Abstract
Background
Prostate cancer is currently diagnosed by random biopsies resulting in the discovery of multiple low risk cancers that often lead to overtreatment.
Multiparametric magnetic resonance imaging (mpMRI) may have the potential to identify patients at low risk for cancer, thus obviating the need for biopsy.
Methods
We reviewed 800 consecutive patients who underwent a 3 Tesla mpMRI of the prostate with endorectal coil from March 2007 to November 2011.
Two radiologists independently reviewed all suspicious lesions using T2-weighted, diffusion weighted, spectroscopic, and dynamic contrast enhanced MRI sequences.
Patients with only low suspicion lesions (maximum of two positive parameters on mpMRI) who subsequently underwent TRUS/MRI-fusion targeted biopsy were selected for analysis.
Results
One hundred and twenty-five patients with only low suspicion prostatic lesions on mpMRI were identified.
On TRUS/MRI-fusion biopsy, 77 of these patients (62%) had no cancer detected, 38 patients had Gleason 6 disease, and 10 patients had Gleason 7 (3+4) disease.
Thirty patients with cancer detected on biopsy qualified for active surveillance using 2011 NCCN guidelines.
No cases of high risk (≥ Gleason 4+3) cancer were identified on biopsy and of the fifteen patients that underwent radical prostatectomy at our institution, none were pathologically upgraded to high risk cancer.
Thus, for patients with only low suspicion lesions, 88% (107 patients) either had no cancer or clinically insignificant disease.
Conclusion
Our results demonstrate that low suspicion lesions on mpMRI are associated with either negative biopsies or low grade tumors suitable for active surveillance.
Such patients have a low risk of harboring high risk prostate cancers.
Introduction
Prostate cancer (CaP) is a common malignancy in the United States1. Screening for CaP with digital rectal exam and serum prostate specific antigen (PSA) often leads to detection of cancer via ultrasound (TRUS) guided biopsy. However, TRUS guided biopsies are not directed at specific areas of abnormality and therefore, may miss clinically significant disease and inaccurately stage the cancer in patients. Therefore, there is currently a need for improving the screening and staging algorithm for CaP.
The use of multiparametric magnetic resonance imaging (mpMRI) has gained attention for its role in the localization of prostate cancer. It has been shown that mpMRI correlates with histopathologic tumor location on final pathology2. By fusing mpMRI to TRUS, it is possible to superimpose pre-biopsy MRI images onto real time transrectal ultrasound images to allow for targeted biopsy. This method yielded a superior cancer detection rate of 54.4% compared to 27–40% for the standard random TRUS biopsy3. In a similar study, the level of suspicion for cancer on mpMRI correlated with the D’Amico classification4. Moreover, MRI has shown to better stage patients before treatment when compared to TRUS biopsy5. This suggests that lesions identified by mpMRI vary in significance according to the number of pulse sequences that are positive. Those lesions seen on only one or two sequences are often associated with the absence of cancer or the presence of low grade cancers. Therefore, mpMRI could potentially serve a greater role in identification and management of low risk prostate cancer in these patients.
At our institution, prostate lesions are graded in three suspicion levels: low, moderate and high, depending on the number of positive MRI parameters2. These parameters include T2-weighted (T2W), diffusion weighted (DW-MRI), spectroscopy (MRSI) and dynamic contrast enhanced MRI (DCE-MRI). Low suspicion lesions are defined as being positive on two or fewer of the four parameters (typically T2W and DW-MRI) whereas moderate suspicion lesions are defined as being positive on three of four parameters (typically, T2W, DW-MRI and DCE-MRI) and high suspicion lesions are defined as being positive on all four parameters. We hypothesize that patients with only low suspicion lesions represent a special low risk group of men with either no disease or clinically insignificant disease, allowing them to be managed conservatively. In this report, we analyze a cohort of patients with low suspicion lesions on mpMRI who subsequently underwent image guided biopsy using a TRUS/MRI fusion guided platform to determine the prevalence of high risk prostate cancer in this group.
Materials and Methods
This prospective study was approved by the Institutional Review Board of the National Cancer Institute of the National Institutes of Health. All patient information was protected according to the Health Insurance Portability and Accountability Act (HIPPA). Patients eligible for this study were appropriately consented and informed for the potential harms and benefits.
Between May 2007 and November 2011, all patients with a high clinical suspicion for prostate cancer (based on elevated PSA, strong family history, referral for evaluation from community doctor) underwent multiparametric magnetic resonance imaging. Mean age was 60 years (range 36–81, median 59) and mean PSA was 7.11 ng/mL (range 0.3–64.7, median 5.7). Further patient demographics are presented in Table 1.
Table 1.
Patient Demographics
| N (%) | |
|---|---|
| Patients | 125 |
| Mean Age (years) | 60 |
| Range | 36–81 |
| Race | |
| Caucasian | 101 (81) |
| Black | 17 (14) |
| Hispanic | 4 (3) |
| Asian | 3 (2) |
| Mean PSA (ng/mL) | 7.11 |
| Range | 0.3 – 64.7 |
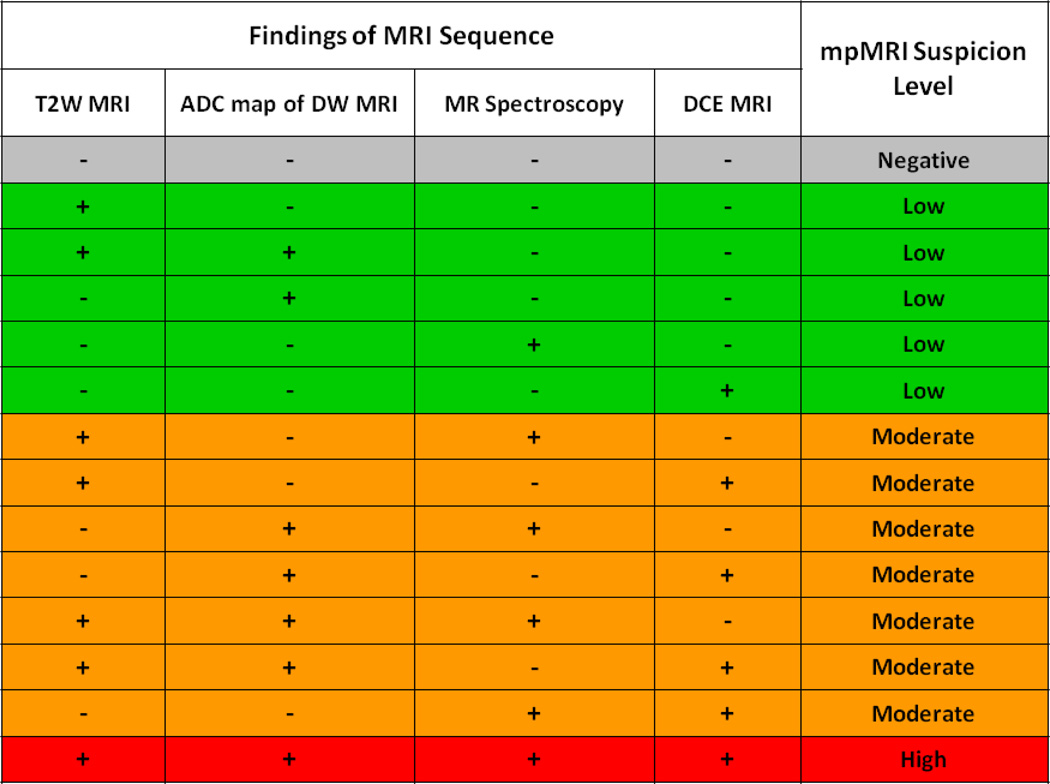
MpMRI was performed at 3.0 Telsa (Achieva, Philips Healthcare, Best, The Netherlands). MRI acquisition was performed using a combination of a 6-channel cardiac surface coil (SENSE, Philips Healthcare) placed over the pelvis and an endorectal coil (BPX-30, Medrad, Pittsburgh, Pennsylvania) as previously described3. MRI sequences included tri-planar T2-weighted MRI, axial diffusion weighted MRI (DW MRI), 3D MR spectroscopy (MRSI), and axial dynamic contrast enhanced MRI (DCE MRI). MpMRI subsequently underwent blinded centralized radiologic evaluation by two experienced genitourinary radiologists (BT and PLC). For multi-parametric MRI analysis of the peripheral zone lesions, on T2W MR images and apparent diffusion coefficient (ADC) maps of DW MRI, the criterion for a “visible” lesion was a well circumscribed, round-ellipsoid low-signal-intensity region within the prostate gland6. The 3D-MR spectroscopy analysis evaluated choline/citrate (Cho/Cit) ratios within the image voxels in the lesion core sites. Voxels were considered abnormal when the (Cho/Cit) ratio was 3 or more standard deviations above the mean healthy Cho/Cit ratio value (≥0.373), which was defined as 0.13 +/− 0.081 based on results previously recorded from 433 healthy voxels from peripheral zone regions6. DCE MR images were evaluated by direct visual interpretation of raw dynamic enhanced T1W images and the diagnostic criterion for prostate cancer was defined as a focus of asymmetric, early and intense enhancement with rapid wash out compared to the background6. For central gland lesions, on T2W MRI images and ADC maps of DW-MRI the criterion for a “visible” lesion was homogenous low signal intensity lesion with irregular margins and no capsule, often invading the pseudocapsule, with lenticular extension into the urethra, or anterior fibromuscular zone6. The criteria for lesion analysis in the peripheral zone were the same for MR spectroscopy and DCE MRI sequences. Intraprostatic lesions were categorized as low suspicion if two or fewer parameters (typically T2W and DW MRI were positive, moderate suspicion if three modalities (typically T2W and DW MRI and DCE-MRI) were positive, and high suspicion if all four modalities were positive (Figure 1). Additionally, the prostate gland was manually contoured planimetrically by an experienced radiologist and MRI derived prostate volumes were calculated.
Figure 1.
Multiparametric MRI scorecard for suspicious lesions. Suspicion level for lesions is determined by number of positive parameters on imaging.
Patients with lesions suspicious for cancer on MRI were enrolled in our image guided prostate biopsy protocol which fuses the MRI to the TRUS. All patients undergoing the outpatient, office-based prostate biopsy were given a 3 day course of antibiotic prophylaxis and a cleansing Fleets enema the morning of the procedure. Lidocaine jelly and injectable lidocaine for analgesia was used to obtain a peri-prostatic block. Initially, all patients underwent a standard “extended sextant” 12-core transrectal ultrasound biopsy. During this TRUS biopsy, the physician was blinded to the location of suspicious lesions previously detected on the mpMRI. In the same biopsy session, patients underwent a TRUS/MRI fusion guided biopsy of suspicious lesions found on mpMRI. An electromagnetic field generator was placed above the pelvis in order to track the transrectal ultrasound probe real-time during the biopsy. Following a 2-dimensional sweep of the rectal probe, the realtime US image was fused to the previous MRI image, allowing the operator to guide the biopsy needle to previously identified suspicious lesions. A minimum of two biopsy cores were taken from each lesion (one in the axial plane, one in the sagittal plane). The details of the biopsy platform and description of the biopsy technique have been previously described3,7,8. All biopsies underwent blinded centralized pathologic evaluation by a single GU pathologist.
Descriptive statistics were used to describe patient characteristics, including patient age, PSA, PSA density, MRI volumes, and lesions. A Student’s t-test was used to determine any differences between the biopsy positive and biopsy negative patients for lesions per patient, MRI prostate volume, PSA, and PSA density.
Results
Patient Demographics
A total of 800 patients underwent mpMRI of the prostate between 2007 and 2011. Clinical suspicion of prostate cancer (elevated PSA, family history, or referral from community center) was suspected in all patients who presented for evaluation. We identified 125 (16%) patients with only low suspicion lesions on mpMRI for analysis in this series.
3T multi-parametric MRI and MRI/US Fusion Biopsy Results
Of the total cohort of 125 patients, 77 (62%) did not have cancer on either on the TRUS or MRI targeted biopsy. Forty-eight patients (38%) had cancer detected on biopsy (4 patients were positive on MRI targeted fusion biopsy alone, 24 positive on TRUS alone, and 20 positive on both MRI targeted and TRUS biopsy, Table 4). When evaluating for disparities between disease positive and negative patients, no differences were noted in the mean number of lesions per patient, PSA, prostate volume, or PSA density (Table 2).
Table 4.
MRI-guided vs. TRUS Biopsy Results
| Gleason 6 | Gleason 7 (3+4) | Total | |
|---|---|---|---|
| MRI-guided biopsy only | 4 | 1* | 4 |
| TRUS biopsy only | 21 | 3* | 24 |
| Both platforms combined | 13 | 6 | 20 |
| Total | 38 | 10 | 48 |
Low volume Gleason 7 disease (<2 cores, <40% each core)
Table 2.
Patient Characteristics
| Biopsy Negative |
Biopsy Positive |
P-value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Patients | 77 (62) | 48 (38) | - |
| Lesions | 155 (58) | 112 (42) | .09 |
| Lesions/Patient | 2.01 | 2.54 | - |
| MRI Prostate Volume (mL) | 52.29 | 59.88 | .188 |
| PSA (ng/mL) | 6.04 | 7.79 | .144 |
| PSA Density (ng/mL/mL) | 0.135 | 0.143 | 0.73 |
Gleason Grade
Of the 48 patients who had positive biopsies, 38 (79%) patients had Gleason 6 (3+3) cancer while 10 (21%) patients had Gleason 7 (3+4) cancer (Table 3). The average number of positive TRUS cores was 2.59 per patient (range 1–9). The mean percentage involvements of TRUS biopsy and MR-guided biopsy cores were 20.7% (range 1–80%) and 21.65% (range 3–70%), respectively. No patients had primary Gleason 4 disease or higher (Table 4).
Table 3.
MpMRI Low Suspicion Biopsy Results
| N | |
|---|---|
| Patients with MR low suspicious lesions | 125 |
| Patients negative on TRUS or MRI guided biopsy | 77 |
| Patients positive on TRUS or MRI guided biopsy | 48 |
| Gleason 6 Cancer on biopsy | 38 |
| Gleason 7 (3+4) Cancer on biopsy | 10 |
| Patients qualified for Active Surveillance via NCCN | 30 |
| Patients currently on an Active Surveillance protocol | 27 |
| Patients underwent whole gland therapy (surgery/radiation) | 21 |
| Patients underwent radical prostatectomy at institution | 15 |
| Patients with ≥ GS 4+3 cancer found on post-operative pathology | 0 |
Post Operative Pathology Correlation
Twenty-one patients (18 prostatectomy, 3 external beam radiation) chose to undergo whole gland therapy at the time of analysis. Fifteen patients chose to undergo surgical intervention at our institution, while 3 patients underwent surgery at other institutions. Of the patients who underwent surgery at our institution, all were found to have either Gleason 6 (3+3) or Gleason 7 (3+4) disease on post operative pathology upon review by an experienced GU pathologist. No patients were found to have primary Gleason 4 disease or greater, extracapsular extension, or seminal vesicle invasion. All patients had pT2 disease on post-operative pathology.
Active Surveillance Patient Qualification
Of the 48 patients found to have cancer on biopsy, 9 patients with Gleason 7 disease were disqualified from active surveillance using 2011 NCCN guidelines due to disease grade. Furthermore, 4 patients with Gleason 6 disease found on MRI guided fusion biopsy alone were also disqualified because no existing guidelines incorporate this novel biopsy system. Of the remaining patients, 30 (29 patients with Gleason 6 disease, 1 patient with Gleason 3+4 disease) qualified for active surveillance using 2011 NCCN guidelines (either “very low risk” or “low risk”). At the time of analysis, 27 patients were still on an active surveillance at the National Cancer Institute. Three patients opted to undergo surgical intervention following cancer diagnosis due to personal preference.
Discussion
PSA screening has resulted in a dramatic increase in the number of men diagnosed with prostate cancer. This has led to concerns that overdiagnosis of indolent cancers is occurring at great human and medical costs. Many small low grade tumors are inconsequential, yet lead to definitive treatment. Multiparametric MRI offers the ability to survey the entire gland for suspicious lesions9. Many lesions depicted by MRI are low in suspicion. In order to determine the value of detecting low suspicion prostate cancer with mpMRI, only patients with a clinical suspicion (elevated PSA, family history, outside referral) of prostate cancer underwent imaging and received a biopsy in our protocol.
The results from this study show that patients with only low suspicion lesions on mpMRI had a very low likelihood of harboring high risk disease. Among the 125 patients found to have only low suspicion lesions on mpMRI, 77 did not have cancer by standard or MRI guided biopsy while 38 patients had Gleason 6 (3+3) cancer. Thus, 115/125 (92%) of patients with only low suspicion lesions either did not have cancer or had low risk cancers. Of the 38 patients with Gleason 6 cancer, 29 patients were classified as either “very low” risk or “low” risk under the 2011 NCCN guidelines, qualifying them for active surveillance. One patient with Gleason 3+4 disease also belonged in this group as he had low volume disease and was 75 years of age with a life expectancy of less than 10 years, qualifying him for active surveillance. Thus, using the 2011 NCCN guidelines, 88% (107 patients) of patients with only low suspicion lesions on mpMRI had either no cancer or qualified for active surveillance. With further evidence that low risk and low volume prostate cancer should be followed with active surveillance10, these patients may avoid radical whole gland treatments along with their associated morbidities.
A total of 18 patients in our cohort did not qualify for active surveillance. Three patients were disqualified from active surveillance for having PSA ≥ 10 ng/mL but met all other parameters (all had small focus of Gleason 6 cancer). Upon further analysis, these patients had a mean prostate volume of 103 mL (range 69–146) on manual MRI planimetric volume estimation. Based on previously published studies, it is known that PSA correlates with prostate size at large volumes11. One patient had areas of chronic inflammation on biopsy, another potential explanation for the high PSA. Since these patients met all other criteria for active surveillance and the high PSA’s are likely explained by their large prostate volumes or inflammation, they may not necessarily have needed to be disqualified from active surveillance. In addition, 4 patients had Gleason 6 cancer detected on MRI guided biopsy alone. Since no guidelines have been established for this novel method of biopsy, these patients were not considered for active surveillance. However, all four patients were classified as “low risk” based on D’Amico classification12 (all had 1 core of Gleason 6 disease) and could be appropriate patients for active surveillance.
Nine patients in our cohort had Gleason 3+4 cancer detected on biopsy and were not eligible for active surveillance. Classical NCCN guidelines categorize most patients with Gleason 3+4 disease as “intermediate” risk, thereby disqualifying them from active surveillance. However, there is mounting evidence that patients with intermediate risk prostate cancer are appropriate for active surveillance13. Using the validated University of California, San Francisco Cancer of the Prostate Risk Assessment Score (CAPRA)14–16, all remaining 9 patients with Gleason 3+4 disease scores were between 3–5, categorizing them as “intermediate” risk. Cooperberg et al. reported that patients with intermediate risk (Gleason 7 or CAPRA risk 3–5) prostate cancer are not necessarily prone to higher rates of progression and thus, careful active surveillance is a legitimate option for appropriately selected patients in this group13.
Studies have shown significant pathological upgrading on radical prostatectomy, with recent studies reporting the number between 20.3 – 54%17,18. This potentially creates a problem in accurately following a patient with supposedly low risk disease. In our study, we found that in the 15 patients who underwent radical prostatectomy at our institution, disease in the prostate was limited to either Gleason 6 or 7 (3+4) tumors. Five patients were upgraded from Gleason 6 disease to Gleason 3+4 disease on post-operative histology, consistent with pathologic upgrading from biopsies noted in prior studies17,18. While some patients were pathologically upgraded on pathology to Gleason 7 (3+4), no patients were upgraded or discovered to have high risk cancer (Gleason 4+3 or greater) on pathology. In an attempt to elucidate MRI’s role in selecting patients with low risk disease using post operative pathology, Guzzo et al. reported that tumor identification on endorectal MRI was not predictive of adverse pathologic features for men who otherwise qualified for active surveillance. However, patients in that study were imaged with a 1.5 Telsa scanner and only T2-weighted sequences were used19. In contrast, by correlating the MRI with patient-specific MRI-based prostate molds to generate whole-mount histologic sections that correlate to axial MRI images, Turkbey et al. demonstrated that the combination of additional MRI parameters significantly increased sensitivity in detecting suspicion lesions2. Patients in our study were imaged using mpMRI and a 3 Telsa scanner, providing for more accuracy when identifying and characterizing each lesion. Though a limited number of patients underwent prostatectomy at our institution, no patients were found to have high risk cancer on pathology, providing further confidence that select patients with low suspicion lesions are appropriate for active surveillance.
Multiparametric MRI is emerging as a tool for urologists in the localization and management of prostate cancer. In the future, mpMRI may be able to localize prostate cancer without the use of a biopsy. If a patient has only low suspicion lesions, the probability they have no cancer or will qualify for active surveillance is quite high (88%). Unlike Choi et al., who reported that patients on active surveillance are imaged less compared to those who choose surgery20, we believe that mpMRI has a significant role in the oncologic management of patients with low risk disease. Twenty-seven of the 30 patients (90%) who qualified for active surveillance remain on a surveillance protocol at the National Cancer Institute, which includes MRI at the time of biopsy.
Our study has several limitations. First, this study was conducted only at one institution with a high level of expertise in mpMRI. We believe that validation of our hypothesis in a matched cohort in another institution would be of value. Additionally, the multi-parametric MRI scoring system used is a simple and non-weighted system in which all sequences had an equal contribution in assessing the suspicion level of lesions. While emerging data suggest specific parameters can correlate to the aggressiveness of prostate cancer21, a clear, weighted MRI scoring system has not been validated. In our current scoring system, each parameter was considered either positive or negative whereas a 5 point scale can used for grading each parameter in the weighted scale. The reproducibility of this 5 point scale, however, has not been well established. Currently, there is an international effort to develop such a grading system for mpMRI22.
Conclusion
Multi-parametric MRI is a non-invasive technique for localization of prostate cancer that allows the clinician to stratify the risk of prostate cancer based on the MR-determined suspicion of the lesions. Our results indicate that low suspicion lesions on multi-parametric MRI tend to predict for the absence of high risk cancer and the presence of low grade disease or no disease. In those individuals with low suspicion-only lesions, constituting about 16% of our population, the risk of significant disease is low enough to consider deferring biopsy or if cancer is found by biopsy, choosing active surveillance. These single institution results should be verified in multiple institutions which will help elucidate the role of mpMRI in the management of low risk prostate cancer.
Acknowledgements
This research was supported [in part] by the Intramural Research Program of the National Cancer Institute, NIH
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010 Sep-Oct;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011 Nov;186(5):1818–1824. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011 Oct;186(4):1281–1285. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rastinehad AR, Baccala AA, Jr, Chung PH, et al. D'Amico risk stratification correlates with degree of suspicion of prostate cancer on multiparametric magnetic resonance imaging. J Urol. 2011 Mar;185(3):815–820. doi: 10.1016/j.juro.2010.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hambrock T, Hoeks C, Hulsbergen-van de Kaa C, et al. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol. 2012 Jan;61(1):177–184. doi: 10.1016/j.eururo.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 6.Akin O, Sala E, Moskowitz CS, et al. Transition zone prostate cancers: features, detection, localization, and staging at endorectal MR imaging. Radiology. 2006 Jun;239(3):784–792. doi: 10.1148/radiol.2392050949. [DOI] [PubMed] [Google Scholar]
- 7.Xu S, Kruecker J, Guion P, et al. Closed-loop control in fused MR-TRUS image-guided prostate biopsy. Med Image Comput Comput Assist Interv. 2007;10(Pt 1):128–135. doi: 10.1007/978-3-540-75757-3_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu S, Kruecker J, Turkbey B, et al. Real-time MRI-TRUS fusion for guidance of targeted prostate biopsies. Comput Aided Surg. 2008 Sep;13(5):255–264. doi: 10.1080/10929080802364645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed HU, Kirkham A, Arya M, et al. Is it time to consider a role for MRI before prostate biopsy? Nat Rev Clin Oncol. 2009 Apr;6(4):197–206. doi: 10.1038/nrclinonc.2009.18. [DOI] [PubMed] [Google Scholar]
- 10.Albertsen P. Further support for active surveillance in the management of low-volume, low-grade prostate cancer. Eur Urol. 2010 Dec;58(6):836–837. doi: 10.1016/j.eururo.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson DJ, St. Sauver JL, Parker AS, et al. Estimation of Prostate Size in Community-dwelling Men. Urology. 2011;77(2):422–426. doi: 10.1016/j.urology.2010.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen MH. Cancer-specific mortality after surgery or radiation for patients with clinically localized prostate cancer managed during the prostate-specific antigen era. J Clin Oncol. 2003 Jun 1;21(11):2163–2172. doi: 10.1200/JCO.2003.01.075. [DOI] [PubMed] [Google Scholar]
- 13.Cooperberg MR, Cowan JE, Hilton JF, et al. Outcomes of Active Surveillance for Men With Intermediate-Risk Prostate Cancer. Journal of Clinical Oncology. 2010;29(2):228–234. doi: 10.1200/JCO.2010.31.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.May M, Knoll N, Siegsmund M, et al. Validity of the CAPRA score to predict biochemical recurrence-free survival after radical prostatectomy. Results from a european multicenter survey of 1,296 patients. J Urol. 2007 Nov;178(5):1957–1962. doi: 10.1016/j.juro.2007.07.043. discussion 1962. [DOI] [PubMed] [Google Scholar]
- 15.Cooperberg MR, Freedland SJ, Pasta DJ, et al. Multiinstitutional validation of the UCSF cancer of the prostate risk assessment for prediction of recurrence after radical prostatectomy. Cancer. 2006 Nov 15;107(10):2384–2391. doi: 10.1002/cncr.22262. [DOI] [PubMed] [Google Scholar]
- 16.Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol. 2005 Jun;173(6):1938–1942. doi: 10.1097/01.ju.0000158155.33890.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gofrit ON, Zorn KC, Taxy JB, et al. Predicting the risk of patients with biopsy Gleason score 6 to harbor a higher grade cancer. J Urol. 2007 Nov;178(5):1925–1928. doi: 10.1016/j.juro.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 18.Cookson MS, Fleshner NE, Soloway SM, Fair WR. Correlation between Gleason score of needle biopsy and radical prostatectomy specimen: accuracy and clinical implications. J Urol. 1997 Feb;157(2):559–562. [PubMed] [Google Scholar]
- 19.Guzzo TJ, Resnick MJ, Canter DJ, et al. Endorectal T2-weighted MRI does not differentiate between favorable and adverse pathologic features in men with prostate cancer who would qualify for active surveillance. Urol Oncol. 2011 Aug 17; doi: 10.1016/j.urolonc.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Choi WW, Williams SB, Gu X, Lipsitz SR, Nguyen PL, Hu JC. Overuse of Imaging for Staging Low Risk Prostate Cancer. The Journal of Urology. 2011;185(5):1645–1649. doi: 10.1016/j.juro.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 21.Turkbey B, Shah VP, Pang Y, et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology. 2011 Feb;258(2):488–495. doi: 10.1148/radiol.10100667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012 Apr;22(4):746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]