Abstract
The safety and immunogenicity of an authentic recombinant (ar) of the live, attenuated MP-12 Rift Valley fever (RVF) vaccine virus with a large deletion of the NSm gene in the pre-Gn region of the M RNA segment (arMP-12ΔNSm21/384) was tested in 4 – 6 month old Bos taurus calves. Phase I of this study evaluated the neutralizing antibody response, measured by 80% plaque reduction neutralization (PRNT80), and clinical response of calves to doses of 1×101 through 1×107 plaque forming units (PFU) administered subcutaneously (s.c.). Phase II evaluated the clinical and neutralizing antibody response of calves inoculated s.c. or intramuscularly (i.m.) with 1×103, 1×104 or 1×105 PFU of arMP-12ΔNSm21/384. No significant adverse clinical events were observed in the animals in these studies. Of all specimens tested, only one vaccine viral isolate was recovered and that virus retained the introduced deletion. In the Phase I study, there was no statistically significant difference in the PRNT80 response between the dosage groups though the difference in IgG response between the 1×101 PFU group and the 1×105 PFU group was statistically significant (p <0.05). The PRNT80 response of the respective dosage groups corresponded to dose of vaccine with the 1×101 PFU dose group showing the least response. The Phase II study also showed no statistically significant difference in PRNT80 response between the dosage groups though the difference in RVFV-specific IgG values was significantly increased (P<0.001) in animals inoculated i.m. with 1×104 or 1×105 PFU versus those inoculated s. c. with 1×103 or 1×105 PFU. Although the study groups were small, these data suggest that 1×104 or 1×105 PFU of arMP-12ΔNSm21/384 administered i.m. to calves will consistently stimulate a presumably protective PRNT80 response for at least 91 days post inoculation. Further studies of arMP-12ΔNSm21/384 are warranted to explore its suitability as an efficacious livestock vaccine.
Keywords: Rift Valley fever, RVF MP-12-NSm deletion vaccine, arMP-12ΔNSm21/384, calves
Introduction
Rift Valley fever virus (RVFV, family Bunyaviridae, genus Phlebovirus) poses a major public health as well as economic threat and outbreaks have led to restrictions of economic importance on the movement and slaughter of animals in the affected regions [1–3]. Livestock, especially sheep and cattle, serve as amplifying hosts for the virus and are a link between competent mosquito vectors and humans [4]. Additionally, herdsmen, abattoir workers and humans living in close contact with their livestock are at risk of infection from the products of abortion and exposure to blood and tissues from viremic animals. Strong protection against infection in humans and livestock can be achieved through vaccination. Although the only RVFV vaccine for human use is a formalin-inactivated product, several live-attenuated vaccines for livestock use have been developed, including the excessively abortigenic and teratogenic Smithburn vaccine currently used in South Africa [5,6]. A highly immunogenic vaccine that is safe for pregnant animals and possess characteristics that allow the differentiation of infected from a vaccinated animals (DIVA) will aid in avoiding embargoes and minimize preventative culling and unnecessary loss of animals but such a vaccine has been difficult to develop. Recently a recombinant virus generated by reverse genetics techniques and lacking portions of the NSm and NSs genes of virulent RVFV strain ZH-501 was tested in rats and sheep, a relevant target livestock species, and may prove to be an efficacious DIVA vaccine [7,8]. MP-12, a live attenuated strain of RVFV developed for use as a vaccine in humans, has been successfully tested in multiple animal systems as well as humans without significant adverse events [9,10,11,12,13]. We chose to test a deletion mutant of this strain as a potential livestock vaccine. Additionally, any in-vivo reassortants leading to recovery of the deleted function would not be expected to generate a virulent virus [14,15].
RVFV is an enveloped virus containing three RNA segments: L, M and S [16,17,18]. MP-12 has independent attenuating mutations in both the L and M segments [14]. The M segment encodes the NSm protein, a 78-kDa protein of unknown function and major viral envelope proteins, Gn/Gc. Gn/Gc are essential for virus assembly, while NSm and the 78-kDa protein are not required for virus replication in cell culture [19]. Using a reverse genetics system of MP-12 strain, an attenuated strain of RVFV [20], we have generated and characterized arMP-12ΔNSm21/384, which lacks NSm gene at the pre-Gn region in the M segment and retains the independent attenuating mutations of both the L and M segments. Our previous study testing immunogenicity and virulence of arMP-12ΔNSm21/384 in pregnant sheep revealed that arMP-12ΔNSm21/384 was highly immunogenic at doses of 1×103 through 1×105 PFU and was non-abortigenic and non- teratogenic when inoculated into ewes in early gestation [21]. The large deletion in the pre-Gn region in the M RNA segment of arMP-12ΔNSm21/384 should also provide the appropriate characteristic for a DIVA vaccine, and we are currently exploring this potential.
Encouraged by the excellent immunogenicity and safety of arMP-12ΔNSm21/384 in pregnant sheep, we report here the results of safety and immunogenicity testing of arMP-12ΔNSm21/384 in economically important and RVFV infection-susceptible 4 – 6 month old Bos taurus calves.
Materials and Methods
Animals
Healthy, 4 – 6 month old Bos taurus heifer and steer calves were used in the present study. The calves were seronegative to both bovine viral diarrhea and bovine leukemia virus by antigen capture enzyme-linked immunosorbent assay (ELISA) analyses done at the Texas Veterinary Medical Diagnostic Laboratory, College Station, Texas and had no detectable neutralizing antibodies to RVFV by PRNT80 at the time of vaccination. The animal experiments were performed under an Institutional Animal Care and Use Committee approved protocol #2010-192.
Viruses
The MP-12-based vaccine candidate used in these studies, arMP-12ΔNSm21/384, was generated by reverse genetics techniques and possesses a large deletion in the pre-Gn region in the M RNA segment of MP-12. [15,22]. The parent virus, authentic RVF MP-12, is the attenuated RVFV vaccine prepared for use in humans by the U. S. Army Medical Research Institute of Infectious Diseases [9].
Experimental Design
The calves were housed in an ABSL2 Ag biocontainment facility where they were randomized into test groups and acclimated to the facility for 14 days. The studies were conducted in two phases: Phase I examined the immune and clinical responses to escalating doses of arMP-12ΔNSm21/384 administered subcutaneously (s.c.) and Phase II tested selected doses of vaccine given s.c. or intramuscularly (i.m.). In Phase I, six groups of 3 or 4 calves each were inoculated s.c. with doses of 1×101, 102, 103, 104, 105 or 1×107 PFU of arMP-12ΔNSm21/384 and were observed for 49 days post inoculation. In Phase II, groups of 3 calves each were inoculated s.c. or i.m. with 1×103, 1×104 or 1×105 PFU of arMP-12ΔNSm21/384 and observed for 91 days post inoculation. Whole blood was collected prior to inoculation on Day-7 and on days 0 through 7, 10, 14, 21, 28, 35, 49 and in Phase II, days 77 and 91 post inoculation. Rectal temperatures were recorded each time blood was collected and their health status was documented daily. At the end of the respective studies, the calves were euthanized with pentobarbital sodium (120 mg/kg i.v.). All calves were healthy and clinically normal at the termination of the respective studies.
Specimen preparation
Serum for an 80% plaque-reduction neutralization test (PRNT80), IgG ELISA, virus isolation and virus plaque assay was harvested from whole blood after low speed centrifugation and stored at − 80C.
Reverse transcription polymerase chain reaction (RT-PCR)
Viral RNA extraction and analysis from 100 μl of tissue culture supernate was done as previously described [21]. RT-PCR was performed with Platinum Taq Polymerase (Invitrogen) using the following primer sets; S20F (ACA CAA AGC TCC CTA GAG AT) and S1058R (TGC GTT CGG CTT CTG CAA GC) for the S-segment detection, and M19F (ACA CAA AGA CGG TGC ATT A) and M1041R (ACT GCA AAG GGC ACA ACC TC) for the M-segment detection.
Virus isolation and viral plaque assay
Virus isolation from undiluted serum was accomplished by culturing samples in Vero E-6 cells in 25 cm2 flasks as previously described [10,11]. The flasks were observed for cytopathic effect daily for 10 days before blind passage in fresh Vero E-6 cells. Virus titers were determined by plaque assay in Vero cell monolayers as previously described [10,11].
Immunology Methods
Serum neutralizing antibody was determined using PRNT80 as previously described [11]. Sera were tested for RVFV-specific IgG antibodies using ELISA as previously described [23,24]. Sera for IgG were tested at a dilution of 1:100. The cutoff value for assigning a positive IgG result was determined from a panel of five sera from RVFV IgG negative animals calculated in an adjusted OD414 value greater than 3 SD.
Statistical Analysis
All calculations were done using Prism Version 5.0d analysis software (Graphpad Software Inc). Analysis of mean PRNT80 titers and mean serum IgG values were done using a one-way analysis of variance and a post hoc Tukey’s multiple comparison test with a significance level of α=0.05.
Results
Phase I
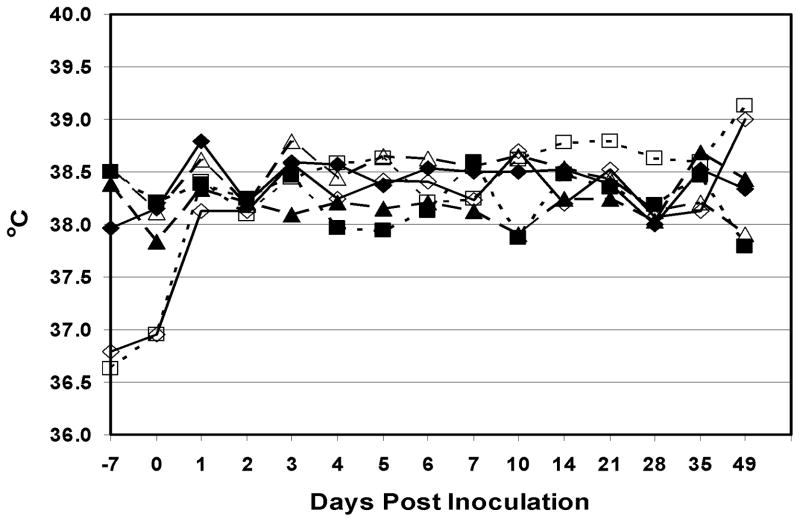
This Phase tested the clinical and immunological response of calves inoculated with escalating doses of arMP-12ΔNSm21/384. The animals remained healthy, and no significant adverse clinical events were detected in this study. All the animals in 1×104 and 1×105 PFU dosage groups showed markedly lower and higher rectal temperatures, at the beginning and at the end of the study, respectively, than animals in the other dosage groups (Figure 1). However, no individual animal was considered febrile (rectal temperature in excess of 39.5°C for several days). Table 1 shows the serum neutralizing antibody and IgG responses of each animal in Phase I. Time until all calves in the respective dosage groups had detectable neutralizing antibody generally corresponded to dose of vaccine with the two highest dose groups seroconverting earlier than the other groups. All calves in the 1×105 and 1×107 PFU dose groups had PRNT80 titers of ≥ 1:20 on day 10 whereas calves in the 1×102, 1×103 and 1×104 PFU dose groups did not all seroconvert with PRNT80 titers of ≥ 1:20 until day 14 and only 2 calves in the 1×101 PFU dose group had detectable PRNT80 titers ≥ 1:20 on day 21. Calf #91, inoculated with 1×101 PFU, was the only calf in that dosage group that failed to develop a PRNT80 titer of ≥ 1:20. That one calf eventually developed a PRNT80 titer of 1:10, the minimal detectable limit of the assay, but no RVFV-specific IgG was detected.
Figure 1.
Mean rectal temperatures of calves in the Phase I studies that were inoculated s.c. with either 1×101 (n=3)(◆), 1×102 (n=3)(■), 1×103 (n=3)(▲),1×104 (n=4)(◇), 1×105 (n=4)(□) or 1×107 (n=3)(Δ) PFU of arMP-12ΔNSm21/384.
Table 1.
Serum neutralizing antibody (PRNT) responses and RVFV IgG OD414 values of calves in the Phase I studies that were inoculated s.c. with either 1×101, 1×102, 1×103, 1×104, 1×105 or 1×107 PFU of arMP-12ΔNSm21/384. No statistically significant differences were calculated between the dosage treatments for PRNT response. The difference in IgG response between the 1×101 PFU group and the 1×105 PFU group was statistically significant (p<0.05). The negative cutoff value for IgG was 0.22.
| Days Post Inoculation | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine Dose: 1 × 101 pfu | ||||||||||||||||
| No.† | 0 | 7 | 10 | 14 | 21 | 28 | 35 | 49 | ||||||||
| PRNT‡ | IgG‡‡ | PRNT | IgG | PRNT | IgG | PRNT | IgG | PRNT | IgG | PRNT | IgG | PRNT | IgG | PRNT | IgG | |
| 89 | <1:10 | 0.02 | <1:10 | 0.00 | <1:10 | 0.02 | 40 | 0.04 | 80 | 0.09 | 80 | 0.07 | 80 | 0.13 | 160 | 0.19 |
| 90 | <1:10 | −0.03 | <1:10 | −0.03 | <1:10 | 0.00 | <1:10 | 0.02 | 20 | 0.12 | 80 | 0.23 | 20 | 0.21 | 80 | 0.45 |
| 91 | <1:10 | 0.04 | <1:10 | 0.03 | <1:10 | 0.02 | <1:10 | 0.06 | <1:10 | 0.10 | <1:10 | 0.00 | <1:10 | −0.03 | 10 | 0.01 |
| Vaccine Dose: 1 × 102 pfu | ||||||||||||||||
| 92 | <1:10 | 0.14 | <1:10 | 0.07 | <1:10 | 0.07 | 20 | 0.09 | 20 | 0.10 | 20 | 0.16 | 20 | 0.06 | 80 | 0.06 |
| 93 | <1:10 | 0.20 | <1:10 | 0.10 | <1:10 | 0.29 | 1280 | 0.32 | 640 | 0.45 | 320 | 0.34 | 320 | 0.79 | 1280 | 0.74 |
| 94 | <1:10 | 0.10 | <1:10 | −0.13 | <1:10 | −0.01 | 80 | 0.02 | 20 | −0.03 | 80 | 0.12 | 40 | 0.18 | 80 | 0.24 |
| Vaccine Dose: 1 × 103 pfu | ||||||||||||||||
| 95 | <1:10 | 0.16 | <1:10 | 0.24 | 20 | 0.33 | 80 | 0.57 | 320 | 0.83 | 320 | 0.96 | 320 | 0.80 | 1280 | 1.24 |
| 96 | <1:10 | 0.04 | <1:10 | 0.14 | 20 | 0.10 | 160 | 0.52 | 80 | 0.37 | 80 | 0.34 | 160 | 1.13 | 320 | 0.82 |
| 97 | <1:10 | 0.03 | <1:10 | 0.03 | <1:10 | 0.06 | 80 | 0.37 | 320 | 0.53 | 320 | 0.82 | 320 | 0.98 | 640 | 1.18 |
| Vaccine Dose: 1 × 104 pfu | ||||||||||||||||
| 101 | <1:10 | 0.08 | <1:10 | 0.07 | <1:10 | 0.28 | 80 | 0.22 | 20 | 0.14 | 20 | 0.17 | 20 | 0.19 | 20 | 0.17 |
| 102 | <1:10 | −0.19 | <1:10 | 0.14 | 80 | 0.36 | 80 | 0.59 | 320 | 0.52 | 320 | 0.89 | 320 | 1.25 | 320 | 1.47 |
| 103 | <1:10 | 0.01 | <1:10 | −0.13 | <1:10 | −0.01 | 320 | 0.13 | 80 | 0.29 | 80 | 0.58 | 320 | 0.69 | 320 | 0.89 |
| 104 | <1:10 | −0.21 | <1:10 | −0.03 | 20 | 0.07 | 20 | 0.09 | 20 | 0.09 | 20 | 0.18 | 20 | 0.17 | 80 | 0.26 |
| Vaccine Dose: 1 × 105 pfu | ||||||||||||||||
| 105 | <1:10 | 0.05 | <1:10 | 0.09 | 80 | 0.26 | 80 | 0.46 | 80 | 1.02 | 160 | 1.23 | 640 | 2.18 | 1280 | 2.38 |
| 106 | <1:10 | 0.05 | <1:10 | 0.24 | 20 | 0.43 | 320 | 0.63 | 320 | 1.03 | 320 | 1.37 | 1280 | 1.92 | 1280 | 2.46 |
| 107 | <1:10 | 0.04 | <1:10 | 0.14 | 320 | 0.49 | 320 | 0.65 | 80 | 1.06 | 80 | 1.46 | 320 | 1.71 | 320 | 2.04 |
| 108 | <1:10 | 0.04 | <1:10 | 0.52 | 80 | 0.77 | 80 | 1.15 | 320 | 1.50 | 80 | 1.90 | 1280 | 2.19 | 1280 | 2.37 |
| Vaccine Dose: 1 × 107 pfu | ||||||||||||||||
| 98 | <1:10 | −0.13 | <1:10 | 0.18 | 20 | 0.29 | 40 | 0.62 | 80 | 0.55 | 80 | 0.93 | 80 | 0.67 | 320 | 0.94 |
| 99 | <1:10 | 0.09 | 20 | 0.14 | 80 | 0.29 | 160 | 0.42 | 80 | 0.66 | 320 | 1.51 | 160 | 1.40 | 320 | 1.59 |
| 100 | <1:10 | −0.02 | 20 | 0.24 | 80 | 0.49 | 160 | 0.73 | 80 | 0.59 | 320 | 1.03 | 160 | 1.17 | 320 | 1.16 |
Calf ear tag number.
Data are expressed as the reciprocal of plaque-reduction neutralization 80% antibody titer. Minimum detection limit of the assay is 1:10.
Adjusted OD414values of sera diluted 1:100
Unexpectedly, a virus titer of 3 × 102 PFU/ml, determined by direct plaque assay, was detected in the serum of calf #93 in the 1×102 PFU dose group on day 7 post inoculation. That was the only instance of viremia detected in any of the calves and this viremic calf also had a rectal temperature of 39.5°C on that day but its rectal temperature was 38°C the next day. RT-PCR analysis showed that the recovered virus retained the introduced deletion in the pre-Gn region. The calf that was viremic on day 7 developed a PRNT80 titer of 1:1280, which was the highest titer of any calf in this Phase of the study, on day 14, whereas the other two animals in that group had PRNT80 titers of 1:20 and 1:80 respectively on day 14. The viremic calf maintained the highest PRNT80 titer of that dosage group for the duration of the study.
There was no statistically significant difference in PRNT80 response between the dosage groups though the difference in IgG response between the 1×101 PFU group and the 1×105 PFU group was statistically significant (p <0.05). IgG was measured at a serum dilution of 1:100. Table 1 shows that the animals that had PRNT80 titers ≤ 1:80 after day 14, especially the calves in the 1×101 and 1×102 dosage groups, had IgG OD values below the calculated cutoff value of 0.22 in this assay. Conversely, there were instances where PRNT80 titers were <1:10 yet IgG OD values were at or exceeded the negative cutoff value and one instance of a PRNT80 titer of 1:320 yet the IgG OD value was 0.13 (calf #103 day 14). The neutralizing antibody response in the calves in Phase I appeared to be dose dependent. The calves receiving 1×102 PFU or less of the attenuated vaccine candidate generally had lower neutralizing antibody responses and lower IgG OD values than the higher dosage groups. We did not measure serum IgM nor did we titrate IgG, because we were most interested in the neutralizing antibody response as an indicator of protection since we were not able to challenge the vaccinated calves with virulent virus.
Phase II
The objectives of this Phase were to compare the s.c. and i.m. routes of inoculation using three selected doses of the arMP-12ΔNSm21/384 vaccine candidate based on PRNT80 and IgG responses of calves in Phase I and to confirm the dose response. Animals were vaccinated with doses of 1×103, 1×104 and 1×105 PFU. Clinical observations were unremarkable and the only evidence of post inoculation pyrexia was calf #85, inoculated with 1×104 PFU i.m., that had a slight nasal discharge and a rectal temperature recording of 40.7°C on post inoculation day 4. The next day the calf’s rectal temperature was 38°C and did not exceed 38.9° C for the remainder of the study. No post inoculation viremias were detected in any animal through day 10.
Table 2 shows the serum neutralizing antibody and IgG responses of each animal in Phase II. Neutralizing antibody was detected earliest on day 7 in calves #84 and #85 in the 1×104 PFU i.m. group and calves #87 and #88 in the 1×105 PFU i.m. group. All inoculated animals developed PRNT80 titers of ≥ 1:20 by post inoculation day 21 with all but calf #80, in the 1×103 PFU i.m. group, seroconverting by day 14. That calf had a PRNT80 titer of 1:20 on day 21 but became undetectable until day 77 where the titer remained at 1:20 for the remainder of the study. RVFV IgG was detected in that animal on day 21 also but IgG OD values remained above the calculated negative cutoff value of 0.23 even though a PRNT80 titer of <1:10 was recorded on days 28 through 49. There was no statistically significant difference in PRNT80 response among the dosage groups, whereas the difference in RVFV-specific IgG values was significantly increased (P<0.001) in animals inoculated i.m. with 1×104 or 1×105 PFU of arMP-12ΔNSm21/384 versus animals inoculated s. c. with 1×103 or 1×105 PFU.
Table 2.
Serum neutralizing antibody (PRNT) responses and RVFV IgG OD414 values of calves in the Phase II studies that were inoculated s.c. or i.m. with 1×103, 1×104 or 1×105 PFU of arMP-12ΔNSm21/384. No statistically significant differences were calculated between the dosage treatments. RVFV-specific IgG values were significantly increased (P<0.001) in animals inoculated i.m. with 1×104 or 1×105 PFU of arMP-12ΔNSm21/384 versus animals inoculated s. c. with 1×103 or 1×105 PFU. Negative cutoff value (dashed line) = 0.23
| Days Post Inoculation | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine Dose: 1 × 103 pfu SC | ||||||||||||||||||||
| No.† | 0 | 7 | 10 | 14 | 21 | 28 | 35 | 49 | 77 | 91 | ||||||||||
| PRNT‡ | IgG‡‡ | PRNT | IgG | PRNT | IgG | PRNT | IgG | PRNT | IgG | PRNT | IgG | PRNT | IgG | PRNT | IgG | PRNT | IgG | PRNT | IgG | |
| 71 | <1:10 | 0.03 | <1:10 | 0.02 | <1:10 | 0.01 | 20 | 0 | 80 | 0.13 | 80 | 0.19 | 80 | 0.16 | 80 | 0.15 | 80 | 0.14 | 20 | 0.11 |
| 72 | <1:10 | 0.08 | <1:10 | 0.03 | <1:10 | 0.12 | 80 | 0.08 | 80 | 0.24 | 80 | 0.26 | 80 | 0.36 | 80 | 0.35 | 80 | 0.33 | 80 | 0.37 |
| 73 | <1:10 | 0.07 | <1:10 | 0.12 | 20 | 0.17 | 320 | 0.6 | 1280 | 0.47 | 1280 | 0.79 | 1280 | 0.92 | 1280 | 1.05 | 320 | 0.95 | 320 | 0.52 |
| Vaccine Dose: 1 × 104 pfu SC | ||||||||||||||||||||
| 74 | <1:10 | 0.09 | <1:10 | 0.06 | 20 | 0.18 | 80 | 0.33 | 80 | 0.41 | 320 | 0.64 | 1280 | 0.94 | 1280 | 0.73 | 320 | 0.75 | 320 | 1.03 |
| 75 | <1:10 | 0.08 | <1:10 | 0.13 | 20 | 0.2 | 80 | 0.34 | 80 | 0.45 | 80 | 0.51 | 80 | 0.6 | 80 | 0.66 | 160 | 0.81 | 80 | 0.84 |
| 76 | <1:10 | 0.1 | <1:10 | 0.2 | 80 | 0.43 | 320 | 0.58 | 80 | 0.82 | 320 | 1.37 | 640 | 1.4 | 1280 | 1.59 | 1280 | 1.56 | 80 | 0.64 |
| Vaccine Dose: 1 × 105 pfu SC | ||||||||||||||||||||
| 77 | <1:10 | 0.1 | <1:10 | 0.1 | <1:10 | 0.08 | 80 | 0.18 | 160 | 0.29 | 80 | 0.51 | 320 | 0.61 | 320 | 0.65 | 320 | 0.67 | 320 | 0.70 |
| 78 | <1:10 | 0.04 | <1:10 | 0.22 | 20 | 0.29 | 80 | 0.54 | 160 | 0.76 | 320 | 0.94 | 320 | 1.12 | 1280 | 0.84 | 320 | 0.87 | 320 | 1.02 |
| 79 | <1:10 | 0 | <1:10 | 0.05 | 20 | 0.15 | 80 | 0.38 | 80 | 0.53 | 320 | 0.7 | 1280 | 0.78 | 320 | 0.59 | 320 | 0.93 | 80 | 0.42 |
| Vaccine Dose: 1 × 103 pfu IM | ||||||||||||||||||||
| 80 | <1:10 | 0.12 | <1:10 | 0.12 | <1:10 | 0.17 | <1:10 | 0.23 | 20 | 0.38 | <1:10 | 0.47 | <1:10 | 0.42 | <1:10 | 0.45 | 20 | 0.44 | 20 | 0.30 |
| 81 | <1:10 | −0.13 | <1:10 | 0.26 | 20 | 0.58 | 80 | 1.11 | 320 | 1.49 | 80 | 1.94 | 1280 | 1.96 | 1280 | 1.95 | 320 | 2.07 | 320 | 2.03 |
| 82 | <1:10 | 0.24 | <1:10 | 0.18 | 20 | 0.58 | 80 | 1.21 | 640 | 1.12 | 1280 | 1.7 | 1280 | 2.24 | 1280 | 2.26 | 1280 | 2.40 | 320 | 2.37 |
| Vaccine Dose: 1 × 104 pfu IM | ||||||||||||||||||||
| 83 | <1:10 | 0.15 | <1:10 | 0.22 | 20 | 0.53 | 80 | 0.97 | 1280 | 1.46 | 80 | 1.94 | 1280 | 2.04 | 320 | 2.14 | 320 | 2.37 | 320 | 2.30 |
| 84 | <1:10 | 0.12 | 40 | 0.29 | 80 | 0.53 | 80 | 1.08 | 320 | 1.39 | 320 | 1.55 | 80 | 1.73 | 320 | 2.04 | 320 | 2.07 | 640 | 1.76 |
| 85 | <1:10 | 0.2 | 20 | 0.19 | 160 | 0.62 | 160 | 0.99 | 1280 | 1.22 | 320 | 1.64 | 320 | 1.93 | 1280 | 1.98 | 80 | 1.54 | 1280 | 2.16 |
| Vaccine Dose: 1 × 105 pfu IM | ||||||||||||||||||||
| 86 | <1:10 | 0.2 | <1:10 | 0.34 | 20 | 0.87 | 80 | 1.68 | 320 | 0.92 | 80 | 1.42 | 80 | 1.86 | 80 | 2.01 | 80 | 1.93 | 80 | 1.78 |
| 87 | <1:10 | 0.28 | 20 | 0.33 | 20 | 0.96 | 160 | 1.72 | 1280 | 2.29 | 1280 | 2.21 | 2560 | 2.82 | 1280 | 2.59 | 320 | 2.90 | 640 | 2.58 |
| 88 | <1:10 | 0.23 | 80 | 0.3 | 80 | 0.7 | 80 | 1.09 | 80 | 1.56 | 320 | 1.53 | 320 | 1.62 | 320 | 1.79 | 80 | 1.85 | 80 | 1.96 |
Calf ear tag number.
Data are expressed as the reciprocal of plaque-reduction neutralization 80% antibody titer. Minimum detection limit of the assay is 1:10.
Adjusted OD414 values of sera diluted 1:100
Discussion
The objective of the present study was to investigate the safety and immunogenicity of a deletion mutant virus, arMP-12ΔNSm21/384, in an economically important and RVFV infection-susceptible livestock species, young Bos taurus calves, 4 – 6 months of age. This vaccine candidate was previously tested in pregnant sheep and did not induce abortion or fetal abnormalities [21]. The present study did not compare this vaccine to any other vaccine primarily because there are no other approved livestock vaccines against RVFV available in the United States. Furthermore, available vaccines, developed and intended for human use such as MP-12 or TSI-GSD 200 do not possess DIVA characteristics. Additionally, both MP-12 and TSI-GSD 200 have been tested extensively in sheep and cattle [10,11,12,24,25]. Initially, in Phase I, we performed a dose escalation study to determine if there was an optimum dose range for the vaccine. We chose to inoculate subcutaneously because most veterinary vaccines for livestock are administered by that route to avoid tissue reactions in the deeper tissues that will devalue the carcass. We then selected doses of 1×103, 1×104 and 1×105 PFU based on their immunogenicity to assess whether the subcutaneous route of inoculation was superior to intramuscular inoculation. The 1×107 dose was considered excessive and did not present any clear advantages over the doses selected.
The study groups were necessarily small due to space limitations in the biocontainment facility and testing for protection against virulent virus challenge was not feasible. The Phase I study demonstrated that as little as 1 × 101 PFU administered s.c. produced potentially protective neutralizing antibody titers in 2 of 3 calves inoculated but a minimum vaccine dose of 1 × 103 PFU administered s.c. would probably be necessary to provide satisfactory protection. The low viremia titer, 3×102 PFU/ml of serum, detected on day 7 in calf #93 inoculated with 1×102 PFU in Phase I was unexpected but not totally surprising as previously we recovered low titers of vaccine virus from ewes inoculated with 1 × 104 and 1 × 105 PFU of this vaccine [21]. A transient and low viremia titer of 3×102 PFU/ml is unlikely sufficient to be transmitted by an arthropod vector and would most likely not pose a threat to naïve animals by this route of transmission [27]. It is conceivable however, if a vaccinated animal was slaughtered shortly after vaccination when viremic due to the vaccine, the butcher or abbatoir worker could possibly be exposed. While it is illogical that a recently vaccinated animal under normal circumstances would be slaughtered for human consumption, the vaccine is a BSL-2 agent generated from MP-12, and would probably not pose a threat to an immunologically competent individual.
The elevated rectal temperatures of the calves in Phase II prior to inoculation are possibly reflective of initial handling stress. The elevated rectal temperatures beginning on day 35 possibly reflect changes in environment as the calves were moved to outside quarantine pens and were subjected to elevated environmental temperatures. While there were no statistically significant differences in the mean PRNT80 titers of the s.c. and i.m. groups, the i.m.-inoculated calves responded much quicker to immunization and their RVFV-specific IgG responses were markedly greater than the s.c.-inoculated calves.
The use of a live, attenuated vaccine based solely on a gene deletion requires careful consideration due to the possibility of reversion to virulence. This could occur by genetic recombination or reassortment in the field with another virus resulting in a vaccine virus recovering the deleted phenotype and becoming virulent. This has happened with a poultry vaccine [28], and if it occurred with a RVF vaccine could be catastrophic. The arMP-12ΔNSm21/384 attenuated vaccine is unique since it retains the MP-12 attenuations based on single-nucleotide polymorphism (SNP) mutations on L and M segments and is stable by both phenotypic and genetic sequence analysis [14,15]. The NSm deletion is not expected to affect this stability and safety may be insured since acquisition of NSs or NSm from another phlebovirus would be expected to result in an attenuated virus.
The small study groups in the present study preclude drawing definitive conclusions on optimum dose and route of inoculation for arMP-12ΔNSm21/384 in cattle but it is apparent that a broad range of doses will elicit an immune response that will likely be protective against virulent virus exposure in cattle as was seen in a previous study in cattle using MP-12 [12]. These data suggest that arMP-12ΔNSm21/384 is a candidate for a DIVA capable RVFV animal vaccine and warrant a thorough evaluation of long-term protective immunity in livestock.
Figure 2.
Mean rectal temperatures of calves in the Phase II studies that were inoculated (A) s.c. with 1×103 (n=3)(◇), 1×104 (n=3)(□) or 1×105 (n=3)(Δ) PFU of arMP-12ΔNSm21/384 and calves that were inoculated (B) i.m. with 1×103 (n=3)(◆), 1×104 (n=3)(■) or 1×105 (n=3)(▲) PFU of arMP-12ΔNSm21/384.
Highlights.
We tested a recombinant RVF MP-12 vaccine (arMP-12ΔNSm21/384) in 4–6 month old Bos taurus calves.
No significant adverse clinical events were observed in the animals in these studies.
The arMP-12ΔNSm21/384 vaccine was immunogenic at doses of 1×101 through 1×107 PFU.
Vaccine doses of 1×104 or 1×105 PFU stimulated a presumably protective PRNT80 response for at least 91 days post inoculation.
Acknowledgments
We thank Dr. George Bettinger for his critical review of the manuscript, Ms. Nicolette Ward for her technical assistance and Dr. Clay Ashley and the staff at the TAMU Veterinary Research Park for their assistance in handling and maintaining the animals.
Support: This study was supported by funds awarded to LGA from Texas AgriLife Research Project 203367-00000-10000 and funds from the U. S. Department of Homeland Security, National Center of Excellence for Foreign Animal and Zoonotic Disease (FAZD) Defense Project ONR-N00014-04-1-0660. A portion of this work (JCM, NL, SM, and CJP) was supported by a grant through the National Institutes of Health (NIH) grant number NIH-NIAID-DMID-02-24 Collaborative Grant on Emerging Viral Diseases. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
In conducting the research described in this report, the investigators adhered to the guidelines of the Institutional Animal Care and Use Committee of Texas A & M University and the recommendations in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, National Academy of Sciences, 1996). The facilities used are fully accredited by the American Association for Accreditation of Laboratory Animal Care. This study was conducted under an approved Texas A & M University animal use protocol number #2010-192.
Author Contributions
Conceived and designed the experiments: JCM, LGA, CJP. Performed the experiments: JCM, LGA, RCL, JW, RP, PK, NL, SM. Wrote the paper: JCM, LGA, RCL, SM. All authors have approved this manuscript.
Competing Interests: The authors have declared that no competing interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hightower A, Kinkade C, Nguku PM, Anyangu A, Mutonga D, et al. Relationship of Climate, Geography, and Geology to the Incidence of Rift Valley Fever in Kenya during the 2006–2007 Outbreak. Am J Trop Med Hyg. 2012;86:373–380. doi: 10.4269/ajtmh.2012.11-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anyamba A, Linthicum KJ, Small J, Britch SC, Pak E, et al. Prediction, assessment of the Rift Valley fever activity in East and Southern Africa 2006–2008 and possible vector control strategies. Am J Trop Med Hyg. 2010;83:43–51. doi: 10.4269/ajtmh.2010.09-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dar O, McIntyre S, Hogarth S, Heymann D. Rift Valley fever and a new paradigm of research and development for zoonotic disease control. Emerg Infect Dis. 2013;19:189–193. doi: 10.3201/eid1902.120941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bird BH, Nichol ST. Breaking the chain:Rift Valley fever virus control via livestock vaccination. Curr Opin Virol. 2012 Jun;2(3):315–23. doi: 10.1016/j.coviro.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Bird BH, Ksiazek TG, Nichol ST, MacLachlan NJ. Rift Valley fever virus. J Am Vet Med Assoc. 2009;234(7):883–893. doi: 10.2460/javma.234.7.883. [DOI] [PubMed] [Google Scholar]
- 6.von Teichman B, Engelbrecht A, Zulu G, Gungu B, Pardini A, Bouloy M. Safety and efficacy of Rift Valley fever Smithburn and Clone 13 vaccines in calves. Vaccine. 2011;29:5771–5777. doi: 10.1016/j.vaccine.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 7.Bird BH, Albarino CG, Hartman AL, Erickson BR, Ksiazek TG, Nichol ST. Rift Valley fever virus lacking the NSs and NSm genes is highly attenuated, confers protective immunity from virulent virus challenge, and allows for differential identification of infected and vaccinated animals. J Virol. 2008;82:2681–2691. doi: 10.1128/JVI.02501-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bird BH, Maartens LH, Campbell S, Erasmus BJ, Erickson BR, Dodd KA, Spiropoulou CF, Cannon D, Drew CP, Knust B, McElroy AK, Khristova ML, Albarino CG, Nichol ST. Rift Valley fever virus vaccine lacking the NSs and NSm genes is safe, nonteratogenic, and confers protection from viremia, pyrexia, and abortion following challenge in adult and pregnant sheep. J Virol. 2011;85(24):12901–12909. doi: 10.1128/JVI.06046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bettinger GE, Peters CJ, Pittman P, Morrill JC, Ranadive M, Kormann RN, Lokugamage N. Rift Valley fever MP-12 vaccine: a university, government, & industry collaborative development. Rift Valley Fever Workshop.2009. [Google Scholar]
- 10.Morrill JC, Jennings GB, Caplen H, Turell MJ, Johnson AJ, et al. Pathogenicity and immunogenicity of a mutagen-attenuated Rift Valley fever virus immunogen in pregnant ewes. Am J Vet Res. 1987;48:1042–1047. [PubMed] [Google Scholar]
- 11.Morrill JC, Carpenter L, Taylor D, Ramsburg HH, Quance J, Peters CJ. Further evaluation of a mutagen-attenuated Rift Valley fever vaccine in sheep. Vaccine. 1991;9(1):35–41. doi: 10.1016/0264-410x(91)90314-v. [DOI] [PubMed] [Google Scholar]
- 12.Morrill JC, Mebus CA, Peters CJ. Safety and efficacy of a mutagen-attenuated Rift Valley fever virus vaccine in cattle. Am J Vet Res. 1997;58(10):1104–1109. [PubMed] [Google Scholar]
- 13.Morrill JC, Mebus CA, Peters CJ. Safety of a mutagen-attenuated Rift Valley fever virus vaccine in fetal and neonatal bovids. Am J Vet Res. 1997;58(10):1110–1114. [PubMed] [Google Scholar]
- 14.Lokugamage N, Freiberg AN, Morrill JC, Ikegami T. Genetic Subpopulations of Rift Valley Fever ZH548, MP-12 and Recombinant MP-12 Strains. J Virol. 2012;86:13566–13575. doi: 10.1128/JVI.02081-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikegami T, Won S, Peters CJ, Makino S. Rescue of infectious Rift Valley fever virus entirely from cDNA, analysis of virus lacking the NSs gene, and expression of a foreign gene. J Virol. 2006 Mar;80(6):2933–40. doi: 10.1128/JVI.80.6.2933-2940.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikegami T, Makino S. The pathogenesis of Rift Valley fever. Viruses. 2011;3:493–519. doi: 10.3390/v3050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bishop DH, Calisher CH, Casals J, Chumakov MP, Gaidamovich SY, et al. Bunyaviridae. Intervirology. 1980;14:125–143. doi: 10.1159/000149174. [DOI] [PubMed] [Google Scholar]
- 18.Walter CT, Barr JN. Recent advances in the molecular and cellular biology of bunyaviruses. J Gen Virol. 2011;92:2467–2484. doi: 10.1099/vir.0.035105-0. [DOI] [PubMed] [Google Scholar]
- 19.Won S, Ikegami T, Peters CJ, Makino S. NSm and 78-kilodalton proteins of Rift Valley fever virus are nonessential for viral replication in cell culture. J Virol. 2006;80:8274–8278. doi: 10.1128/JVI.00476-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caplen H, Peters CJ, Bishop DH. Mutagen-directed attenuation of Rift Valley fever virus as a method for vaccine development. J Gen Virol. 1985;66:2271–2277. doi: 10.1099/0022-1317-66-10-2271. [DOI] [PubMed] [Google Scholar]
- 21.Morrill JC, Laughlin RC, Lokugamage N, Pugh R, Sbrana E, Weise WJ, Adams LG, Makino S, Peters CJ. Safety and Immunogenicity of Recombinant Rift Valley Fever MP-12 Vaccine Candidates in Sheep. Vaccine. 2013;31:559–565. doi: 10.1016/j.vaccine.2012.10.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Won S, Ikegami T, Peters CJ, Makino S. NSm protein of Rift Valley fever virus suppresses virus-induced apoptosis. J Virol. 2007;81:13335–13345. doi: 10.1128/JVI.01238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meegan JM, Yedloutschnig RJ, Peleg BA, et al. Enzyme-linked immunosorbent assay for detection of antibodies to Rift Valley fever virus in ovine and bovine sera. Am J Vet Res. 1987;48(7):1138–41. [PubMed] [Google Scholar]
- 24.Ksiazek TG, Jouan A, Meegan JM, et al. Rift Valley fever among domestic animals in the recent West African outbreak. Res Virol. 1989;140(1):67–77. doi: 10.1016/s0923-2516(89)80086-x. [DOI] [PubMed] [Google Scholar]
- 25.Yedloutschnig RJ, Dardiri AH, Walker JS, Peters CJ, Eddy GA. Immune response of steers, goats and sheep to inactivated Rift Valley fever vaccine. Proc U S Animal Health Assoc Annual Meeting. 1979;83:253–260. [PubMed] [Google Scholar]
- 26.Harrington DG, Lupton HW, Crabbs CL, et al. Evaluation of a formalin-inactivated Rift Valley fever vaccine in sheep. Am J Vet Res. 1980;41:1559–1564. [PubMed] [Google Scholar]
- 27.Turell MJ, Gargan TP, Bailey CL. Replication and dissemination of Rift Valley fever virus in Culex pipiens. Am J Trop Med Hyg. 1984;33:176–181. doi: 10.4269/ajtmh.1984.33.176. [DOI] [PubMed] [Google Scholar]
- 28.Lee SW, Markham PF, Coppo MJ, Legione AR, Markham JF, Noormohammadi AH, Browning GF, Ficorilli N, Hartley CA, Devlin JM. Attenuated vaccines can recombine to form virulent field viruses. Science. 2012 Jul 13;337(6091):188. doi: 10.1126/science.1217134. [DOI] [PubMed] [Google Scholar]