Abstract
Arsenic (As), a ubiquitous environmental toxicant, has recently been linked to disrupted immune function and enhanced infection susceptibility in highly exposed populations. Drinking water As levels above the EPA maximum contaminant level occur in our US study area and are a particular health concern for pregnant women and infants. As part of the New Hampshire Birth Cohort Study, we investigated whether in utero exposure to As affects risk of infant infections. We prospectively obtained information on four-month-old infants (n=214) using a parental telephone survey on infant’s infections and symptoms, including respiratory infections, diarrhea and specific illnesses, as well as the duration and severity of infections. Using logistic regression and Poisson models, we evaluated the association between maternal urinary As during pregnancy and infection risks adjusted for potentially confounding factors. Maternal urinary As concentrations were related to total number of infections requiring a physician visit (Relative Risk (RR) per one-fold increase in As in urine =1.5; 95% confidence interval (CI)=1.0, 2.1) or prescription medication (RR=1.6; 95% CI =1.1, 2.4), as well as lower respiratory infections treated with prescription medication (RR=3.3; 95% CI =1.2, 9.0). Associations were observed with respiratory symptoms (RR=4.0; 95% CI =1.0, 15.8), upper respiratory infections (RR=1.6; 95% CI =1.0, 2.5), and colds treated with prescription medication (RR=2.3; 95% CI =1.0, 5.2). Our results provide initial evidence that in utero As exposure may be related to infant infection and infection severity and provide insight into the early life impacts of fetal As exposure.
Keywords: Arsenic, infant respiratory infection, prenatal exposure, pregnancy, US cohort
1. Introduction
Infectious diseases remain the primary cause of mortality in young children, resulting in nearly 4.4 million child deaths under age 5 years in 2010, despite major advancements in immunization and sanitation programs (Schuchat 2012; WHO 2010). Even infants born in industrialized countries, such as the US, experience a high burden of infection-related morbidity and mortality, especially before the age of one year and primarily from respiratory infections and diarrhea (Mehal et al. 2012; Tregoning and Schwarze 2010). We are now beginning to appreciate the potential impact of environmental agents on childhood infection susceptibility and to understand the effects of toxicants, such as arsenic (As) in altering the body’s response to infection (Birnbaum and Jung 2010; Feingold et al. 2010; Karagas 2010).
Non-occupational As exposure occurs primarily via contaminated drinking water, typically from unregulated, private wells (Council 2001; Karagas et al. 2002; Karagas et al. 1998), and some foods including rice and poultry (Gilbert-Diamond et al. 2011; Nachman et al. 2012). Millions are chronically exposed to As worldwide (Argos et al. 2010; Council 2001; MM Rahman et al. 2009), including in the US, where detectable urinary levels of inorganic As were found in over half of the 2003- 2004 National Health and Nutrition Examination Survey (NHANES) participants over age 6 years (Caldwell et al. 2009). While chronic As exposure has been most widely associated with carcinogenicity (Council 2001; Karagas et al. 2001; Karagas et al. 2002; MM Rahman et al. 2009), as well as higher mortality rates (Argos et al. 2010), the potential for adverse effects of As are of particular concern in vulnerable populations, such as pregnant women and infants (Karagas 2010; Vahter 2009). During pregnancy, As passes easily from mother to fetus through the placenta, resulting in in utero exposure levels roughly equivalent to those of the mother during this critical developmental period (Vahter 2009). Studies, primarily from As-endemic areas of the globe, have reported increased risks of spontaneous abortions, stillbirths, infant mortality, preterm birth, low birth weight, and growth restriction (Ahmad et al. 2001; Hopenhayn et al. 2003; Huyck et al. 2007; Milton et al. 2005; Rahman et al. 2010; Rahman et al. 2007; A Rahman et al. 2009; Vahter 2009; von Ehrenstein et al. 2006).
Recent attention has turned toward growing evidence of the immune modulating effects of As (summarized in Tables 3 and S1). As-exposed model organisms from mice to zebrafish exhibit altered expression of immune response genes and are less able to clear viral and bacterial infections, even at low levels of exposure (i.e. 2 and 10 ug/L in water) (Kozul et al. 2009a; Kozul et al. 2009b; Nayak et al. 2007). It has been well established that As treatment in vitro acts as an inhibitor of both lymphocyte proliferative responses and cytokine production following immune challenge and can induce oxidative stress responses and apoptosis of lymphocytes (Conde et al. 2007; Das et al. 2011; Galicia et al. 2003; Lau et al. 2004a; Lau et al. 2004b; Lemarie et al. 2006; Martin-Chouly et al. 2011; Nain and Smits 2010; Patterson et al. 2004; Stepnik et al. 2005; Vega et al. 2004). In cross-sectional studies, As-exposed adults displayed hallmarks of immune dysfunction, including impaired macrophage functionality, decreased lymphocyte proliferative response, reduced cytokine production (i.e. IFN-γ, a cytokine known to be an important mediator of immune responses to microbial infections), abnormal proportions of T-cell populations, as well as elevated oxidative stress markers, which may promote lymphocyte apoptosis (Banerjee et al. 2009; Biswas et al. 2008; Fry et al. 2007; Hernandez-Castro et al. 2009; Soto-Pena et al. 2006). A study located in a region of Mexico with elevated rates of As drinking water contamination found that highly exposed children (>50 ug/L urinary As, as compared to the low exposure group, i.e. <50 µg/L) exhibited altered immune function measures, including increased granulocyte macrophage colony stimulating factor (GM-CSF) secretion by mononuclear cells and reductions in proliferative responses to mitogen stimulation, CD4+ T-cell subpopulations, and IL-2 secretion (Soto-Pena et al. 2006). Further, microarray analyses provide evidence for the occurrence of these immunopathological effects at the gene regulatory level, including As-associated differential regulation of immune-related genes, pro-inflammatory cytokines (e.g. IL-1β) and cellular stress-response genes that correspond to pathways implicated in disrupted immune function (Andrew et al. 2008; Fry et al. 2007; Wu et al. 2003). Similarly, in a prospective study of infants in Thailand, cord blood gene expression signatures from infants born to mothers who were exposed to As during pregnancy (≥0.5µg/g toenail As; corresponding to chronic consumption of ≥10µg/L water) had abnormal activation of cellular stress and inflammation gene networks when compared to infants of unexposed mothers (<0.5µg/g toenail As) (Fry et al. 2007). Many of these genes are involved in immune response and function, including production of cytokines IL-8 and IL-1β, thus supporting a role for As in immune perturbation (Fry et al. 2007). In a recent prospective study from Bangladesh, maternal As exposure related to decreased cord blood levels of signal-joint T-cell receptor excision circles (sjTRECs), a molecular indicator of thymic development and lymphocyte maturation (Ahmed et al. 2012). Taken together, these results suggest that As exposure in humans may impair immune function, potentially leading to enhanced infection susceptibility.
Table 3.
Summary of literature on in utero or early life arsenic exposure and changes in immune and immune-related outcomes in infants and children.
| Outcome | Results | References | ||
|---|---|---|---|---|
| Cord Blood | ↑ | IL-1β/NFκB (SOC3, CXCL1), STAT1/HIF1α (DUSP1), and apoptosis/stress response/TNFα (EGR-1, IER2, JUNB, OSM, PTGS2) pathways Δ 8-OHdG #* , apoptosis related genes (GADD45A, NOD1, CASP2, CASP8, CD70, TRADD)* |
Fry et al., 2007; Ahmed et al., 2011; Ahmed et al., 2012 |
|
| U | IL-8, TNFα, IL-1β# | |||
| ↓ | sjTRECs in CD4+ / CD8+ cells #*, antioxidant (ALB, TXNDC2, SOD3, APOE), oxidative stress (ANGPTL7, NME5, MBL2, MTL5) peroxidase (CYGB, DUOX2, EPX, LPO, PXDNL, PXDN, GPX5) and ROS metabolism (NOS2A, AOX1) pathways* |
|||
| Placenta | ↑ | Expression of 8-oxoG #* , IL-1β # , Leptin ,# IFN- γ , TNFα |
Ahmed et al., 2011; Ahmed et al., 2012 |
|
| ↓ | CD3+ T-cells ; CD64+ monocytes/ macrophages | |||
| Peripheral Blood Mononuclear Cells |
↑ | PBMC apoptosis; Nitric oxide and oxygen superoxide anion production; GM-CSF secretion$ |
Rocha-Amador et al., 2011; Luna et al., 2010; Soto-Pena et al., 2006 |
|
| ↓ | Lymphocyte proliferative response; T-helper cell proportion; CD4+: CD8+ ratio; secretion of IL-2, IFN-γ$ |
|||
| Clinical manifestations in infancy (up to age 12 months) |
↑ | Diarrhea, lower respiratory infection, severe lower respiratory infection, acute respiratory infection # |
Rahman et al., 2011; Raqib et al., 2009; Moore et al., 2009 |
|
| ↓ | Infant thymic index at 2, 6 and 12 months #% | |||
U: U-shaped expression pattern, i.e. highest expression at lowest and highest As exposures.
Based on maternal toenail arsenic during pregnancy Based on urinary arsenic measured at gestational week 8
Based on urinary arsenic measured at gestational week 30
Based on urinary arsenic measured at gestational weeks 8 and 30 (averaged)
Based on concomitant urinary arsenic in children ages of 6–12 years
Based on cord blood arsenic measured at birth
A small number of recent studies have reported associations between prenatal As exposure and increased infection among pregnant women and infants (Tables 3 and S1). In As-endemic Bangladesh, a prospective study of 140 mother-child dyads found that elevated urinary As concentration in pregnancy related to maternal fever and diarrhea during pregnancy and an increased risk of acute respiratory infection in male infants (Raqib et al. 2009). A subsequent study of 1552 pregnant women in Bangladesh found maternal urinary As concentrations during pregnancy were associated with increased risk of lower respiratory tract infections and diarrhea in infants (Rahman et al. 2011). An ecological study from Chile reported elevated standardized mortality ratios for bronchiectasis -- a chronic lung disease often resulting from prolonged or repeated lung infection -- for individuals born around the time of peak drinking water As concentrations (Smith et al. 2006). Thus, accumulating evidence indicates that high levels of As exposure may enhance infection risks for infants exposed prenatally.
In the state of New Hampshire, USA, roughly 40% of all households rely on private wells as their primary water source, of which over 1 of every 10 wells contains As levels exceeding the EPA’s maximum contaminant level (MCL) of 10 µg/L (Karagas et al. 2002; Karagas et al. 1998). Therefore, we investigated whether in utero exposure to lower, environmentally present levels of As increases infant infections in the first 4 months after birth in the New Hampshire Birth Cohort Study (NHBCS), an ongoing prospective study of infants born to mothers using private well water in their homes.
2. Methods
2.1. The New Hampshire Birth Cohort
In January 2009, we began recruiting 18–45 year old pregnant women receiving prenatal care at study clinics in New Hampshire, USA as described previously (Gilbert-Diamond et al. 2011). Women were screened for eligibility at an initial prenatal care visit and were enrolled at 24–28 weeks gestation if they reported using water from a private, unregulated well in their home since their last menstrual period and were not planning a change in residence prior to delivery. Only singleton, live births were included in the cohort.
2.2. Study Questionnaires and Medical Record Review
Women who agreed to participate were asked to complete a medical history and lifestyle questionnaire, which included questions about sociodemographic factors (age, race/ethnicity, marital status, level of education), reproductive history (previous pregnancies, complications, birth outcomes), and general health history. Women were asked about habits, including tobacco and alcohol use, as well as regarding their home water source, use of water filters and amount of water consumption.
Two weeks post-delivery, participants were sent a second questionnaire to obtain updated information about changes in key exposures, and information about pregnancy complications and infections. Participants also consented to a review of prenatal, labor and delivery, and infant medical records, which allowed additional information to be recorded about prenatal infections, medication use, birth outcomes and delivery details, as well as the general health of the women and their infants after birth.
2.3. Infant Infection and Allergy Follow-Up Telephone Survey
Mothers were contacted by telephone at four months postpartum and asked a series of questions to determine whether their child had any infections in the first four months of life (e.g. influenza, otitis media, respiratory syncytial virus) or symptoms of illness (e.g. fever, diarrhea, cough). For positive responses, women were questioned about the duration of any symptoms, and whether the illness resulted in a doctor visit or treatment with prescription medication.
2.4. Home Water Sampling
Upon enrollment, participants were asked to provide water samples from their home tap (i.e. kitchen), using a commercially washed, mineral free, high-density polyethylene collection bottle (compliant with EPA standards for water collection) and instructions to minimize contamination. They were provided with mailing materials to return the samples to the study office, where they were stored at −20 °C until analysis by inductively coupled plasma mass spectrometry (ICP-MS) at the Trace Element Analysis Core at Dartmouth, as previously described (Gilbert-Diamond et al. 2011).
2.5. Urine Collection and Analysis
Women were asked to provide a spot urine sample upon enrollment (24–28 weeks gestation) using a pre-labeled, acid-washed, urine specimen container. Urine samples were stored upright at 4 °C, and sent via courier to the Pathology Department at Dartmouth Hitchcock Medical Center for processing within 24 hours of collection, after which they were aliquoted and stored at −80 °C. The original collection vial was shipped on dry ice to the University of Arizona Hazard Identification Core for analysis as described elsewhere (Gilbert-Diamond et al. 2011) using a high-performance liquid chromatography (HPLC) ICP-MS system (Larsen et al. 1993; Le et al. 2000; Wei et al. 2001). This method can quantitatively determine levels of AsIII, Asv , dimethylarsinic acid (DMAv ), monomethylarsonic acid (MMA v), and arsenobetaine. Detection limits ranged from 0.10 to 0.15 µg/L for individual As species and samples that registered below the detection limit were assigned a value equal to the detection limit divided by the square root of two.
2.6. Statistical Analysis
Individual maternal arsenic exposure at 24–28 weeks gestation was assessed by calculating total urinary As concentration by summing inorganic As (AsIII and AsV) and the metabolic products MMAV and DMAV, as previously reported (Gilbert-Diamond et al. 2011). We excluded arsenobetaine from this calculation, which is thought to be nontoxic and pass through the body unmetabolized (Tseng 2009).
We used logistic regression models to assess the relation between ln-transformed urinary maternal pregnancy As and types of common infections as separate outcomes, such as rhinorrhea, colds, otitis media, influenza, respiratory syncytial virus (RSV), upper respiratory tract infections (includes rhinorrhea, colds, otitis media, conjunctivitis), or lower respiratory tract infections (includes RSV, pertussis, bronchitis, bronchiolitis, pneumonia) and acute respiratory (includes cough, difficulty breathing, wheeze) and gastrointestinal (i.e. diarrhea) symptoms, similar to previous studies (Rahman et al. 2011; Raqib et al. 2009). Using Poisson models, we evaluated the relation between ln-transformed maternal urinary As and number of reported infections overall, as well as those lasting more than 2 days, resulting in a doctor visit or warranting prescription medication treatment. To generate a graphical representation of infection count results, a scatter plot smoother was used to compare with estimated Poisson regression curves (Friedman 1984).
All models were adjusted for available covariates that could potentially influence infection risk based on a priori considerations, including maternal age, maternal smoking and secondhand smoke exposure during pregnancy, birth weight, gestational age, parity, breast-feeding and day care attendance. Gestational age was calculated using first trimester ultrasound gestational age estimates or, if an ultrasound estimate was unavailable, last menstrual period date. A proportion of individuals were missing values for gestational age due to incomplete records, which were imputed using the average of the observed values. Maternal age was calculated at time of birth and both smoking and secondhand smoke were defined as any reported use or exposure during any trimester.
3. Results
A total of 214 mother- infant pairs were available for the analysis of maternal pregnancy urinary arsenic and infant infections at four months of age as of January 19, 2012.
3.1. Demographic Data
The mean (SD) age of the women in this study was 31.4 (4.8) years at the time of delivery (Table 1). The majority of women (87.0%) reported that they did not smoke during pregnancy and also were unexposed to second-hand smoke (87.3%). In the cohort of infants, slightly more than half were female (54%) and the mean (SD) birth weight was 3456.9 (546.3) grams, just slightly higher than the average birth weight in the US of 3389 grams at term (Donahue et al. 2010). The average (SD) gestational age at birth was 39.7 (1.7) weeks. At four months of age, most children (64%) were not in daycare and received all care in the home and 42% of mothers reported exclusive breast-feeding and close to half (49%) reported some combination of breast and formula feeding. Infections (Table 2) were prevalent, with 85% reporting at least one infection in the first four months of life, of which half resulted in a doctor visit and close to a quarter (27%) of infections were treated with prescription medication.
Table 1.
Selected sample characteristics for mothers and infants participating in the New Hampshire Birth Cohort Study (n = 214).
| Maternal Variables | Mean (range) or % |
|---|---|
| Maternal Age, years | 31.5 (18.6–44.6) |
| <20 | 1.4% |
| 20–29 | 33.2% |
| 30–35 | 45.3% |
| >35 | 20.1% |
| Education Level* | |
| <11th grade | 2.0% |
| High school graduate/ GED | 8.2% |
| Junior college, some college, technical school | 17.9% |
| College graduate | 44.4% |
| Postgraduate schooling | 27.5% |
| Relationship status* | |
| Single | 10.7% |
| Married | 85.7% |
| Separated or divorced | 3.6% |
| Smoking during pregnancy* | |
| Yes | 5.6% |
| No | 87.0% |
| Secondhand smoke exposure* | |
| Yes | 4.7% |
| No | 87.3% |
| Parity* | |
| Nulliparous | 44.4% |
| Multiparous | 54.2% |
| Infant Variables | |
| Infant Sex | |
| Male | 45.7% |
| Female | 54.3% |
| Birth Weight, grams* | 3456.9 (1380.0–5318.0) |
| Gestational age, weeks* | 39.6 (31.1–44.9) |
| Childcare Setting at 4 months | |
| Home | 64.5% |
| Daycare | 35.5% |
| Type of feeding at 4 months | |
| Breast-fed only | 42.1% |
| Formula-fed only | 9.3% |
| Combination | 48.6% |
Sum of subjects less than total sample size due to missing values. Eighteen subjects were missing education level and relationship status, sixteen were missing smoking and secondhand smoke exposure, three were missing parity, four were missing birth weight and twenty-nine were missing gestational age, for which missing values were imputed using the average of observed values.
Table 2.
Frequencies and relative risk estimates (95% CI)† for infant infections in the first four months of life, per one-fold increase in maternal urinary arsenic on a natural log scale, at ~24–28 weeks pregnancy (n = 214).
| Infections | At least one infection |
Lasting 2 or more days |
With a physician visit |
Treated with prescription medication |
|---|---|---|---|---|
| RR (95% CI) Total no. of cases |
||||
| Respiratory tract infections (RTI) | ||||
| Any Upper RTI | 1.1 (0.8, 1.6) 133 |
1.2 (0.9, 1.7) 111 |
1.1 (0.8, 1.6) 53 |
1.6 (1.0, 2.5) 28 |
| Cold, runny or stuffed nose |
1.0 (0.8, 1.4) 126 |
1.1 (0.8, 1.5) 103 |
1.0 (0.7, 1.4) 39 |
2.3 (1.0, 5.2) 9 |
| Eye infection (conjunctivitis) |
1.4 (0.8, 2.4) 17 |
1.4 (0.8, 2.6) 14 |
1.6 (0.9, 2.9) 14 |
1.2 (0.7, 2.1) 14 |
| Ear infection (otitis media) |
1.1 (0.5, 2.6) 8 |
1.1 (0.5, 2.6) 8 |
1.6 (0.7, 3.8) 7 |
1.6 (0.7, 3.8) 7 |
| Any Lower RTI (i.e. bronchitis, pneumonia, bronchiolitis, RSV, pertussis) |
1.4 (.7, 3.1) 9 |
1.4 (0.7, 3.1) 9 |
1.4 (0.7, 3.1) 9 |
3.3 (1.2, 9.0) 7 |
| Acute symptoms, conditions, illnesses | ||||
| Respiratory (i.e. cough, wheeze, difficulty breathing) |
1.1 (0.8, 1.6) 74 |
1.3 (0.9, 1.9) 57 |
1.3 (0.8, 2.0) 27 |
4.0 (1.0, 15.9) 5 |
| Gastrointestinal (i.e. diarrhea) |
1.2 (0.7, 2.0) 21 |
1.9 (0.9, 3.9) 10 |
3.5 (0.8, 15.4) 6 |
* 1 |
Based on logistic regression;
too few cases to analyze
3.2. As Exposure
At 24 to 28 weeks gestation, the median maternal total urinary As concentration measured was 3.7 µg/L and the mean (SD) concentration was 6.0 (7.5) µg/L, with a range of 0.45- 58.3 µg/L. The overall average drinking water As concentration was 5.2 µg/L (range 0.01–67.5 µg/L).
3.3. In Utero As Exposure and Infant Infections
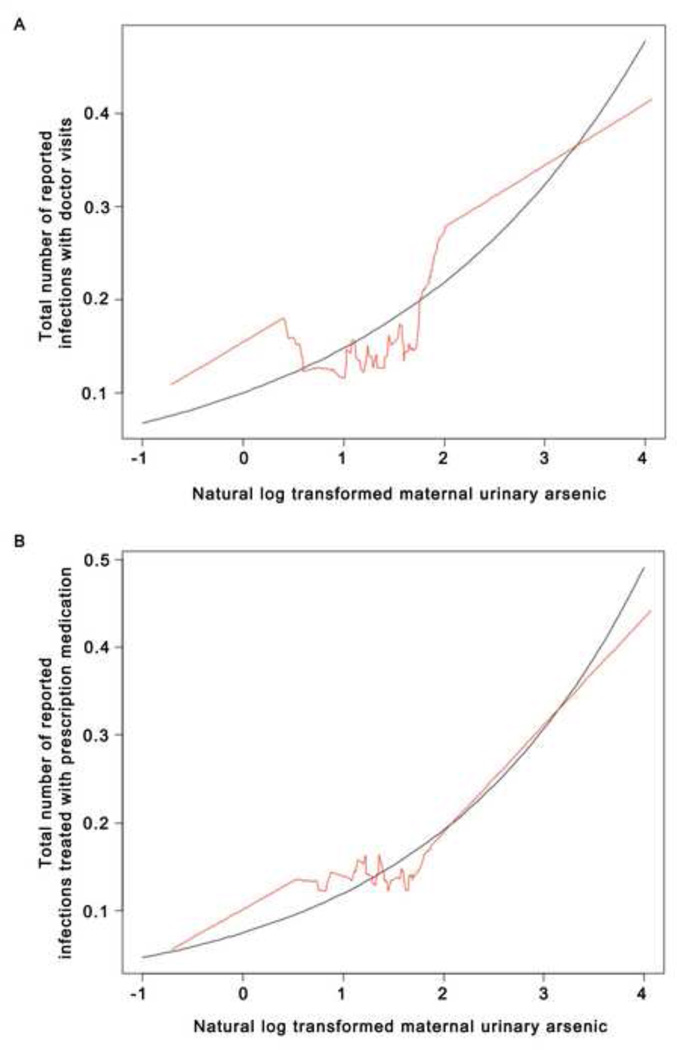
As summarized in Table 2, in Poisson models, maternal urinary As concentration during pregnancy (per one-fold increase in As in urine) was related to the number of reported infant infections in the first four months of life requiring a doctor visit (RR =1.5; 95% CI =1.0, 2.1) or treated with a prescription medication (RR=1.6; 95% CI =1.1, 2.4), after adjustment for maternal age, infant sex, gestational age, birth weight, breast feeding, day care attendance and parity. Figure 1 shows the plots of the smoothed observed data and fitted model parameters from the Poisson models indicating increasing trends in the number of reported infant infections with higher maternal urinary As concentrations. The relationship between maternal urinary As and number of infections lasting more than two days was suggestive of a similar trend, but not significant (RR=1.3; 95% CI =0.9, 1.8) (data not shown).
Figure 1.
Graphical representation of Poisson models of natural log transformed maternal urinary As and (a) total number of reported infant infections resulting in a doctor visit (RR = 1.5; CI =1.0, 2.1) and (b) total number of reported infant infections resulting in prescription medication treatment (RR = 1.6; CI =1.1, 2.4). Shown are the fitted Poisson models (black) and the smoothed scatterplots (red) of data.
In logistic regression models, after adjustment for maternal age, infant sex, gestational age, birth weight, breast feeding, day care attendance and parity, maternal urinary As concentrations during pregnancy (per one-fold increase in As in urine) were associated specifically with infant lower respiratory infections treated with a prescription medication (RR=3.3; 95% CI =1.2, 9.0), and respiratory symptoms including coughing, wheezing or difficulty breathing (RR=4.0; 95% CI =1.0, 15.8) treated with a prescription medication, and with attenuated, and not statistically significant estimates for those requiring a visit to the doctor or lasting two or more days.
Additional associations were observed with upper respiratory infections (RR=1.6; 95% CI =1.0, 2.5), and specifically infant colds, including rhinorrhea or nasal congestion, treated with a prescription medication (RR=2.3; 95% CI =1.0, 5.2), but with less statistical precision. Diarrhea symptoms lasting two or more days (RR=1.9; 95% CI =0.9, 3.9) or requiring a doctor’s visit (RR= 3.5; 95% CI =0.8, 15.4) also were positively associated with higher maternal urinary As concentrations, but we could not exclude the possibility of chance.
4. Discussion
In our US-based study, we found maternal As exposure during pregnancy to be related to infant infections in the first four months of life, and specifically those resulting in a doctor visit, requiring prescription medications, and infections of the lower respiratory tract.
To our knowledge, our findings are the first to report such an association in a prospective study in the United States. Remarkably, despite differences between study sites and populations, our results concur with two prospective studies from Bangladesh that found associations between maternal As exposure and increased infant infection morbidity. The earlier study of 140 mother and infant pairs by Raqib and colleagues observed increases in acute respiratory infections in infants in relation to maternal urinary As, which also related to thymic index and decreased presence of immune modulators IL-7 and lactoferrin in breast milk (Raqib et al. 2009). A larger, subsequent study followed 1552 pregnant women and their infants for 12 months after birth (Rahman et al. 2011). Similar to our study, in Bangladesh As exposure-related respiratory infections, particularly lower respiratory infections were elevated; close to a 70% increase in the relative risk of lower respiratory infections and more than a 50% increase in severe lower respiratory infections was observed among infants whose mothers had urinary As concentrations in the highest quintile of exposure (262–977 µg/L), compared with those in the lowest quintile (< 39 µg/L) (Rahman et al. 2011). The highest versus lowest quintile of exposure also was associated with a 20% increase in the relative risk of diarrhea in these infants (Rahman et al. 2011). While overall exposure levels and effect sizes in our study were lower than those of Bangladesh, we observed analogous trends in our data for more severe infections and diarrhea symptoms in infants with higher in utero As exposure.
Our results suggest that exposure to As in utero may affect the risk of severe infant infections reflected by infections requiring doctor visits or warranting prescription medication. In animal work, low doses of As (2, 10 and 100 µg/L in water), comparable to exposure levels in our study population, enhanced virulence of infections, weakened immune response, and increased pathogen load (Kozul et al. 2009a; Nayak et al. 2007). Epidemiological (Banerjee et al. 2009; Biswas et al. 2008; Fry et al. 2007; Hernandez-Castro et al. 2009; Soto-Pena et al. 2006) and experimental (Conde et al. 2007; Das et al. 2011; Lau et al. 2004b; Lemarie et al. 2006; Martin-Chouly et al. 2011; Nain and Smits 2010; Patterson et al. 2004; Stepnik et al. 2005; Vega et al. 2004) studies (summarized in Tables 3 and S1) provide further evidence of As’s ability to exert measurable immunopathological effects, in part by reducing lymphocyte proliferative response, macrophage functionality and certain T-cell populations. However, data on the effects of As on the immune system and subsequent clinical outcomes in infants and children is very limited, and prospective studies at low As exposure levels common to the US are lacking.
There are both strengths and limitations of our study. Our study was based on a biomarker of in utero exposure -- maternal urinary As-- and carefully collected prospective data, including infection occurrences, as well as information on potentially confounding factors. However, we lacked information on postnatal infant exposure to As (i.e. from food or water sources); yet based on previous studies, including our own study of infant formula, ingested sources may not contribute appreciably to exposure (Jackson et al. 2012b). Accuracy of mothers’ recollections of infections and their doctors’ diagnoses is a potential source of bias; however, we would not expect that reporting or diagnostic inaccuracies would be related to maternal arsenic exposure causing differential misclassification. We attempted to minimize misclassification by asking about the severity of infections (e.g., whether the infection required a doctor visit or prescription medicine). While under-or over-reporting remains a possibility, this too was unlikely related to exposure status (i.e., maternal urinary As concentrations). In future analyses as we obtain more information from our mothers and medical records, it will also be important to consider maternal infections and conditions such as asthma and allergies, which could potentially impact the incidence of infections in infants. Also, while private wells in the US provide drinking water that is generally free of pathogens, it is possible that illness could result from home water consumption, but we expect that such occurrences are sporadic and unlikely to be related to the presence of arsenic in well water. It is conceivable that the effects of in utero As exposure may differ by the sex of the infant. We did not observe any appreciable differences by gender, i.e., for lower respiratory infections involving a physician visit (data not shown); but as our study size is still relatively small, we lacked statistical power to detect such differences. We also are limited in the precision of our analyses, but we were able to observe significant increases in As-exposure related infection risks, and as this cohort grows, will be able to determine the robustness of our results, and the impact of As exposure on infections later in childhood.
Acute respiratory infections are among the most common causes of morbidity and mortality among children worldwide (Tregoning and Schwarze 2010; WHO 2010). Lower respiratory infections accounted for the majority of hospitalizations for childhood infections in the US in 2003, which together resulted in 1 million hospital days for infants at a cost of $690 million (Yorita et al. 2008). Respiratory syncytial virus (RSV), a lower respiratory infection often involved in bronchiolitis (CDC 2012), alone is responsible for the hospitalization of 1 in 50 US infants before their first birthday (Zhou et al. 2012). There is evidence that early-life respiratory infections increase the risks of later-childhood asthma, allergic sensitization, and wheezing (Lemanske et al. 2005; Tregoning and Schwarze 2010; Wright 2002). At much higher relative exposure levels (>800µg/L in drinking water) compared to our study, studies have suggested that in utero or early life As exposure decreases lung function and bronchiectasis-related mortality in adulthood (Dauphine et al. 2011; Smith et al. 2006).
5. Conclusions
During the critical developmental period that begins in fetal life and continues postnatally, the immune system has distinct sensitivities to environmental perturbations compared to that of an adult (Dietert and Piepenbrink 2006; Vahter 2009). Our initial findings suggest that maternal As exposure during pregnancy may increase risk of infant infections early in life, including infections that require medical treatment. Millions of people are exposed to elevated As concentrations in drinking water within the United States and worldwide, while others may be exposed via dietary sources (Gilbert-Diamond et al. 2011; Jackson et al. 2012a). Further, there is mounting evidence of As’s effects on immune function. Thus, in light of the health risks and costs of early life infections, our findings highlight the need to understand the potential clinical and public health implications of environmental As exposure in early childhood.
Supplementary Material
Highlights.
-
▸
Gestational As related to risk of infant infections involving a physician visit
-
▸
Associations also were specific to infections prescribed medication
-
▸
Strongest associations were observed for lower respiratory tract infections
-
▸
Our findings parallel those observed in more highly exposed populations
-
▸
As exposure at levels found in the US may enhance susceptibility to infant infections
Acknowledgments
The authors would like to thank our participants and study staff, without whom this work would not be possible.
Funding Sources: This work was supported by grants P20ES018175 (NIEHS) and RD83459901 (USEPA).
This study was reviewed and approved by the Committee for the Protection of Human Subjects (CPHS) at Dartmouth College, Hanover, NH and all participants in the study provided informed consent in accordance with CPHS guidelines.
Relevant abbreviations
- (As)
Arsenic
- (EPA)
Environmental Protection Agency
- (MCL)
Maximum contaminant level
- (NHBCS)
New Hampshire Birth Cohort Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing financial interests.
Appendix. Supplementary Information.
References
- Ahmad SA, Sayed MH, Barua S, Khan MH, Faruquee MH, Jalil A, et al. Arsenic in drinking water and pregnancy outcomes. Environmental health perspectives. 2001;109(6):629–631. doi: 10.1289/ehp.01109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Ahsan KB, Kippler M, Mily A, Wagatsuma Y, Hoque AM, et al. In utero arsenic exposure is associated with impaired thymic function in newborns possibly via oxidative stress and apoptosis. Toxicological sciences : an official journal of the Society of Toxicology. 2012 doi: 10.1093/toxsci/kfs202. [DOI] [PubMed] [Google Scholar]
- Andrew AS, Jewell DA, Mason RA, Whitfield ML, Moore JH, Karagas MR. Drinking-water arsenic exposure modulates gene expression in human lymphocytes from a U.S. population. Environmental health perspectives. 2008;116(4):524–531. doi: 10.1289/ehp.10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos M, Kalra T, Rathouz PJ, Chen Y, Pierce B, Parvez F, et al. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet. 2010;376(9737):252–258. doi: 10.1016/S0140-6736(10)60481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee N, Banerjee S, Sen R, Bandyopadhyay A, Sarma N, Majumder P, et al. Chronic arsenic exposure impairs macrophage functions in the exposed individuals. Journal of clinical immunology. 2009;29(5):582–594. doi: 10.1007/s10875-009-9304-x. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Jung P. Evolution in environmental health: incorporating the infectious disease paradigm. Environmental health perspectives. 2010;118(8):a327–a328. doi: 10.1289/ehp.1002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas R, Ghosh P, Banerjee N, Das JK, Sau T, Banerjee A, et al. Analysis of T-cell proliferation and cytokine secretion in the individuals exposed to arsenic. Human & experimental toxicology. 2008;27(5):381–386. doi: 10.1177/0960327108094607. [DOI] [PubMed] [Google Scholar]
- Caldwell KL, Jones RL, Verdon CP, Jarrett JM, Caudill SP, Osterloh JD. Levels of urinary total and speciated arsenic in the US population: National Health and Nutrition Examination Survey 2003–2004. Journal of exposure science & environmental epidemiology. 2009;19(1):59–68. doi: 10.1038/jes.2008.32. [DOI] [PubMed] [Google Scholar]
- CDC CfDCaP. [accessed Nov 1 2012]];Respiratory Syncytial Virus. Available: http://www.cdc.gov/rsv/
- Conde P, Acosta-Saavedra LC, Goytia-Acevedo RC, Calderon-Aranda ES. Sodium arsenite-induced inhibition of cell proliferation is related to inhibition of IL-2 mRNA expression in mouse activated T cells. Archives of toxicology. 2007;81(4):251–259. doi: 10.1007/s00204-006-0152-7. [DOI] [PubMed] [Google Scholar]
- Council NR. Arsenic in Drinking Water. Washington, D.C: National Academy Press; 2001. [Google Scholar]
- Das S, Pan D, Bera AK, Rana T, Bhattacharya D, Bandyapadyay S, et al. Sodium arsenite mediated immuno-disruption through alteration of transcription profile of cytokines in chicken splenocytes under in vitro system. Molecular biology reports. 2011;38(1):171–176. doi: 10.1007/s11033-010-0091-5. [DOI] [PubMed] [Google Scholar]
- Dauphine DC, Ferreccio C, Guntur S, Yuan Y, Hammond SK, Balmes J, et al. Lung function in adults following in utero and childhood exposure to arsenic in drinking water: preliminary findings. International archives of occupational and environmental health. 2011;84(6):591–600. doi: 10.1007/s00420-010-0591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietert RR, Piepenbrink MS. Perinatal immunotoxicity: why adult exposure assessment fails to predict risk. Environmental health perspectives. 2006;114(4):477–483. doi: 10.1289/ehp.8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue SMA, Kleinman KP, Gillman MW, Oken E. Trends in Birth Weight and Gestational Length Among Singleton Term Births in the United States: 1990–2005. Obstetrics & Gynecology. 2010;115(2, Part 1):357–364. doi: 10.1097/AOG.0b013e3181cbd5f5. 310. 1097/AOG.1090b1013e3181cbd1095f1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold BJ, Vegosen L, Davis M, Leibler J, Peterson A, Silbergeld EK. A niche for infectious disease in environmental health: rethinking the toxicological paradigm. Environmental health perspectives. 2010;118(8):1165–1172. doi: 10.1289/ehp.0901866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JH. A variable span smoother. Laboratory for Computational Statistics Stanford University Technical Report. 1984;(No. 5) [Google Scholar]
- Fry RC, Navasumrit P, Valiathan C, Svensson JP, Hogan BJ, Luo M, et al. Activation of inflammation/NF-kappaB signaling in infants born to arsenic-exposed mothers. PLoS genetics. 2007;3(11):e207. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galicia G, Leyva R, Tenorio EP, Ostrosky-Wegman P, Saavedra R. Sodium arsenite retards proliferation of PHA-activated T cells by delaying the production and secretion of IL-2. International immunopharmacology. 2003;3(5):671–682. doi: 10.1016/S1567-5769(03)00049-3. [DOI] [PubMed] [Google Scholar]
- Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, et al. Rice consumption contributes to arsenic exposure in US women. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(51):20656–20660. doi: 10.1073/pnas.1109127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Castro B, Doniz-Padilla LM, Salgado-Bustamante M, Rocha D, Ortiz-Perez MD, Jimenez-Capdeville ME, et al. Effect of arsenic on regulatory T cells. Journal of clinical immunology. 2009;29(4):461–469. doi: 10.1007/s10875-009-9280-1. [DOI] [PubMed] [Google Scholar]
- Hopenhayn C, Ferreccio C, Browning SR, Huang B, Peralta C, Gibb H, et al. Arsenic exposure from drinking water and birth weight. Epidemiology. 2003;14(5):593–602. doi: 10.1097/01.ede.0000072104.65240.69. [DOI] [PubMed] [Google Scholar]
- Huyck KL, Kile ML, Mahiuddin G, Quamruzzaman Q, Rahman M, Breton CV, et al. Maternal arsenic exposure associated with low birth weight in Bangladesh. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2007;49(10):1097–1104. doi: 10.1097/JOM.0b013e3181566ba0. [DOI] [PubMed] [Google Scholar]
- Jackson BP, Taylor VF, Karagas MR, Punshon T, Cottingham KL. Arsenic, organic foods, and brown rice syrup. Environmental health perspectives. 2012a;120(5):623–626. doi: 10.1289/ehp.1104619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson BP, Taylor VF, Punshon T, Cottingham KL. Arsenic concentration and speciation in infant formulas and first foods. Pure and applied chemistry Chimie pure et appliquee. 2012b;84(2):215–223. doi: 10.1351/PAC-CON-11-09-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagas MR. Arsenic-related mortality in Bangladesh. Lancet. 2010;376(9737):213–214. doi: 10.1016/S0140-6736(10)61002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagas MR, Stukel TA, Morris JS, Tosteson TD, Weiss JE, Spencer SK, et al. Skin cancer risk in relation to toenail arsenic concentrations in a US population-based case-control study. American journal of epidemiology. 2001;153(6):559–565. doi: 10.1093/aje/153.6.559. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Stukel TA, Tosteson TD. Assessment of cancer risk and environmental levels of arsenic in New Hampshire. International journal of hygiene and environmental health. 2002;205(1–2):85–94. doi: 10.1078/1438-4639-00133. [DOI] [PubMed] [Google Scholar]
- Karagas MR, Tosteson TD, Blum J, Morris JS, Baron JA, Klaue B. Design of an epidemiologic study of drinking water arsenic exposure and skin and bladder cancer risk in a U.S. population. Environmental health perspectives. 1998;106(Suppl 4):1047–1050. doi: 10.1289/ehp.98106s41047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozul CD, Ely KH, Enelow RI, Hamilton JW. Low-dose arsenic compromises the immune response to influenza A infection in vivo. Environmental health perspectives. 2009a;117(9):1441–1447. doi: 10.1289/ehp.0900911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozul CD, Hampton TH, Davey JC, Gosse JA, Nomikos AP, Eisenhauer PL, et al. Chronic exposure to arsenic in the drinking water alters the expression of immune response genes in mouse lung. Environmental health perspectives. 2009b;117(7):1108–1115. doi: 10.1289/ehp.0800199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen EH, Pritzl G, Hansen SH. Speciation of 8 arsenic compounds in human urine by high-performance liquid-chromatography with inductively-coupled plasma-mass spectrometric detection using antimonate for internal chromatographic standardization. J Anal At Spectrom. 1993;8(4):557–563. [Google Scholar]
- Lau AT, He QY, Chiu JF. A proteome analysis of the arsenite response in cultured lung cells: evidence for in vitro oxidative stress-induced apoptosis. The Biochemical journal. 2004a;382(Pt 2):641–650. doi: 10.1042/BJ20040224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AT, Li M, Xie R, He QY, Chiu JF. Opposed arsenite-induced signaling pathways promote cell proliferation or apoptosis in cultured lung cells. Carcinogenesis. 2004b;25(1):21–28. doi: 10.1093/carcin/bgg179. [DOI] [PubMed] [Google Scholar]
- Le XC, Lu XF, Ma MS, Cullen WR, Aposhian HV, Zheng BS. Speciation of key arsenic metabolic intermediates in human urine. Anal Chem. 2000;72(21):5172–5177. doi: 10.1021/ac000527u. [DOI] [PubMed] [Google Scholar]
- Lemanske RF, Jr, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. The Journal of allergy and clinical immunology. 2005;116(3):571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Lemarie A, Morzadec C, Bourdonnay E, Fardel O, Vernhet L. Human macrophages constitute targets for immunotoxic inorganic arsenic. J Immunol. 2006;177(5):3019–3027. doi: 10.4049/jimmunol.177.5.3019. [DOI] [PubMed] [Google Scholar]
- Martin-Chouly C, Morzadec C, Bonvalet M, Galibert MD, Fardel O, Vernhet L. Inorganic arsenic alters expression of immune and stress response genes in activated primary human T lymphocytes. Molecular immunology. 2011;48(6–7):956–965. doi: 10.1016/j.molimm.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Mehal JM, Esposito DH, Holman RC, Tate JE, Callinan LS, Parashar UD. Risk factors for diarrhea-associated infant mortality in the United States, 2005–2007. The Pediatric infectious disease journal. 2012;31(7):717–721. doi: 10.1097/INF.0b013e318253a78b. [DOI] [PubMed] [Google Scholar]
- Milton AH, Smith W, Rahman B, Hasan Z, Kulsum U, Dear K, et al. Chronic arsenic exposure and adverse pregnancy outcomes in bangladesh. Epidemiology. 2005;16(1):82–86. doi: 10.1097/01.ede.0000147105.94041.e6. [DOI] [PubMed] [Google Scholar]
- Nachman KE, Raber G, Francesconi KA, Navas-Acien A, Love DC. Arsenic species in poultry feather meal. The Science of the total environment. 2012;417–418:183–188. doi: 10.1016/j.scitotenv.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Nain S, Smits JE. Pathological, immunological and biochemical markers of subchronic arsenic toxicity in rats. Environmental toxicology. 2010 doi: 10.1002/tox.20635. [DOI] [PubMed] [Google Scholar]
- Nayak AS, Lage CR, Kim CH. Effects of low concentrations of arsenic on the innate immune system of the zebrafish (Danio rerio) Toxicological sciences : an official journal of the Society of Toxicology. 2007;98(1):118–124. doi: 10.1093/toxsci/kfm072. [DOI] [PubMed] [Google Scholar]
- Patterson R, Vega L, Trouba K, Bortner C, Germolec D. Arsenic-induced alterations in the contact hypersensitivity response in Balb/c mice. Toxicology and applied pharmacology. 2004;198(3):434–443. doi: 10.1016/j.taap.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Rahman A, Persson LA, Nermell B, El Arifeen S, Ekstrom EC, Smith AH, et al. Arsenic exposure and risk of spontaneous abortion, stillbirth, and infant mortality. Epidemiology. 2010;21(6):797–804. doi: 10.1097/EDE.0b013e3181f56a0d. [DOI] [PubMed] [Google Scholar]
- Rahman A, Vahter M, Ekstrom EC, Persson LA. Arsenic exposure in pregnancy increases the risk of lower respiratory tract infection and diarrhea during infancy in Bangladesh. Environmental health perspectives. 2011;119(5):719–724. doi: 10.1289/ehp.1002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Vahter M, Ekstrom EC, Rahman M, Golam Mustafa AH, Wahed MA, et al. Association of arsenic exposure during pregnancy with fetal loss and infant death: a cohort study in Bangladesh. American journal of epidemiology. 2007;165(12):1389–1396. doi: 10.1093/aje/kwm025. [DOI] [PubMed] [Google Scholar]
- Rahman A, Vahter M, Smith AH, Nermell B, Yunus M, El Arifeen S, et al. Arsenic exposure during pregnancy and size at birth: a prospective cohort study in Bangladesh. American journal of epidemiology. 2009;169(3):304–312. doi: 10.1093/aje/kwn332. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Ng JC, Naidu R. Chronic exposure of arsenic via drinking water and its adverse health impacts on humans. Environmental geochemistry and health. 2009;31(Suppl 1):189–200. doi: 10.1007/s10653-008-9235-0. [DOI] [PubMed] [Google Scholar]
- Raqib R, Ahmed S, Sultana R, Wagatsuma Y, Mondal D, Hoque AM, et al. Effects of in utero arsenic exposure on child immunity and morbidity in rural Bangladesh. Toxicology letters. 2009;185(3):197–202. doi: 10.1016/j.toxlet.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Schuchat AaJP. [accessed 7-10-2012 2012];Child Health: Bacterial and other infectious diseases. 2012 Available: http://www.cdc.gov/reproductivehealth/ProductsPubs/DatatoAction/pdf/Chltl.pdf.
- Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, et al. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environmental health perspectives. 2006;114(8):1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Pena GA, Luna AL, Acosta-Saavedra L, Conde P, Lopez-Carrillo L, Cebrian ME, et al. Assessment of lymphocyte subpopulations and cytokine secretion in children exposed to arsenic. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20(6):779–781. doi: 10.1096/fj.05-4860fje. [DOI] [PubMed] [Google Scholar]
- Stepnik M, Stanczyk M, Arkusz J, Lewinska D. Assessment of apoptosis in thymocytes and splenocytes from mice exposed to arsenate in drinking water: cytotoxic effects of arsenate on the cells in vitro. Journal of environmental science and health Part A, Toxic/hazardous substances & environmental engineering. 2005;40(2):369–384. doi: 10.1081/ese-200045629. [DOI] [PubMed] [Google Scholar]
- Tregoning JS, Schwarze J. Respiratory Viral Infections in Infants: Causes, Clinical Symptoms, Virology, and Immunology. Clinical Microbiology Reviews. 2010;23(1):74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CH. A review on environmental factors regulating arsenic methylation in humans. Toxicology and applied pharmacology. 2009;235(3):338–350. doi: 10.1016/j.taap.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Vahter M. Effects of arsenic on maternal and fetal health. Annual review of nutrition. 2009;29:381–399. doi: 10.1146/annurev-nutr-080508-141102. [DOI] [PubMed] [Google Scholar]
- Vega L, Montes de Oca P, Saavedra R, Ostrosky-Wegman P. Helper T cell subpopulations from women are more susceptible to the toxic effect of sodium arsenite in vitro. Toxicology. 2004;199(2–3):121–128. doi: 10.1016/j.tox.2004.02.012. [DOI] [PubMed] [Google Scholar]
- von Ehrenstein OS, Guha Mazumder DN, Hira-Smith M, Ghosh N, Yuan Y, Windham G, et al. Pregnancy outcomes, infant mortality, and arsenic in drinking water in West Bengal, India. American journal of epidemiology. 2006;163(7):662–669. doi: 10.1093/aje/kwj089. [DOI] [PubMed] [Google Scholar]
- Wei HY, Brockhoff-Schwegel CA, Creed JT. A comparison of urinary arsenic speciation via direct nebulization and on-line photo-oxidation-hydride generation with IC separation and ICP-MS detection. J Anal At Spectrom. 2001;16(1):12–19. [Google Scholar]
- WHO WHO. [accessed Nov 1 2012];Global Health Observatory, causes of child mortality. 2010 Available: http://www.who.int/gho/child_health/mortality/causes/en/
- Wright AL. Epidemiology of asthma and recurrent wheeze in childhood. Clinical reviews in allergy & immunology. 2002;22(1):33–44. doi: 10.1007/s12016-002-0004-z. [DOI] [PubMed] [Google Scholar]
- Wu MM, Chiou HY, Ho IC, Chen CJ, Lee TC. Gene expression of inflammatory molecules in circulating lymphocytes from arsenic-exposed human subjects. Environmental health perspectives. 2003;111(11):1429–1438. doi: 10.1289/ehp.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorita KL, Holman RC, Sejvar JJ, Steiner CA, Schonberger LB. Infectious disease hospitalizations among infants in the United States. Pediatrics. 2008;121(2):244–252. doi: 10.1542/peds.2007-1392. [DOI] [PubMed] [Google Scholar]
- Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng PY, Steiner C, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54(10):1427–1436. doi: 10.1093/cid/cis211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.