Abstract
CD47 is a “self marker” that is usually overexpressed on the surface of cancer cells to enable them to escape immunosurveillance. Recognition of CD47 by its receptor, signal regulatory protein α (SIRPα), which is expressed in the macrophages, inhibits phagocytic destruction of cancer cells by the macrophages. In this study, we have first shown that clinical isolates of human melanoma significantly upregulate CD47, possibly as a mechanism to defend themselves against the macrophages. We then exploited RNA interference (RNAi) technology to test the hypothesis that knocking down CD47 in the tumor cells will render them targets for macrophage destruction; hence, creating a novel anti-cancer therapy. Anti-CD47 siRNA was encapsulated in a liposome-protamine-hyaluronic acid (LPH) nanoparticle (NP) formulation to address the challenge of targeted delivery of siRNA-based therapeutics in vivo. Efficient silencing of CD47 in tumor tissues with systemic administration of LPH(CD47) also significantly inhibited the growth of melanoma tumors. In a lung metastasis model, LPH(CD47) efficiently inhibited lung metastasis to about 27% of the untreated control. Moreover, no hematopoietic toxicity was observed in the animals that received multiple doses of LPH(CD47). Our findings indicate CD47 as a potential prognostic marker for melanoma development as well as a target for therapeutic intervention with RNAi-based nanomedicines.
Introduction
Malignant melanoma is notorious for its aggressive invasiveness and metastasis, which always results in a poor prognosis for patients.1 Although melanoma cells exhibit a well-defined immunogenicity,2,3 the overall results of the clinical immunotherapies are not satisfactory.4 The paradoxical observations suggest an immunosuppressive microenvironment in the tumor that is mediated by different mechanisms, stemming from the secretion of immunosuppressive factors5,6 by stromal cells or lymphocytes. The microenvironment may also be created by the acquired immune tolerance of the melanoma cells.5,7
CD47 is a ubiquitous membrane receptor that belongs to the immunoglobulin (Ig) superfamily.8 The ligation between CD47 and signal regulatory protein α (SIRPα), which is expressed on the macrophages, transduces an inhibitory signal that suppresses the phagocytic activity of macrophages.9 The ubiquitous expression of CD47 on the cell surface labels the cells with “the marker of self,” a mechanism through which macrophage discriminates between “self” and “foreign” cells for contractile engulfment. CD47 expression levels are reported to be elevated in the cancerous cells of several leukemic cell lines,10,11 bladder-tumor initiating cells,12 and lymphomas,13 which correlates with an adverse clinical prognosis. It is well acknowledged that inflammation and escape from immunosurveillance plays a critical role in promoting tumor progression. Over time, cancer cells can develop to express phenotypes that are less immunogenic.14 In this context, the overexpression of CD47 is probably an outcome of immunoediting, which ensures that cancer cells escape elimination by the innate immune response of the macrophages.
A number of recent studies have demonstrated the therapeutic efficacy of a function-blocking, anti-CD47, monoclonal antibody (mAbs) in the treatment of myeloma,15 leiomyosarcoma,16 lymphoma,13,17 leukemia,18 and breast cancers.19 The administration of anti-CD47 mAbs inhibited tumor growth and prevented the metastasis by blocking the CD47/SIRPα interaction that protected the cancer cells from phagocytosis. Although antibody-based therapy seemed to be efficacious, safety concerns arose due to the ubiquitous expression of CD47, particularly on hematopoietic cells. For example, mice that received an acute infusion of antibodies exhibited temporary anemia19 or neutropenia.11 To avoid these undesired side effects, we exploited RNA interference (RNAi) therapy to target CD47 by systemically delivering siRNA formulated in the liposome-protamine-hyaluronic acid (LPH) nanoparticles (NPs). The LPH-NP is a well-established in vivo siRNA delivery system that is optimized for systemic delivery of siRNA to the tumor site with high specificity and efficiency.20,21
In the present study, we first examined the CD47 expression level in clinical human melanoma samples. With the confirmation of overexpression of CD47 in the melanoma, we hypothesized that the anti-phagocytic signal could be blocked by knockdown of CD47 with siRNA targeting CD47, which was systemically delivered using LPH-NPs. The hypothesis was tested in immune-tolerant murine melanoma cell line B16F10. The physiological effects on the melanoma cell after CD47 knockdown and its interaction with primary macrophages were studied in vitro. The pharmacodynamics of CD47 targeting RNAi therapy was evaluated in syngeneic solid tumor and lung metastatic models. Last but not least, the systemic toxicities of the mice that received multiple doses of LPH-NP were examined.
Results
CD47 expression is elevated in human melanoma tumors
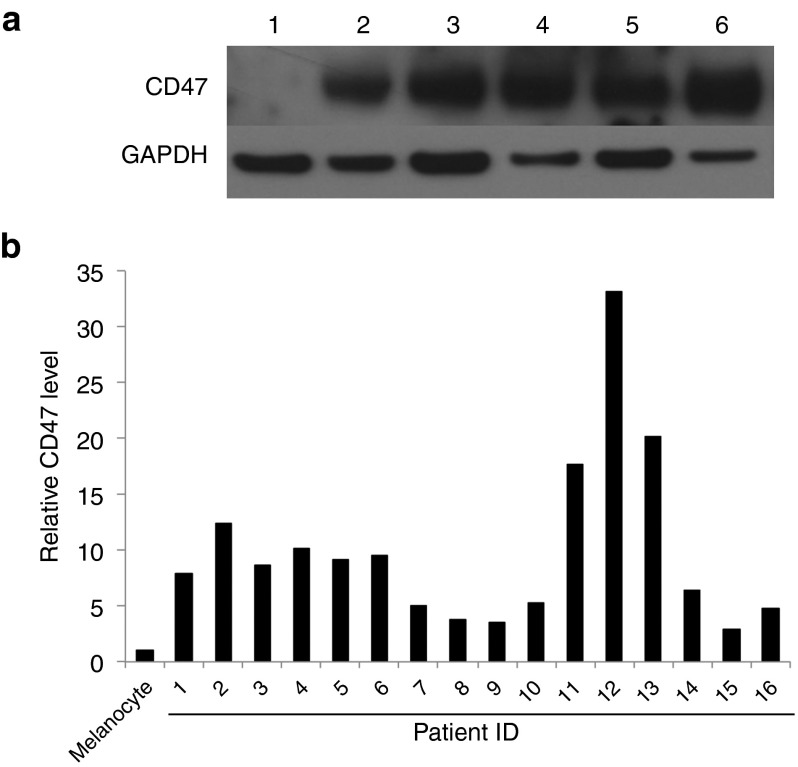
The expression level of CD47 was evaluated within a panel of 16 clinical samples from patients with malignant or metastatic melanomas. The expression level of CD47 on normal, human epidermal melanocytes was used as a reference control. Results from western-blot analyses (Figure 1a) indicated that the expression level of CD47 was statistically higher (P = 0.0005) in all of the clinical melanoma samples than in the melanocytes (two-sided Wilcoxon signed-rank test) (Figure 1b). The overexpression of CD47 is not only observed in leukemia,18 but also in some solid tumors.19 In these tumors, the CD47 expression level could be considered an adverse prognostic factor.19
Figure 1.
CD47 is elevated in clinical melanoma samples. (a) Western-blot analysis of representative melanocyte and melanoma patients samples (1, Melanocyte; 2–6, clinical samples). GAPDH was used as a loading control. (b) Quantification of relative CD47 levels of clinical melanoma samples compared with the melanocyte, based on densitometry analysis. The expression levels of CD47 on clinical melanoma samples are statistically higher than that of the melanocyte according to the two-sided Wilcoxon signed-rank test.
In vitro delivery of siRNA targeting CD47 in LPH-NPs silences the target gene in B16F10 cells
siRNA against CD47 was encapsulated into LPH-NPs through a stepwise, self-assembly process based on a well-established protocol.20 Briefly, the core of the NP was formed by mixing negatively charged siRNA/hyaluronic acid and positively charged protamine at certain ratio so that the complex was slightly negatively charged. The core was then coated with preformed cationic liposome prepared with DOTAP and Cholesterol (1/1 mol/mol), resulting in high surface charge. After post-insertional pegylation, the final NPs were ~70 nm in diameter, with a surface charge of 20 mV, as measured by a Zetasizer (Figure 2a). The decreased surface charge of the LPH-NP compared with that of a cationic liposome (~45 mV), indicates extensive PEGylation (Supplementary Figure S1). Such PEGylation reduces protein absorption and consequently decreases uptake by the reticuloendothelial system,22 extending the circulation time in the blood. A TEM analysis of the LPH-NP after negative staining with uranyl acetate confirmed the particle size (Figure 2b). The efficiency for decreasing the expression of CD47 mediated by the LPH-NP (CD47) was evaluated in vitro in the murine melanoma cell line, B16F10. Twenty-four hours after transfection, CD47 protein levels were examined by western-blot analysis (Figure 2c). The delivery of anti-CD47 siRNA by commercial transfection agent TRANSIT, resulted in significant target gene knockdown, which showed sequence specificity of siRNA for this study. Moreover, the LPH-NPs loaded with anti-CD47 siRNA, rather than control siRNA, decreased CD47 protein levels, indicating the LPH formulation did not cause any non-specific downregulation of the target gene.
Figure 2.

In vitro transfection of LPH-NP knockdown CD47 expression in B16F10 cells. (a) Representative of size and zeta potential of LPH-NP determined by dynamic light scattering and coupled Doppler Laser Densitometry. The NPs were determined to be ~70 nm in diameter with a surface charge of 20 mV. (b) Representative of TEM image of LPH-NP after uranyl acetate negative staining. Scale bar: 100 nm. (c) Western-blot analysis of CD47 protein level (top) in the B16F10 melanoma cells 24 hours after transfection after treatment. GAPDH was used as a loading control (bottom). Lane 1: untreated; Lane 2: cells transfected with LPH(con siRNA); Lane 3: cells transfected with positive control TransIT (CD47 siRNA); Lane 4: cells transfected with LPH(CD47 siRNA). (d) Flowcytometry analysis of B16F10 cells treated with the LPH-NPs. Cells were stained with FITC-conjugated, anti-CD47 antibody 24 hours after transfection. The data was analyzed with FlowJo software.
The silencing effect was semiquantitatively confirmed using flow cytometric analysis (Figure 2d). Cells were stained with FITC-conjugated, anti-CD47 antibodies before analysis. The number of CD47 receptors on the cell-surface was then measured by the intensity of FITC fluorescence. The flowcytometry data showed that with 100% transfection efficiency, anti-CD47 siRNA delivered by LPH (CD47) significantly reduced the CD47 level to ~3.6% compared with untreated cells. Minimal expression levels of CD47 on the red blood cells or platelets are sufficient for protecting cells from being engulfed by the macrophages.9 Therefore, complete removal of CD47 from mRNA is desired to create an opportunity for the macrophage to recognize the abnormal cancer cells and eradicate them.
Silencing of CD47 triggered the phagocytosis by macrophages
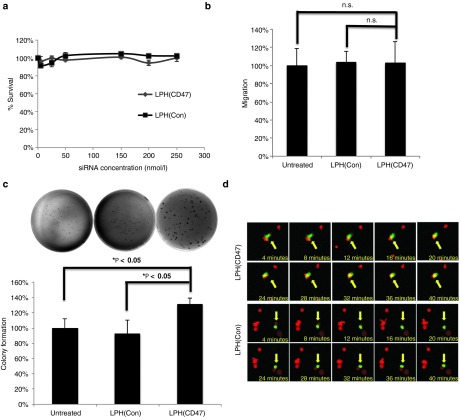
As an emerging therapeutic target, the physiological functions of CD47 have not been well elucidated. To evaluate the impact of CD47 silencing in vitro, a series of viability and invasion studies were performed on B16F10 cells after being transfected with LPH(CD47). First, loss of CD47 did not directly impair the short-term proliferative capacity of cells based on the results of an MTS assay that was performed 2 days after transfection (Figure 3a). By comparison, in untreated cells, the NP formulation with control siRNA did not cause any cytotoxicity. A matrigel invasion assay was performed to examine if the knockdown of CD47 would affect the migration and invasion abilities of the B16F10 cells. The results showed no significant difference between the untreated cells and cells treated with anti-CD47 siRNA, indicating no direct enhancing or inhibition of invasiveness occurred (Figure 3b). A soft agar colony formation assay was conducted to evaluate the anchorage-independent growth. The number of colonies counted in the assay demonstrated that the silencing of CD47 enhanced neoplasticity of the B16F10 cells. Furthermore, the colonies formed by cells with CD47 knockdown were larger in size (Figure 3c). In addition, a dose-response cell proliferation assay was performed on the cells treated with various concentrations of anti-CD47 siRNA delivered by LPH-NPs. The data showed an enhanced proliferation in a dose-response manner. Higher anti-CD47 siRNA concentration led to higher proliferation capacity, which showed the proliferative capacity of the tumor cells are reversely correlated to the CD47 expression level (Supplementary Figure S2).
Figure 3.
The downregulation of CD47 enhanced the colony formation and induced phagocytosis of melanoma cells by macrophages. (a) Cell proliferation of B16F10 cells transfected with anti-CD47 or control siRNA at different concentrations (n = 3). Neither the deficiency of CD47 or the delivery vector caused any cytotoxicity to B16F10 cells. (b) Quantification of matrigel-based migration assay (n = 3). The loss of CD47 did not prohibit the migration of the B16F10 cells (Student's t-test; n.s., not significant; P > 0.05). (c) Top: representative images of agarose gel colony formation assay. Bottom: quantification of colonies based on manual counting (n = 3) (Student's t-test, *P < 0.05). The silencing of CD47 in B16F10 cells led to an elevated level of anchorage-independent colony formation capacity. (d) Silencing of CD47 triggered phagocytosis of macrophages in vitro. Top: representative images of GFP-labeled murine macrophage interacting with RFP-labeled B16F10 after transfection with CD47 siRNA or control siRNA.
To illustrate that silencing of CD47 enabled the phagocytosis of immune-system tolerant B16F10 cells in vitro, cells labeled with red fluorescent protein (RFP) were transfected with LPH(CD47) and incubated with primary murine macrophages that had been harvested from green fluorescent protein (GFP)-expressing, transgenic C57 mice. The interaction between the RFP-expressing B16F10 cells and the GFP-expressing macrophages was visualized using confocal microscopy (Figure 3d). The results indicated a phagocytic index of 14% was induced by CD47 siRNA treatment versus 2% in control siRNA treated groups. These results were consistent with previous reports that blockade the interaction between the SIRPα and CD47 compromises the immune tolerance of the B16F10 cells,19 making them vulnerable to murine macrophage recognition and engulfment (Figure 3d).
Systemic delivery of anti-CD47 siRNA in LPH inhibited melanoma solid tumor development
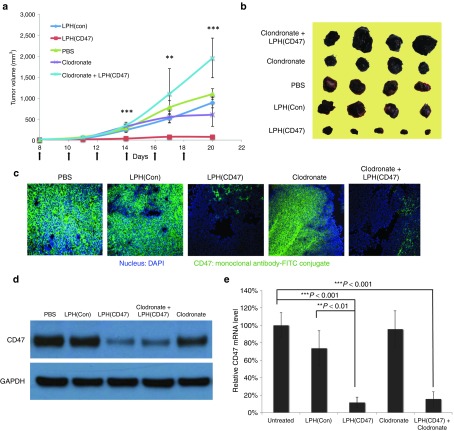
Having demonstrated that the silencing of CD47 could induce phagocytosis in vitro, the efficacy of systemically delivering anti-CD47 siRNA was evaluated in immune-competent syngeneic mice. The flanks of C57 mice were inoculated with 2 × 105 murine melanoma B16F10 cells. The mice received intravenous (IV) injections of LPH(CD47) NPs every other day for a total of six injections. The delivery of CD47 siRNA in an LPH-NP formulation effectively inhibited solid tumor growth (Figure 4a,b). The tumor volume was reduced by ~93% at the endpoint compared to the untreated controls (P < 0.0001). As was reported by Chono et al.,20 the control siRNA loaded LPH-NP was minimally immunostimulatory over a broad range of dose (0.15–1.2 mg/kg), which was also tested in C57BL/6 mice. Therefore, the low dose we used in animal studies should not induce significant levels of inflammatory cytokines, which could possibly enhance anti-tumor effect of the NPs. To demonstrate that the tumor growth inhibition was mediated by the macrophages, clodronate encapsulated in the liposomes was intraperitoneally injected into the mice to deplete the macrophages before treatment with LPH(CD47). Liposomal clodronate has been developed and successfully applied in the selective depletion of macrophages in some immune23,24 or cancer therapies.25 We observed that the depletion of the macrophages completely abolished the inhibition effect mediated by LPH(CD47) (P < 0.0001). The combinatorial treatment of liposomal clodronate and LPH(CD47) resulted in larger tumor volumes compared to the untreated tumors (P < 0.001). Levels of CD47 protein knockdown were measured using immunofluorescence staining with FITC-conjugated, anti-CD47 antibody. The epifluorescent image demonstrated an effective silencing of the “self-marker” after treatment with LPH(CD47) (Figure 4c). Western-blot analysis (Figure 4d) and RT-PCR analysis (Figure 4e) of the tumor samples confirmed the efficient reduction of the CD47 protein and mRNA levels in the tumors. In addition, liposomal clodronate treatment did not impact on the CD47 expression in the tumor cells, which further supported that the tumor inhibition was CD47 and macrophage dependent. To assess the delivery specificity of the LPH-NPs, the infiltrated leukocytes in the solid tumors were isolated and stained with FITC-conjugated, anti-CD47 antibody for flowcytometry analysis (Supplementary Figure S3). Results indicated no significant difference regarding the CD47 expression level in leukocytes isolated from CD47 siRNA treated or untreated tumors, suggesting the high selectivity of the siRNA delivery mediated by LPH-NPs and minimal impact on the immune system in the tumor microenvironment.
Figure 4.
Intravenous administration of LPH(CD47) NP inhibited B16F10 solid tumor growth. (a) IV injection LPH(CD47) NP inhibited B16F10 tumor growth. Tumor n = 5–7 per group, error bars show mean ± SEM (**P < 0.01, ***P < 0.0001 one-way analysis of variance). (b) Images of representative of tumors collected from the endpoint of the experiment. (c) Immunofluorescence staining of CD47 on tumor tissues collected. The nucleus was stained with DAPI and CD47 was stained with FITC-conjugated, anti-CD47 antibody. (d) Western-blot analysis of CD47 protein level in the tumors after treatment. (e) Quantitation of CD47 mRNA level in the tumors after treatment (Student's t-test, **P < 0.01, ***P < 0.001).
Systemic delivery of LPH-NPs containing anti-CD47 siRNA to suppressed tumor metastasis
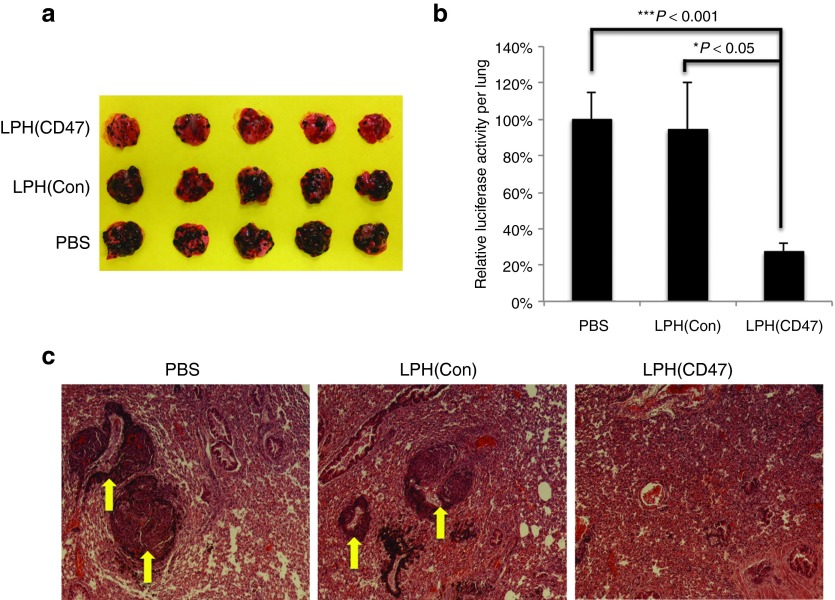
To evaluate if targeted delivery of anti-CD47 siRNA to cancer cells could be applied therapeutically against the homing of circulating cells in the lungs, we employed a well-established B16F10 metastasis model, which spontaneously developed lung metastasis after IV inoculation.21,26,27 LPH(CD47) were IV administered to the animals every 3 days beginning 3 days after inoculation (dose = 0.6 mg/kg) for a total of 5 injections. As shown in Figure 5a, there were fewer micrometastases in the lungs treated with LPH(CD47) than in the untreated control groups. Because the B16F10 melanomas were stably transfected with the firefly luciferase gene, the tumor metastasis could be quantified by measuring luciferase activity in the homogenates of the lungs (Figure 5b). The results demonstrated decreased homing of melanoma cells, ~27% of that of the untreated control. The histological analysis of the lung tissues after treatment using hematoxylin and eosin stain showed fewer and smaller lung micrometastases observed in LPH(CD47)-treated animals (Figure 5c), as were indicated by melanin-containing cells in the lung. However, the tissues appeared dense and fibrotic which suggested that parenchyma of the lung tissues have been replaced or filled with stromal cells recruited by the tumor cells regardless of the treatments by the time the experiment was terminated. On the basis of these results, we conclude that the delivery of control siRNA did not elicit any therapeutic effects. To confirm the LPH(CD47) mediated therapeutic effect, we have performed a dose-dependence study with this lung metastasis model by dosing the animals with various amounts of anti-CD47 siRNA (0.2–0.6 mg/kg) encapsulated in the LPH-NPs, following the exactly same protocol. The results indicated a dose-responsive trend of the tumor growth inhibition by showing a lower tumor loading with a higher drug dose, which supported the hypothesis that knockdown of CD47 in the tumor cells inhibited formation and proliferation of lung metastases (Supplementary Figure S4).
Figure 5.
Intravenous administration of LPH(CD47) NP inhibited the growth of B16F10 lung metastasis. (a) Images of B16F10 metastasis bearing lung harvested on day 18 after inoculation. (b) Quantitation of tumor load on the lung lobe determined by luciferase activity (n = 5) (Student's t-test, *P < 0.05, ***P < 0.001). (c) Representative images of hematoxylin and eosin staining of the lung tissues harvested from treated animals. Arrows indicate micrometastases in the lung tissue.
Systemic delivery of anti-CD47 siRNA in LPH-NPs caused no systemic organ damage or anemia
One of the over-arching goals of pharmaceutical sciences is to reduce undesired adverse effects through improvement of the pharmacokinetic profiles and biodistributions of therapeutic agents. To evaluate whether systemic administration of CD47 siRNA caused any side effects to circulating hematopoietic cells, a hematology examination was performed on the blood samples collected from animals receiving injections of LPH(CD47) every 48 hours (dose = 0.6 mg/kg) for a total of 6 injections. The cell counts of LPH(CD47) treated mice were not significantly different from the control groups (Figure 6a).
Figure 6.
Repeated administration of LPH(NP) NP caused minimal major organ toxicity or hematotoxicity. (a) Hematology test of whole blood collected from mice treated with multiple dose of LPH-NPs loaded with anti-CD47 siRNA (n = 5). (b) Blood biochemistry test of serum collected from mice treated with multiple doses of LPH-NPs. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; HCT, hematocrit; HGB, hemoglobin; PLT, platelet; RBC, red blood cell; WBC, white blood cell.
Mice repeatedly dosed with LPH(CD47) demonstrated that the administration of CD47 siRNA in an LPH formulation did not cause anemia. This might be expected because PEGylated NPs can inhibit uptake of particles by leukocytes.28 The major concern regarding the antibody therapy was resolved through the utilization of the NP formulation, which altered the tropism of the therapeutics and avoided the side effects. Although there was an inevitable amount of accumulation of NPs in the liver and kidney, blood biochemistry tests showed that no detectable damage was caused; aspartate aminotransferase (AST), alanine aminotransferase (ALT) and blood urea nitrogen (BUN) analyses were all within normal range (Figure 6b). A western-blot analysis of all major organs showed that accumulation of LPH(CD47) NPs did not downregulate CD47 expression significantly compared to the untreated control (Supplementary Figure S5).
Discussion
In this study, it was observed that the expression level of CD47 was elevated in clinical melanoma samples compared to that of melanocytes, similar to levels measured in leukemic cells, lymphoma and other cancerous cells. Instead of antibody-based therapy, we used an alternative therapeutic approach, RNA interference. By systemically delivering anti-CD47 siRNA encapsulated in the LPH-NPs, we were able to block the inhibitory signal on the cancer cells that prevented them from being phagocytosed by macrophages, resulting in a significant inhibition of melanoma solid tumor growth and dissemination of metastasis.
High invasiveness and metastasis of melanoma requires the cells to be able to survive or evade the innate immune system once they are detached from the immunosuppressive primary microenvironment and until they establish a metastatic site. Therefore, the capacity to be immunologically tolerant is critical to the metastasis of the melanoma. CD47 is a receptor that is ubiquitously expressed on cell surfaces; it conveys a “self” signal to the macrophages to limit clearance from the system. This transmembrane receptor has received much attention recently because malignant cells exhibit elevated expression of CD47 to counteract the pro-phagocytic stimulus of the abnormal expression profile of surface proteins recognized by macrophages.29 The analysis of CD47 levels in melanoma patients suggested a mechanism of immunologically sculpting tumor development, enabling the escape of the immune surveillance and providing a rational for therapies developed against this molecule. The finding led to the generation of the hypothesis with significant translation potential that silencing the CD47 receptor with siRNA as a therapeutic approach could inhibit melanoma progression and metastasis. Although the interactions between SIRPα and CD47 have been demonstrated across species,15,19 the ligation should be classified as antagonistic to phagocytosis rather than “recognition” of the self-marker.30 Therefore we used the immune-tolerant B16F10 murine melanoma cell line and the host strain C57BL/6 as an in vivo tumor model to validate the anti-cancer efficacy of RNAi therapy against CD47.
Apart from the anti-phagocytic activity, CD47 is also engaged in signal transductions that regulate cellular calcium concentration,31,32 apoptosis,33,34 proliferation,35,36 and even stress resistance.37,38,39 Most of the physiological activities of CD47 were studied by using monoclonal antibodies or peptides, which could be either agonists or antagonists to the particular receptor. Therefore, the reports outlining CD47's roles in cell survival, proliferation, and migration that are seemingly paradoxical, can be attributed to the choices of agents in the studies. For example, some CD47 antibodies not only block the ligation between SIRPα and CD47, they also stimulate antibody-dependent, cell-mediated toxicity.40 In contrast, removal of CD47 with RNA interference provides an alternative approach to demonstrate the physiological functions of CD47.
The results of an MTS assay and a migration assay show that the deficiency of CD47 does not influence the cell viability or invasiveness in the short term (Figure 3a,b). However, an agarose colony formation assay revealed an enhanced cell proliferation capacity, which was independent of external and internal stimuli (Figure 3c), a more reliable means to examine the extent of cell transformation. Interestingly, there were no reports on the characteristics on the CD47 deficient cancer cells, particularly those knocked down by RNAi. However, a series of experiments conducted on CD47 null mice have shown that the absence of CD47 could protect tissues from death caused by ionizing radiation.38 The radio-resistance manifested by the primary cells cultured from the CD47 null mice showed that this effect is cell-autonomous.41 Furthermore, a significant increase in the formation of autophagosomes has been observed in T cells lacking CD47 after irradiation, suggesting a mechanism for enhanced cell survival.42
The in vivo data was consistent with our colony formation data, which demonstrated an enhanced tumor growth induced by CD47 deficiency. This enhancement was observed in subcutaneous models (Figure 4a), when the tumor-associated macrophages were depleted by intraperitoneal injection of liposomal clodronate.25 In a syngeneic model of melanoma, however, the knockdown of CD47 in the presence of macrophages resulted in a significant inhibition of tumor growth (Figure 4a). The subcutaneous model data provided evidence to support that macrophage is the major player in the RNAi therapy targeting CD47. The same therapeutic effect was also demonstrated in a lung metastatic model, which supported the hypothesis that silencing of CD47 expression abolishes the anti-phagocytic signal of the cancer cells, disturbs the homeostasis between the pro- and anti-phagocytic surface markers and inhibits tumor growth and dissemination as a result of enhanced engulfment by macrophages.
siRNA-based therapeutics delivered by LPH-NPs significantly extended the half-life, increased bioavailability22 and, most importantly, stringently controlled the tropism of therapeutics. The NPs accumulated in the proximity of the cancer cells due to the enhanced permeability and retention effects in the tumors with poorly arranged vasculature.43 The surface-functionalized, targeting ligands on the NPs then facilitate the receptor-mediated internalization and promote drug release. Therefore CD47 siRNA could be delivered in a targeted fashion through the exploitation of a ligand for a more unique and tumor-specific receptor than the ubiquitously expressed CD47.
Conversely, antibody-based therapeutics target and exert biological activities on the same molecules that are expressed in the cytoplasmic membrane, regardless of their cellular population. The limitation restricts the application of antibody-based therapy because CD47 is ubiquitously expressed on all cell populations, particularly hematopoietic cells. As was observed, a single injection of mouse anti-CD47 antibody reduced the levels of red blood cells, hemoglobin, hematocrit and platelets to 60–80% of their original levels.19 These levels did not completely recover until 6 days after administration. In contrast, repeated injections of the LPH-NPs loaded with siRNA effectively inhibited tumor growth while maintaining blood parameters even after 6 administrations(Figure 6a). Therefore, this method provides an efficacious and safer approach to target CD47 for cancer therapy.
Conclusion
Our study using melanoma as a model has conclusively demonstrated that CD47 is an excellent target for RNAi-mediated cancer therapy. B16F10 is among the most aggressive murine tumors; it is fast growing and highly metastatic. A single therapeutic agent, i.e., siRNA, cannot control its growth in both solid tumor and metastasis. LPH formulation is a versatile platform that can codeliver siRNA, plasmid DNA and chemodrugs into individual cancer cells to exert synergistic anti-tumor effects.44 Complete eradication of the tumor could be possible if RNAi or gene/chemo therapies are codelivered to target multiple signaling pathways. Thus, LPH(CD47) may represent a starting point of treating very aggressive tumors, such as melanoma.
Materials and Methods
Materials. 1,2-dioleoyl-3-trimethylammonium-propane chloride salt (DOTAP), and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(methoxy (polyethyleneglycol)-2000) ammonium salt (DSPE-PEG2000) were purchased from Avanti Polar Lipids (Alabaster, AL). Hyaluronic acid (HA), cholesterol (Chol), and protamine sulfate (fraction × from salmon) was purchased Sigma-Aldrich (St Louis, MO). All the other chemicals were purchased from Sigma-Aldrich unless otherwise mentioned. DSPE-PEG-AA was synthesized according to the previously established protocol.45 The mouse CD47 siRNA with sequence 5′-GGAAUGACCUCUUUCACCA-3′ and the control siRNA with sequence 5′-AATTCTCCGAACGTGTCACGT-3′ were synthesized by Sigma-Aldrich.
Cell culture. Murine melanoma B1610 cells were used in this study. The cells were purchased from American Tissue Culture Collection. The cells were stably transduced with GL3 firefly luciferase gene with a retroviral vector by Pilar Blancafort's laboratory at the University of North Carolina at Chapel Hill. Cells were maintained in DMEM (Life Technologies, Carlsbad, CA) with 10% fetal bovine serum (Life Technologies).
Experimental animals. Female C57BL/6 mice of 6–8 weeks old were purchased from the National Cancer Institute (Frederick, MD). Female C57BL/6-Tg(UBC-GFP)30Scha/J mice of 6–8 weeks old were purchased from the Jackson laboratory (Bar Harbor, Maine). All animal protocols were approved by the University of North Carolina at Chapel Hill's Institutional Animal Care and Use Committee.
Processing human melanoma samples. Experiments involved human materials were conducted in Gavin P Robertson's laboratory at Pennsylvania State University according to the protocol approved by the institutional Review Board Committee. Samples preparation for western blot was performed according to previously published protocol.46 Briefly, sixteen tissues samples were acquired at surgery, snap frozen in liquid nitrogen and pulverized using a mortar and pestle. Cell debris were pelleted by centrifugation (≥10,000g) and the protein concentrations of the supernatant were quantified. A western-blot analysis was then performed using these samples to assess the CD47 protein level.
Preparation of liposome and LPH. LPH-NPs were formulated through a stepwise self-assembly process based on a well-established protocol.20 DOTAP and Chol (1:1, mol/mol) were dissolved in chloroform and solvent was removed under reduced pressure. The lipid film is hydrated overnight with distilled water to make the final concentration 10 mmol/l DOTAP and cholesterol. The liposome was sequentially extruded through 400 nm, 200 nm, 100 nm, and 50 nm polycarbonate membranes (Millipore, Billerica, MA) to form 80–100 nm unilamellar liposomes. The LPH core was formed by mixing 140 μl of solution A (24 μg siRNA and 24 μg HA in 5% glucose) and 140 µl of solution B (36 μg protamine in 5% glucose). The mixed solution was incubated at room temperature for 10 minutes before 60 μl DOTAP/Chol liposome (10 mmol/l each) was added. PEGylation was performed by adding 30 μl DSPE-PEG (10 mg/ml) and 30 μl DSPE-PEG-AA (10 mg/ml) to the LPR and incubating the mix at 50 °C for 15 minutes. The size and surface charge of the NPs were determined by using a Malvern ZS90 Zetasizer (Worcestershire, UK).
Liposomal clodronate was prepared according a well-established protocol,25 implementing only minor modifications. Soy phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL) (0.4 g) and 0.06 g of cholesterol was dissolved in chloroform and solvent was removed under reduced pressure. The lipid membrane was then rehydrated overnight with phosphate buffer (20 mmol/l, pH 7.4) containing 0.211 g clodronate (Sigma-Aldrich). The liposome was sequentially extruded through 400 nm, 200 nm, 100 nm, and 50 nm polycarbonate membranes (Millipore) to form 80–100 nm unilamellar liposomes. The prepared liposomes contained ~20 mg/ml clodronate.
Western-blot analysis. Clinical samples and melanocyte were lysed with a radioimmunoprecipitation assay buffer. Protein concentration was determined using the Bradford protein assay reagents according to the manufacturer's protocols. Protein samples for western blotting were diluted in 4× sample buffer containing reducing reagent and heated at 95 °C for 5 minutes. Proteins were resolved by electrophoresis on NuPAGE 4–12% Bis-Tris Gels (Life Technologies), transferred to Immobilon-P transfer membrane (Millipore) and probed with anti-CD47 mAb (Santa Cruz, Santa Cruz, CA) or anti-GAPDH mAb (Cell Signaling, Danvers, MA) followed by the horseradish peroxidase-conjugated secondary antibodies, respectively, and detected using the Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific, Rockford, IL). The relative CD47 expression level was determined with ImageJ software (National Institutes of Health, Bethesda, MD) using GAPDH as a loading control.
In vitro transfection. Six-well plates were seeded with 1 × 105 cells per well. LPH loaded with CD47 or control siRNA was added to each well in the presence of OptiMEM medium with final concentration of 250 nmol/l. The medium was replaced with full medium 4 hours after transfection and the cells were incubated overnight. The CD47 protein levels of samples after transfection were determined by western-blot analysis with GAPDH as loading control.
MTS cell proliferation assay. For short-term proliferation assay, 96-well plates were seeded with 5 × 103 cells per well and transfected with LPH(CD47) or LPH(Con) NPs equivalent to 250 nmol/l anti-CD47 siRNA. Twenty-four after transfection, cells were subject to MTS assay (Promega, Madison, MI) according to the manufacture's instruction. For long-term proliferation assay, six-well plates were seeded with 1 × 105 cells per well. Cells were transfected with NPs equivalent to 0–250 nmol/l siRNA and seeded into 96-well plate with a seeding density of 100 cells per well. Cells were then subjective to MTS assay 7 days after transfection.
Colony formation analysis in soft agar. Anchorage-independent cell growth was analyzed by plating 0.35% top agarose containing 1 × 105 LPH-NPs transfected cells mixed with complete medium on a surface of 0.5% bottom agarose mixed with complete medium. Cells were fed twice weekly by adding fresh medium. After 2 weeks of incubation, colonies were stained with crystal violet solution, photographed and counted with Gel Doc Imaging system (Bio-Rad, Hercules, CA). Each experiment was performed in triplicate and repeated three times.
Invasion assay. Six-well BD BioCoat Matrigel Invasion chamber (Becton Dickinson, Franklin Lakes, NJ) were rehydrated for 2 hours in 37 °C with complete medium containing 10% FBS and 1% PS. LPH-NPs transfected cells were suspended with basal medium containing 5 × 104 cells and added to each insert Complete medium was added to the well as a chemo-attractant. After incubation for 24 hours, non-invading cells were removed from the upper side of the membrane by scrubbing. Invasion of cells was detected by staining the cells with crystal violet solution and visualizing the cells under a microscope. Cells were counted under an inverted microscope in four randomly selected fields (magnification ×100), and results were expressed in the form of a bar graph. Assays were performed three times for each treatment.
In vitro phagocytosis assay. The in vitro phagocytosis was determined according to the published protocol with modification.19 Briefly, C57BL/6 were transfected with plasmid DNA encoding Tdtomato RFP (Clontech Laboratories, Mountain View, CA) with Lipofectamine 2000 (Life Technologies) for 2 consecutive days to ensure over 90% transfection activity. The macrophages were collected from GFP-expressing C57BL/6 transgenic mice (the Jackson Laboratory) to ensure CD47-SIPRα compatibility. The inflammatory response of transgenic mice was stimulated by intraperitoneal injection of 3% proteose peptone (Sigma-Aldrich) in 1 ml of Dulbecco's phosphate-buffered saline (DPBS). Three days after stimulation, mice were euthanized and macrophages were harvested from the peritoneal cavity. The RFP-expressing B16F10 cells were transfected with LPH(CD47) or LPH(con). One day after transfection, the primary murine macrophages were incubated with transfected B16F10 cells in the serum free media and observed under the Olympus FV1000 MPE SIM Laser Scanning Confocal Microscope (Olympus, Center Valley, PA). The images with dual channel were acquired every 2 minutes. The phagocytic index was determined as the number of phagocytosed RFP-expressing cells per 100 macrophages (GFP-expressing).
In vivo transfection study LPH-NPs equivalent to 12 μg of CD47 siRNA or control siRNA prepared as aforementioned were IV injected into the subcutaneous-tumor–bearing, nude mice. Mice were killed 24 hours after injection. Organs and tumor tissues were homogenized and the tissue lysates were subject to luciferase assay (Promega, Madison, MI) according to manufacture's instructions.
Tumor growth inhibition/metastasis inhibition assay. B16F10 cells were harvested and suspended in DPBS with concentration 2 × 105 cells/50 μl. C57BL/6 mice of 6–8 weeks old were inoculated with B16F10 cells by subcutaneously injecting 2 × 105 B16F10 cells in 50 μl DPBS on the hind legs. Tumor bearing animals received LPH-NPs treatment started from 8th day after inoculation when the tumor volumes reached 50 mm3. LPH-NPs equivalent to 12 μg of CD47 or control siRNA (0.6 mg/kg), prepared as described above, were IV injected every other day for a totally of 6 treatments. Tumor size was measured with a digital caliper (Thermo Fisher Scientific, Pittsburg, PA) and animal weight was monitored every 3 days. Tumor volume was calculated as (1/2 × length × width × height).
To deplete the tumor-associated macrophages, animals were subject to three intraperitoneal injections of liposomal clodronate with 100 mg/kg for the first dose and 50 mg/kg for the following two doses. The liposomal clodronate treatment started from day 3 after inoculation and lasted 3 consecutive days.
The metastasis model was created by IV injecting 2 × 105 B16F10 cells in 100 μl DPBS into 6–8 week old C57BL/6 mice. LPH-NPs equivalent to 12 µg siRNA (0.6 mg/kg) were IV administered to the mice every 3 days for a total of 5 treatments starting from 3 days after inoculation. Mice were killed and lungs were harvested on day 18 after inoculation. For the dose-dependence assay, animals were treated with LPH-NPs equivalent to 0.2–0.6 mg/kg siRNA following the same treatment regime.
Flow cytometry. B16F10/Luc cells were seeded at 1 × 105 cells/well in six-well plate 24 hours before the transfection. 24 hours after transfection, the cells were washed with PBS and collected by centrifugation. Cell suspensions were stained with FITC-conjugated, anti-CD47 (Santa Cruz) or isotype control antibodies (Becton Dickinson, Franklin Lakes, NJ). CD47 expression was analyzed by flow cytometry on a BD FACSAria instrument (Becton Dickinson). To stain tumor-infiltrating leukocytes, cells were isolated from solid tumors after LPH-NPs treatment.
Quantitative RT-PCR. Quantitative RT-PCR analysis was performed with the SYBR Green kit (Life Technologies) and ABI prism 7500 Sequence Detector (Applied Biosystems, Foster City, CA). Total RNA from tumor tissues were extracted using an RNeasy kit (Qiagen, Valencia, CA). cDNAs were synthesized with a SuperScript II reverse transcriptase kit (Life Technologies). Reactions were run using a standard cycling program; 50 °C for 2 minutes, 95 °C for 10 minutes, 40 cycles of 95 °C for 15 seconds, and 60 °C for 1 minute. The PCR primers to detect mouse CD47 (forward; 5′-GTTCAGCTCAACTACTGT-3′, reverse; 5′-CTCTTATTCGTATGGCTG-3′) and mouse actin (forward; 5′-ATATCGCTGCGCTGGTCGTC-3′, reverse; 5′-AGGATGGCGTGAGGGAGAGC-3′) were synthesized and purified by IDT (Coralville, IA).
Immunofluorescence. Immunofluorescence detection of CD47 on tumor tissues was prepared using paraffin sections (obtained from the UNC Tissue Procurement Core) that were deparaffinized, antigen recovered and blocked at room temperature with 5% BSA/PBS for 1 hour, and incubated with FITC-conjugated, anti-CD47 antibodies (Santa Cruz) overnight at 4 °C. The slides were then washed with PBS and counter-stained with VECTORSHIELD mounting media with DAPI (VECTOR Laboratories, Burlingame, CA).
AST, ALT, BUN assay and hematology assay. Mice that were IV injected with LPH (equivalent to 24 μg CD47 siRNA or control siRNA) every other day for a total of 5 treatments were subjected to a toxicity assay. Sera and whole blood collected from the mice were assayed for AST, ALT, BUN, and hematological parameters performed by UNC facility. Organs were collected and fixed with 4% paraformaldehyde in PBS overnight before stained with hematoxylin and eosin by UNC histology facility.
Statistical analysis. Percentages of GFP-positive cells and MFIs were calculated by BD CellQuest (BD Biosciences) software. Statistical analysis was undertaken using Prism 5.0c Software. A two-tailed t-test or a one-way analysis of variance was performed when comparing two groups or more than two groups, respectively. Statistical significance was defined by a value of P < 0.05.
SUPPLEMENTARY MATERIAL Figure S1. Size and zeta potential characterization of core, membrane-coated NP and pegylated LPH-NP. Figure S2. Dose-dependence cell proliferation assay of B16F10 cells treated with various doses of LPH(CD47) NP. Figure S3. Flowcytometry analysis of tumor-infiltrating leukocytes collected from the tumors tissues of the tumor-bearing mice treated with the LPH-NPs. Figure S4. Quantitation of B16F10 tumor load on the lung determined by luciferase activity after treatment of LPH(CD47) NP with various doses (n = 3). Figure S5. Western-blot analysis of CD47 expression levels in the major organs collected from the untreated animals and the animals that received multiple treatments of LPH(CD47) NP.
Acknowledgments
This work was supported by NIH grants CA129835, CA129421, CA151652, CA151455, and CA149363. We thank Kelly Ann Racette for manuscript editing. The authors declared no conflict of interest.
Supplementary Material
References
- Liang S, Sharma A, Peng HH, Robertson G, Dong C. Targeting mutant (V600E) B-Raf in melanoma interrupts immunoediting of leukocyte functions and melanoma extravasation. Cancer Res. 2007;67:5814–5820. doi: 10.1158/0008-5472.CAN-06-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipponi A, Wieers G, van Baren N, Coulie PG. Tumor-infiltrating lymphocytes: apparently good for melanoma patients. But why. Cancer Immunol Immunother. 2011;60:1153–1160. doi: 10.1007/s00262-011-1026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmiani G, Castelli C, Santinami M, Rivoltini L. Melanoma immunology: past, present and future. Curr Opin Oncol. 2007;19:121–127. doi: 10.1097/CCO.0b013e32801497d7. [DOI] [PubMed] [Google Scholar]
- Umansky V, Sevko A. Melanoma-induced immunosuppression and its neutralization. Semin Cancer Biol. 2012;22:319–326. doi: 10.1016/j.semcancer.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18:11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartsev S, Gabrilovich DI. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev. 2006;25:323–331. doi: 10.1007/s10555-006-9002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivoltini L, Canese P, Huber V, Iero M, Pilla L, Valenti R, et al. Escape strategies and reasons for failure in the interaction between tumour cells and the immune system: how can we tilt the balance towards immune-mediated cancer control. Expert Opin Biol Ther. 2005;5:463–476. doi: 10.1517/14712598.5.4.463. [DOI] [PubMed] [Google Scholar]
- Frazier WA, Gao AG, Dimitry J, Chung J, Brown EJ, Lindberg FP, et al. The thrombospondin receptor integrin-associated protein (CD47) functionally couples to heterotrimeric Gi. J Biol Chem. 1999;274:8554–8560. doi: 10.1074/jbc.274.13.8554. [DOI] [PubMed] [Google Scholar]
- Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci USA. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- Kim D, Wang J, Willingham SB, Martin R, Wernig G, Weissman IL. Anti-CD47 antibodies promote phagocytosis and inhibit the growth of human myeloma cells. Leukemia. 2012;26:2538–2545. doi: 10.1038/leu.2012.141. [DOI] [PubMed] [Google Scholar]
- Edris B, Weiskopf K, Volkmer AK, Volkmer JP, Willingham SB, Contreras-Trujillo H, et al. Antibody therapy targeting the CD47 protein is effective in a model of aggressive metastatic leiomyosarcoma. Proc Natl Acad Sci USA. 2012;109:6656–6661. doi: 10.1073/pnas.1121629109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MP, Tang C, Pachynski RK, Chin R, Majeti R, Weissman IL. Extranodal dissemination of non-Hodgkin lymphoma requires CD47 and is inhibited by anti-CD47 antibody therapy. Blood. 2011;118:4890–4901. doi: 10.1182/blood-2011-02-338020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MP, Alizadeh AA, Tang C, Jan M, Weissman-Tsukamoto R, Zhao F, et al. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res. 2011;71:1374–1384. doi: 10.1158/0008-5472.CAN-10-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chono S, Li SD, Conwell CC, Huang L. An efficient and low immunostimulatory nanoparticle formulation for systemic siRNA delivery to the tumor. J Control Release. 2008;131:64–69. doi: 10.1016/j.jconrel.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhu X, Zhang X, Liu B, Huang L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol Ther. 2010;18:1650–1656. doi: 10.1038/mt.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SD, Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J Control Release. 2010;145:178–181. doi: 10.1016/j.jconrel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner JW, Uchida O, Kajiwara N, Kim EY, Patel AC, O'Sullivan MP, et al. CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat Med. 2005;11:1180–1187. doi: 10.1038/nm1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler P, Aichele P, Odermatt B, Hengartner H, Zinkernagel RM, Schwendener RA. Crucial role of marginal zone macrophages and marginal zone metallophils in the clearance of lymphocytic choriomeningitis virus infection. Eur J Immunol. 1997;27:2626–2633. doi: 10.1002/eji.1830271023. [DOI] [PubMed] [Google Scholar]
- Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjällman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li J, Liu F, Huang L. Systemic delivery of siRNA via LCP nanoparticle efficiently inhibits lung metastasis. Mol Ther. 2012;20:609–615. doi: 10.1038/mt.2011.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SD, Chono S, Huang L. Efficient oncogene silencing and metastasis inhibition via systemic delivery of siRNA. Mol Ther. 2008;16:942–946. doi: 10.1038/mt.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, et al. Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med. 2010;2:63ra94. doi: 10.1126/scitranslmed.3001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Parthasarathy R, Sen S, Boder ET, Discher DE. Species- and cell type-specific interactions between CD47 and human SIRPalpha. Blood. 2006;107:2548–2556. doi: 10.1182/blood-2005-04-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Brown EJ, Fazeli B. A 50-kDa integrin-associated protein is required for integrin-regulated calcium entry in endothelial cells. J Biol Chem. 1993;268:19931–19934. [PubMed] [Google Scholar]
- Sick E, Niederhoffer N, Takeda K, Landry Y, Gies JP. Activation of CD47 receptors causes histamine secretion from mast cells. Cell Mol Life Sci. 2009;66:1271–1282. doi: 10.1007/s00018-009-8778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen RD, Hestdal K, Olafsen MK, Lie SO, Lindberg FP. CD47 signals T cell death. J Immunol. 1999;162:7031–7040. [PubMed] [Google Scholar]
- Manna PP, Frazier WA. CD47 mediates killing of breast tumor cells via Gi-dependent inhibition of protein kinase A. Cancer Res. 2004;64:1026–1036. doi: 10.1158/0008-5472.can-03-1708. [DOI] [PubMed] [Google Scholar]
- Sick E, Boukhari A, Deramaudt T, Rondé P, Bucher B, André P, et al. Activation of CD47 receptors causes proliferation of human astrocytoma but not normal astrocytes via an Akt-dependent pathway. Glia. 2011;59:308–319. doi: 10.1002/glia.21102. [DOI] [PubMed] [Google Scholar]
- Congote LF, Temmel N. The C-terminal 26-residue peptide of serpin A1 stimulates proliferation of breast and liver cancer cells: role of protein kinase C and CD47. FEBS Lett. 2004;576:343–347. doi: 10.1016/j.febslet.2004.09.035. [DOI] [PubMed] [Google Scholar]
- Lih CJ, Wei W, Cohen SN. Txr1: a transcriptional regulator of thrombospondin-1 that modulates cellular sensitivity to taxanes. Genes Dev. 2006;20:2082–2095. doi: 10.1101/gad.1441306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, Degraff WG, Tsokos M, et al. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med. 2009;1:3ra7. doi: 10.1126/scitranslmed.3000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxhimer JB, Shih HB, Isenberg JS, Miller TW, Roberts DD. Thrombospondin-1/CD47 blockade following ischemia-reperfusion injury is tissue protective. Plast Reconstr Surg. 2009;124:1880–1889. doi: 10.1097/PRS.0b013e3181bceec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sick E, Jeanne A, Schneider C, Dedieu S, Takeda K, Martiny L. CD47 update: a multifaceted actor in the tumour microenvironment of potential therapeutic interest. Br J Pharmacol. 2012;167:1415–1430. doi: 10.1111/j.1476-5381.2012.02099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Maxhimer JB, Hyodo F, Pendrak ML, Ridnour LA, DeGraff WG, et al. Thrombospondin-1 and CD47 limit cell and tissue survival of radiation injury. Am J Pathol. 2008;173:1100–1112. doi: 10.2353/ajpath.2008.080237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Pantoja DR, Miller TW, Pendrak ML, DeGraff WG, Sullivan C, Ridnour LA, et al. CD47 deficiency confers cell and tissue radioprotection by activation of autophagy. Autophagy. 2012;8:1628–1642. doi: 10.4161/auto.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46 12 Pt 1:6387–6392. [PubMed] [Google Scholar]
- Chen Y, Bathula SR, Li J, Huang L. Multifunctional nanoparticles delivering small interfering RNA and doxorubicin overcome drug resistance in cancer. J Biol Chem. 2010;285:22639–22650. doi: 10.1074/jbc.M110.125906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R, Tyagi P, Li S, Huang L. Anisamide-targeted stealth liposomes: a potent carrier for targeting doxorubicin to human prostate cancer cells. Int J Cancer. 2004;112:693–700. doi: 10.1002/ijc.20452. [DOI] [PubMed] [Google Scholar]
- Huh SJ, Chung CY, Sharma A, Robertson GP. Macrophage inhibitory cytokine-1 regulates melanoma vascular development. Am J Pathol. 2010;176:2948–2957. doi: 10.2353/ajpath.2010.090963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.