese adult male Sprague-Dawley rats. Although a 10-day treatment with the botanicals did not impact feeding or body weight in obese animals, the administration of C aurantium + R rosea did result in a decrease in visceral fat weight. The botanical combination also reduced plasma NE levels and elevated hypothalamic NE levels. This was accompanied by slight improvement in the C aurantium–induced increases in heart rate.
To determine a botanical concentration that impacts food intake, we measured the feeding suppression of each botanical alone or in combination after a 24-hour food deprivation. Although C aurantium (1-10 mg/kg) and R rosea (2-20 mg/kg) alone did not influence food intake, it was determined that a 5.6-mg/kg dose of C aurantium in combination with 20 mg/kg of R rosea produced an approximate 10% feeding suppression. There was not a significant dose-dependent increase in the feeding suppression of the botanical combination when the concentration of C aurantium was increased from 3.2 to 5.6 mg/kg. Moreover, when the concentration of C aurantium was increased to 10 mg/kg, the feeding suppression was not significantly different from vehicle. One unexplored reason for this lack of a dose-dependent response could be that the adverse effects of C aurantium were increased with the 10-mg/kg dose, which is similar to what has been reported by others [18]. To measure whether the suppression of food intake by 5.6 mg/kg of C aurantium + 20 mg/kg of R rosea was caused by visceral illness, kaolin (clay) intake along with food intake was measured for 24 hours after dosing. Kaolin intake is often used as a metric of aversion. Because rats lack the capacity to vomit, they will engage in pica behavior as a result of visceral illness [21,22]. Our results confirmed that C auratium + R rosea reduced standard chow intake without producing visceral illness. It further confirmed that individual doses of R rosea tested did not influence the refeeding response, as demonstrated by others [17]. Using a range of doses (10, 15, and 20 mg/kg), it was determined that R rosea did not alter standard chow intake in nondeprived and deprived (20 hours) feeding conditions, but R rosea (20 mg/kg) reversed the anorectic effects of restraint stress and intracerebroventricular injections of corticotrophin releasing factor [17]. Along these lines and supportive of its putative adaptogenic properties, R rosea (20 mg/kg) has been demonstrated to abolish the binge eating induced by yo-yo dieting and stress in rodents and to attenuate the associated corticosterone elevations [16]. Interestingly, in our study, subchronic treatment (10 days) with C aurantium, not R rosea, was associated with a reduction in plasma corticosterone after a 24-hour food deprivation in animals on high-fat diet. Although C aurantium has not been reported to reduce plasma corticosterone, a citrus flavonoid, naringen, has been demonstrated to possess anxiolytic properties [23]. Our finding of a reduction in plasma corticosterone after subchronic dosing of C aurantium and acute food deprivation warrants further investigation. An examination of how the diurnal rhythm of corticosterone varies with botanical treatments would be informative, as well. Although C aurantium is reported to have antiobesity effects [24], in our study, C aurantium did not result in a reduction in standard chow intake after a 24-hour food deprivation. Reduction in food intake has been demonstrated with C aurantium (2.5-20 mg/kg), although this was after a 7-day treatment period [18]. Future studies are therefore needed to investigate our reported novel interaction between C aurantium + R rosea to suppress food intake after food deprivation. An additional strategy would be to investigate the combinational effects of the bioactive constituents of C aurantium and R rosea. Recent findings have demonstrated that salidroside, an active component of R rosea when combined with Hypericum perforatum (St John’s Wort) produces a synergic effect on binge eating [25].
The botanical combination of C aurantium + R rosea was not effective in reducing food intake and body weight in an animal model of diet-induced obesity. After a 10-day treatment with C aurantium + R rosea, animals did show reductions in visceral fat weight compared with other botanical treatments and the pair-fed group. There was also a trend with the C aurantium + R rosea treatment to reduce visceral fat compared with the vehicle group. In vitro actions of synephrine on rat and human adipose cells have demonstrated only a modest stimulatory effect on the lipolytic pathways, whereas other alkaloids found in C aurantium, such as tyramine and N-methyl tyramine, exhibit antilipolytic properties [26]. Recent findings indicate that citrus flavonoids, specifically nobiletin, possess potent lipolytic actions and could account for the synephrine-independent lipolytic action of C aurantium [27,28]. Notably in our study, treatments with C aurantium (5.6 mg/kg) alone did not reduce white adipose weight compared with vehicle, but a reduction in white adipose tissue weight was observed when C aurantium was combined with R rosea. In addition, obese animals treated with C aurantium + R rosea exhibited a trend for lower leptin levels, which is also suggestive of a reduction in white adipose tissue weight. The discovery of a reduction in white adipose tissue weight by C aurantium + R rosea is entirely novel, and it likely represents a unique mechanism because R rosea individually has not been associated with altering adiposity or stimulating lipolytic mechanisms. One limitation of our findings was that leptin and adipose weight were measured after a 24-hour food deprivation. Although this deprivation was used to normalize the acute metabolic factors caused by the different treatments, it is expected that 24-hour deprivation would decrease leptin and adiposity levels in all groups. Nonetheless, the greater reduction by C aurantium + R rosea is an intriguing finding that requires further investigation.
Treatments with C aurantium have been associated with cardiovascular alterations. Several case reports have linked C aurantium–containing compounds with adverse cardiac effects [29,30] and even ischemic stroke [31]. In male rats, C aurantium (20 mg/kg) produced ventricular alterations and enlargement of the QRS complex on electrocardiograms in 80% of treated rats [18]. In the same study, C aurantium resulted in 50% mortality in rats treated with 20 mg/kg and 30% mortality in rats treated with 10 mg/kg for 15 days [18]. In a recent study by Hansen and colleagues [32], it was found in female rats treated with C aurantium (10 mg/kg) had an increased heart rate 2 hours postdosing and an increased heart rate 1, 2, 4, and 8 hours postdosing with 50 mg/kg. It was also noted that similar to our study, C aurantium treatments (10 or 50 mg/kg) did not alter standard chow intake or body weight compared with vehicle (0.25% methyl-cellulose) for 28 days. The results from our study demonstrate an increase in heart rate after a 10-day treatment with 5.6 mg/kg compared with vehicle in rats exposed to more than 13 weeks of high-fat feeding. In addition to the dietary conditions, another difference between our study and the report by Hansen and colleagues [32] is that we measured hemodynamic parameters at 24 hours postdosing. Another novel finding from our study was that when C aurantium was combined with R rosea, we saw a slight reduction in heart rate. This reduction was still significantly higher than the pair-fed group but was not statistically different from the vehicle-treated group. It should be noted, however, that the baseline values for the hemodynamic measures were likely elevated because of maintenance on a high-fat diet. Notwithstanding, the mechanism of action whereby R rosea improves C aurantium–induced tachycardia needs to be elucidated. Prolonged treatments with R rosea have been shown to improve cardiac output in diabetic rats [33]. R rosea improvements on cardiac output resulted from BP changes after a 21-day treatment (75 mg/kg, intraperitoneal) and were dependent on peroxisome proliferator–activated receptor δ signaling [33]. One mechanism that could have led to improvements in both adipose tissue weight and heart rate is by sympathetic output alterations. Our findings indicate that all botanical treatments used in our study decreased plasma NE compared with vehicle treatment, with the combination producing the lowest levels. This could indicate that C aurantium and R rosea alone can impact sympathetic tone, an effect that is exacerbated when the botanicals are administered in combination. This further indicates that C aurantium is likely to have direct actions on the heart rate, rather than simply an increase in sympathetic tone. One limitation of these interpretations, however, is that animals were fed and maintained on a high-fat diet and these values were not measured in lean controls. The increase in weight gain and high-fat diet has been demonstrated to alter autonomic nervous system output and reactivity [34,35]. Interestingly, hypothalamic NE was specifically elevated in animals treated with C aurantium + R rosea compared with all groups, suggesting alterations in central noradrenergic pathways as a consequence of treatment with the botanical combination. Brain NE was measured by tissue homogenates of the medial hypothalamus. These homogenates would have been inclusive of the paraventricular nucleus and the ventro-medial nucleus, which receives substantial NE input to influence body weight, food intake, and sympathetic output. The increase in medial hypothalamic NE was accompanied by an increase in frontal cortex DA levels. In particular, frontal cortex DA was elevated in the C aurantium + R rosea compared with the C aurantium and pair-fed groups. In vitro studies have indicated that methanol and water extracts of R rosea inhibit the enzymatic action of human monoamine oxidases (MAOs) [36]. Specifically, the isolated bioactive constituent, rosiridin, inhibited the action of MAO A by 16% and MAO B by 83%. Because the 2 MAO isoforms, A and B, regulate the degradation of NE and DA, respectively, one possibility is that R rosea interfered with the degradation of the NE and DA. Future studies will target specific hypothalamic nuclei and the medial prefrontal cortex to determine how C aurantium + R rosea and their bioactive constituents influence central monoamine pathways.
The use of combinational therapy offers the potential for synergistic interactions between compounds to produce a greater degree of weight loss than the sum of the individual effects of each compound [37]. Given that smaller doses of each compound can be used to produce effective weight loss, compounds that interact synergistically also have the potential advantage of minimizing associated dose-dependent adverse effects [38,39]. Based on our initial findings, we accept our hypothesis that combinational doses of C aurantium and R rosea promote improvements in the alterations caused by high-fat feeding and obesity. Notably, these effects appear to result from an interaction between the 2 botanicals and their bioactive constituents, representing a novel mechanism that requires further examination.
Acknowledgments
The authors would like to thank David Ribnicky for his assistance with the botanical extracts, Wes Darcy for his assistance with the CODA system, and Kathy Manger for her editorial comments and corrections. The project was supported by P50AT002776 from the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements, which funds the Botanical Research Center of Pennington Biomedical Research Center and the Department of Plant Biology and Pathology in the School of Environmental and Biological Sciences of Rutgers University.
Abbreviations
- ANOVA
analysis of variance
- BP
blood pressure
- Ca
Citrus aurantium
- CR
Citrus aurantium + Rhodiola rosea
- DA
dopamine
- E
epinephrine
- HVA
homovanillic acid
- MAO
monoamine oxidase
- NE
norepinephrine
- PF
pair fed
- Rr
Rhodiola rosea
- V
vehicle
References
- 1.Haaz S, Fontaine KR, Cutter G, Limdi N, Perumean-Chaney S, Allison DB. Citrus aurantium and synephrine alkaloids in the treatment of overweight and obesity: an update. Obes Rev. 2006;7:79–88. doi: 10.1111/j.1467-789X.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- 2.Dwyer JT, Allison DB, Coates PM. Dietary supplements in weight reduction. J Am Diet Assoc. 2005;105:S80–6. doi: 10.1016/j.jada.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 3.Heber D. Herbal preparations for obesity: are they useful? Prim Care. 2003;30:441–63. doi: 10.1016/s0095-4543(03)00015-0. [DOI] [PubMed] [Google Scholar]
- 4.Andraws R, Chawla P, Brown DL. Cardiovascular effects of ephedra alkaloids: a comprehensive review. Prog Cardiovasc Dis. 2005;47:217–25. doi: 10.1016/j.pcad.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Airriess CN, Rudling JE, Midgley JM, Evans PD. Selective inhibition of adenylyl cyclase by octopamine via a human cloned alpha 2A-adrenoceptor. Br J Pharmacol. 1997;122:191–8. doi: 10.1038/sj.bjp.0701348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim KW, Kim HD, Jung JS, Woo RS, Kim HS, Suh HW, et al. Characterization of antidepressant-like effects of p-synephrine stereoisomers. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:21–6. doi: 10.1007/s002100100416. [DOI] [PubMed] [Google Scholar]
- 7.Pellati F, Benvenuti S. Chromatographic and electrophoretic methods for the analysis of phenethylamine [corrected] alkaloids in Citrus aurantium. J Chromatogr A. 2007;1161:71–88. doi: 10.1016/j.chroma.2007.05.097. [DOI] [PubMed] [Google Scholar]
- 8.Stohs SJ, Preuss HG, Shara M. The safety of Citrus aurantium (bitter orange) and its primary protoalkaloid p-synephrine. Phytother Res. 2011;25:1421–8. doi: 10.1002/ptr.3490. [DOI] [PubMed] [Google Scholar]
- 9.Allison DB, Cutter G, Poehlman ET, Moore DR, Barnes S. Exactly which synephrine alkaloids does Citrus aurantium (bitter orange) contain? Int J Obes (Lond) 2005;29:443–6. doi: 10.1038/sj.ijo.0802879. [DOI] [PubMed] [Google Scholar]
- 10.Arbo MD, Schmitt GC, Limberger MF, Charao MF, Moro AM, Ribeiro GL, et al. Subchronic toxicity of Citrus aurantium L. (Rutaceae) extract and p-synephrine in mice. Regul Toxicol Pharmacol. 2009;54:114–7. doi: 10.1016/j.yrtph.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Stohs SJ, Preuss HG, Shara M. A review of the human clinical studies involving Citrus aurantium (bitter orange) extract and its primary protoalkaloid p-synephrine. Int J Med Sci. 2012;9:527–38. doi: 10.7150/ijms.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tounsi MS, Wannes WA, Ouerghemmi I, Jegham S, Ben Njima Y, Hamdaoui G, et al. Juice components and antioxidant capacity of four Tunisian citrus varieties. J Sci Food Agric. 2010;91:142–51. doi: 10.1002/jsfa.4164. [DOI] [PubMed] [Google Scholar]
- 13.Kelly GS. Rhodiola rosea: a possible plant adaptogen. Altern Med Rev. 2001;6:293–302. [PubMed] [Google Scholar]
- 14.Spasov AA, Wikman GK, Mandrikov VB, Mironova IA, Neumoin VV. A double-blind, placebo-controlled pilot study of the stimulating and adaptogenic effect of Rhodiola rosea SHR-5 extract on the fatigue of students caused by stress during an examination period with a repeated low-dose regimen. Phytomedicine. 2000;7:85–9. doi: 10.1016/S0944-7113(00)80078-1. [DOI] [PubMed] [Google Scholar]
- 15.Bocharov EV, Ivanova-Smolenskaya IA, Poleshchuk VV, Kucheryanu VG, Il’enko VA, Bocharova OA. Therapeutic efficacy of the neuroprotective plant adaptogen in neurode-generative disease (Parkinson’s disease as an example) Bull Exp Biol Med. 2010;149:682–4. doi: 10.1007/s10517-010-1023-z. [DOI] [PubMed] [Google Scholar]
- 16.Cifani C, Micioni Di BM, Vitale G, Ruggieri V, Ciccocioppo R, Massi M. Effect of salidroside, active principle of Rhodiola rosea extract, on binge eating. Physiol Behav. 2011;101:555–62. doi: 10.1016/j.physbeh.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Mattioli L, Perfumi M. Rhodiola rosea L. extract reduces stress- and CRF-induced anorexia in rats. J Psychopharmacol. 2007;21:742–50. doi: 10.1177/0269881106074064. [DOI] [PubMed] [Google Scholar]
- 18.Calapai G, Firenzuoli F, Saitta A, Squadrito F, Arlotta MR, Constantino G, et al. Antiobesity and cardiovascular toxic effects of Citrus aurantium extracts in the rat: a preliminary report. Fitoterapia. 1999;70:586–92. [Google Scholar]
- 19.Larsen PJ, Fledelius C, Knudsen LB, Tang-Christensen M. Systemic administration of the long-acting GLP-1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes. 2001;50:2530–9. doi: 10.2337/diabetes.50.11.2530. [DOI] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego: Academic Press; 1998. [Google Scholar]
- 21.Andrews PL, Horn CC. Signals for nausea and emesis: implications for models of upper gastrointestinal diseases. Auton Neurosci. 2006;125:100–15. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Jonghe BC, Horn CC. Chemotherapy-induced pica and anorexia are reduced by common hepatic branch vagotomy in the rat. Am J Physiol Regul Integr Comp Physiol. 2008;294:R756–65. doi: 10.1152/ajpregu.00820.2007. [DOI] [PubMed] [Google Scholar]
- 23.Yi LT, Li J, Li HC, Su DX, Quan XB, He XC, et al. Antidepressant-like behavioral, neurochemical and neuroendocrine effects of naringenin in the mouse repeated tail suspension test. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:175–81. doi: 10.1016/j.pnpbp.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Moro CO, Basile G. Obesity and medicinal plants. Fitoterapia. 2000;71(Suppl 1):S73–82. doi: 10.1016/s0367-326x(00)00177-5. [DOI] [PubMed] [Google Scholar]
- 25.Micioni Di Bonaventura MV, Vitale G, Massi M, Cifani C. Effect of Hypericum perforatum extract in an experimental model of binge eating in female rats. J Obes. 2012;2012:956137. doi: 10.1155/2012/956137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercader J, Wanecq E, Chen J, Carpene C. Isopropylnorsynephrine is a stronger lipolytic agent in human adipocytes than synephrine and other amines present in Citrus aurantium. J Physiol Biochem. 2011;67:443–52. doi: 10.1007/s13105-011-0078-2. [DOI] [PubMed] [Google Scholar]
- 27.Kim GS, Park HJ, Woo JH, Kim MK, Koh PO, Min W, et al. Citrus aurantium flavonoids inhibit adipogenesis through the Akt signaling pathway in 3T3-L1 cells. BMC Complement Altern Med. 2012;12:31. doi: 10.1186/1472-6882-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YS, Cha BY, Choi SS, Choi BK, Yonezawa T, Teruya T, et al. Nobiletin improves obesity and insulin resistance in high-fat diet–induced obese mice. J Nutr Biochem. 2013;24:156–62. doi: 10.1016/j.jnutbio.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Firenzuoli F, Gori L, Galapai C. Adverse reaction to an adrenergic herbal extract (Citrus aurantium) Phytomedicine. 2005;12:247–8. doi: 10.1016/j.phymed.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Nasir JM, Durning SJ, Ferguson M, Barold HS, Haigney MC. Exercise-induced syncope associated with QT prolongation and ephedra-free Xenadrine. Mayo Clin Proc. 2004;79:1059–62. doi: 10.4065/79.8.1059. [DOI] [PubMed] [Google Scholar]
- 31.Bouchard NC, Howland MA, Greller HA, Hoffman RS, Nelson LS. Ischemic stroke associated with use of an ephedra-free dietary supplement containing synephrine. Mayo Clin Proc. 2005;80:541–5. doi: 10.4065/80.4.541. [DOI] [PubMed] [Google Scholar]
- 32.Hansen DK, George NI, White GE, Pellicore LS, Abdel-Rahman A, Fabricant D. Physiological effects following administration of Citrus aurantium for 28 days in rats. Toxicol Appl Pharmacol. 2012;261:236–47. doi: 10.1016/j.taap.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Cheng YZ, Chen LJ, Lee WJ, Chen MF, Jung Lin H, Cheng JT. Increase of myocardial performance by Rhodiola-ethanol extract in diabetic rats. J Ethnopharmacol. 2012;144:234–9. doi: 10.1016/j.jep.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 34.Chantemele EJ, Mintz JD, Rainey WE, Stepp DW. Impact of leptin-mediated sympatho-activation on cardiovascular function in obese mice. Hypertension. 2011;58:271–9. doi: 10.1161/HYPERTENSIONAHA.110.168427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rocchini AP, Mao HZ, Babu K, Marker P, Rocchini AJ. Clonidine prevents insulin resistance and hypertension in obese dogs. Hypertension. 1999;33:548–53. doi: 10.1161/01.hyp.33.1.548. [DOI] [PubMed] [Google Scholar]
- 36.van Diermen D, Marston A, Bravo J, Reist M, Carrupt PA, Hostettmann K. Monoamine oxidase inhibition by Rhodiola rosea L. roots. J Ethnopharmacol. 2009;122:397–401. doi: 10.1016/j.jep.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Roth JD, Trevaskis JL, Turek VF, Parkes DG. “Weighing in” on synergy: preclinical research on neurohormonal anti-obesity combinations. Brain Res. 2010;1350:86–94. doi: 10.1016/j.brainres.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 38.Roth JD, Coffey T, Jodka CM, Maier H, Athanacio JR, Mack CM, et al. Combination therapy with amylin and peptide YY[3-36] in obese rodents: anorexigenic synergy and weight loss additivity. Endocrinology. 2007;148:6054–61. doi: 10.1210/en.2007-0898. [DOI] [PubMed] [Google Scholar]
- 39.Bello NT, Kemm MH, Ofeldt EM, Moran TH. Dose combinations of exendin-4 and salmon calcitonin produce additive and synergistic reductions in food intake in nonhuman primates. Am J Physiol Regul Integr Comp Physiol. 2011;299:R945–52. doi: 10.1152/ajpregu.00275.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


