Abstract
Background
While adjuvant chemoradiation is commonly used in the United States for treatment of resected pancreatic cancer, there is no consensus on the benefit of this therapy as results from randomized trials are conflicting. We reviewed our experience in a consecutive, unselected series of patients treated with adjuvant 5-FU and radiation therapy for resected pancreatic adenocarcinoma.
Methods
86 patients with resected pancreatic adenocarcinoma who underwent adjuvant therapy from 1998–2005 were identified. Medical records were reviewed. 93% of patients were treated with external beam RT to ≥ 50.4 Gy and 91% of patients received concurrent 5-FU by continuous infusion. 45% of patients went on to receive adjuvant gemcitabine.
Results
Median follow-up was 31 months (range 21–62) among the 20 patients still alive. Fewer than half of patients had positive (33%) or close (<1mm, 15%) resection margins. 81% of the tumors were T3; 66% had involved lymph nodes. The median overall survival (OS) for all patients was 22 months; negative lymph node status (p=0.016) was a significant prognostic factor for improved OS, while treatment with gemcitabine trended towards improved OS (p=0.080). Median disease-free survival (DFS) for all patients was 10 months, and treatment with gemcitabine (p=0.044) or any chemotherapy (p=0.047) were significant predictors of DFS. 75 patients (87%) had disease recurrence, the majority of whom recurred with peritoneal (55%) or liver (53%) metastases. Negative lymph nodes trended towards a lower rate of distant failure (p=0.060).
Conclusions
The median survival of this cohort is greater than that of the chemoradiation arms of the EORTC and ESPAC-1 trials, and comparable to the survival observed on the GITSG chemoradiation arm. Lymph node status and treatment with adjuvant chemotherapy were significant predictors of OS and DFS, respectively. Future survival improvements should be directed at reducing peritoneal and liver metastases. Further randomized trials are required to define the role of adjuvant therapy for pancreatic adenocarcinoma.
Keywords: pancreatic cancer, adjuvant therapy, chemoradiation, gemcitabine, patterns of failure
Introduction
The role of adjuvant therapy for pancreatic adenocarcinoma is not well defined. The rationale for adjuvant chemotherapy and radiation therapy (RT) following resection is to treat residual microscopic disease. However, consensus on the benefit of such therapy remains elusive, as results from randomized trials are conflicting. The early Gastrointestinal Tumor Study Group (GITSG) trials in 1985 and 1987 showed improved survival with a combination of post-operative RT and chemotherapy over observation alone.1, 2 The European Organization for Research and Treatment of Cancer (EORTC) challenged these results in 1999, showing no significant improvement in survival with chemoradiation.3 However, there was still a trend towards benefit of adjuvant chemoradiation therapy, with a 2-year survival of 37% with chemoradiation and 23% with surgery alone, and given that the study may have been underpowered, some argue that the results still support the use of this adjuvant therapy in resected pancreatic cancer.4
The assumption that chemoradiation improves outcomes after resection was further challenged by the ESPAC-1 study, which used a two-by-two factorial design to compare adjuvant chemotherapy, chemoradiation, and chemoradiation plus chemotherapy to surgery alone.5 The results published in 2001 showed no benefit in median survival for those who received chemoradiation compared to those who did not, yet they did suggest a significant survival benefit in patients who received 5-fluorouracil (5-FU) chemotherapy alone. In 2004, the authors published an updated report which showed worse survival for those who received chemoradiation and improved survival for those who received chemotherapy alone.6
Interpretation of the results from these three major studies, especially ESPAC-1, varies considerably due to several limitations including statistical design issues and the suboptimal, relatively out-of-date, chemoradiation protocols.7–9 In the ESPAC-1 study, clinicians were allowed to deliver “background” chemoradiation or chemotherapy separate from the study regimens, and 30% of patients assigned to receive radiation did not receive a uniform dose of radiation or radiation at all. With regard to radiation therapy, current treatment typically consists of radiation doses of 45–54 gray (Gy) rather than the 40 Gy used in the three studies above, and the split course technique is no longer used because of prolonged treatment time and inferior radiobiologic efficiency.8 A number of studies also support the use of concurrent chemotherapy with continuous infusional 5-FU rather than bolus 5-FU administration.10, 11
More recent studies have highlighted the efficacy of four to six months of adjuvant gemcitabine (GEMZAR®, Eli Lilly & Company, Indianapolis, IA, USA) chemotherapy. The most recent update of the CONKO-001 randomized controlled trial showed a statistically significant increase in both median disease-free survival and overall survival in patients with resected pancreatic cancer treated with gemcitabine compared with observation alone.12, 13 This study did not utilize adjuvant radiation therapy. RTOG 97-04 is a phase III study comparing pre- and post-chemoradiation 5-FU vs. pre- and post-chemoradiation gemcitabine.14 The chemoradiation protocol utilized continuous infusion 5-FU concurrent with radiation in both arms. The results showed a similar outcome whether patients were treated with adjuvant gemcitabine or 5-FU before and after 5-FU-based chemoradiation. Among patients with pancreatic head lesions there was a trend in favor of gemcitabine on univariate analysis (median survival of 20.5 months compared to 16.9 months with 5-FU, p=0.09). After adjusting for nodal status, tumor diameter, and margin status on multivariate analysis, patients with head lesions treated with gemcitabine had a statistically significant improvement in survival.
Another significant finding of RTOG 97-04 was the importance of treating patients with appropriate radiation technique. Patients whose treatment met protocol guidelines had improved survival and decreased toxicity compared to those who did not.15 Of note, the ESPAC-1 trial included no central review of radiation technique or quality.
With the small number of randomized controlled trials, conflicting evidence on the benefit of chemoradiation, and the development of novel chemoradiation regimens, more research exploring adjuvant therapy for resected pancreatic cancer is needed. Adjuvant chemoradiation with 5-FU continues to be commonly used in the United States, and remains the standard treatment at our institutions. We sought to review our experience with this treatment in a consecutive, unselected series of patients treated with adjuvant 5-FU and radiation therapy for resected pancreatic adenocarcinoma.
Patients and Methods
A retrospective chart review was conducted on all patients with resected pancreatic adenocarcinoma who underwent adjuvant chemotherapy and radiation therapy at the Brigham and Women’s Hospital/Dana Farber Cancer Institute and Massachusetts General Hospital between October 1, 1998 and December 31, 2005. The institutional review board approved the study protocol. Patients with cancers arising in the common bile duct, ampulla of vater, or duodenum, patients with pancreatic endocrine tumors, acinar cell tumors, and adenosquamous cancers were excluded from the study. Patients were also excluded if they had received any neoadjuvant chemotherapy or intraoperative radiation therapy. Initially, 97 consecutively treated patients meeting the above criteria were identified. Four patients were lost to follow-up following adjuvant chemoradiation and were thus excluded from analysis. An additional seven patients were excluded as they were enrolled and treated on an adjuvant chemoradiation protocol differing from the standard chemotherapeutic regimen being used at the time. This left 86 patients who had resected, pathologically confirmed adenocarcinoma of the pancreas.
Demographic, clinical, laboratory, histopathologic, and radiographic data were gathered from the electronic medical records. All patients underwent pretreatment staging with abdominal computed tomography (CT), either alone or in conjunction with magnetic resonance imaging (MRI), to determine whether their disease was resectable. For patients whose surgery was performed at outside institutions, histopathology slides were obtained and reviewed by our institutional pathology departments. All patients received adjuvant therapy with concurrent chemotherapy and radiation.
Disease recurrence was determined from reviewing hospital records and radiologic reports including CT, MRI, and positron emission tomography (PET) imaging. All patients were followed after adjuvant therapy with interval bloodwork, including cancer antigen 19-9 (CA 19-9) and carcinoembryonic antigen (CEA), as well as imaging every three to four months to assess for disease recurrence and metastases. Both the initial and subsequent sites of recurrence were documented and coded as locoregional (including the pancreatic bed and regional lymph nodes), liver, peritoneal, lung, bone, brain, or other distant site. There were several cases where it was difficult to distinguish between local recurrences in the pancreatic bed and regional lymph nodes on CT, so these sites were grouped together as locoregional recurrences. Long term outcome, disease status, and deaths were obtained from the medical record and the Social Security Death Index.16
Statistical Analysis
Overall survival (OS) and disease-free survival (DFS) as well as time to locoregional recurrence and to distant metastasis were calculated starting from the first day of adjuvant therapy with post-operative chemoradiation. The OS time of a patient still alive at the time of chart review was censored at the date of last follow-up. The DFS time was measured until a patient had evidence of any local or distant recurrence, or was censored at the date of last follow-up in the absence of disease recurrence. The time to locoregional or distant failure was measured until a patient had a recurrence documented at the relevant site or otherwise was censored at the earlier date of death or last follow-up. Survival and failure rates were estimated by the Kaplan-Meier method, and a 95% confidence interval (CI) for the median was based on the sign test. The log-rank test was used to identify patient, tumor and treatment factors associated with the survival and failure outcomes.
Results
Patient Characteristics and Treatment
There were 40 men and 46 women in the cohort, most of whom were Caucasian (81%), with a median age of 62.5 years, as seen in Table 1. The majority of surgical resections were pancreaticoduodenectomies (Whipple procedure, 70%) or distal pancreatectomies (19%.) All but 3 patients had resections performed at institutions with a pancreatectomy volume of ≥20 per year (range 20–202 procedures per year).17 Most tumors were located in the head of the pancreas (76%), median tumor size was 3 cm, and all but 1 patient (99%) had ductal adenocarcinoma. 85% of patients had either T3 (81%) or T4 (3%) classification tumors.18 Most patients (63%) had stage IIB disease (T1-3, N1, M0: either tumor limited to the pancreas, 2 cm or less in greatest dimension [T1], tumor limited to the pancreas, more than 2 cm in greatest dimension [T2], or tumor extends beyond the pancreas but without involvement of the celiac axis or the superior mesenteric artery [T3], regional lymph node metastasis [N1], no distant metastasis [M0]).19 Less than half of the cohort had resection margins that were microscopically positive (33%) or within 1 mm of the surgical margin (15%), and 66% of patients had positive lymph nodes postoperatively. Histology showed that 56% of tumors were moderately differentiated, 56% of tumors had lymphovascular invasion and 79% had perineural invasion. Moderate to poorly differentiated cancers were grouped with poorly differentiated grade for all subsequent survival analyses.
Table 1.
Patient and tumor characteristics (n=86)
| Characteristic | # patients (%) | |
|---|---|---|
| Age (years) | Median: | 62.5 |
| Range: | 34–80 | |
| Gender | M | 40 (53%) |
| F | 46 (47%) | |
| Race | Caucasian | 70 (81%) |
| African-American | 3 (3%) | |
| Hispanic | 3 (3%) | |
| Other/ Unknown | 10 (12%) | |
| Surgery | Pancreaticoduodenectomy (Whipple) | 60 (70%) |
| Pylorus-preserving | 7 (8%) | |
| Distal pancreatectomy | 16 (19%) | |
| Total pancreatectomy | 3 (3%) | |
| Histology | Ductal Adenocarcinoma | 85 (99%) |
| Mucinous adenocarcinoma | 1 (1%) | |
| Tumor size (longest diameter, cm) |
Median: | 3.0 |
| Range: | 1.2–7.0 | |
| Site of tumor | Head | 65 (76%) |
| Head & Body | 1 (1%) | |
| Body | 6 (7%) | |
| Tail | 14 (16%) | |
| Resection Margins | Positive | 28 (33%) |
| <1 mm | 13 (15%) | |
| Negative | 45 (52%) | |
| Postoperative tumor classification |
T2 | 13 (15%) |
| T3 | 70 (81%) | |
| T4 | 3 (3%) | |
| Postoperative nodal classification |
N0 | 29 (34%) |
| N1 | 57 (66%) | |
| Grade/degree of Differentiation |
Poor | 23 (27%) |
| Moderate to Poor | 7 (8%) | |
| Moderate | 48 (56%) | |
| Well | 5 (6%) | |
| Unknown | 3 (3%) | |
| Lymphovascular Invasion |
Yes | 48 (56%) |
| No | 21 (24%) | |
| Unknown | 17 (20%) | |
| Perineural Involvement |
Yes | 68 (79%) |
| No | 4 (5%) | |
| Unknown | 14 (16%) | |
Adjuvant treatment parameters are shown in Table 2. Among the 20 surviving patients, median follow-up was 31 months. Median number of days between resection and start of adjuvant therapy was 55, and most patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 (60%) or 1 (33%) at initiation of therapy. All patients received 5-FU concurrent with radiation therapy, mostly through continuous infusion (91%). Standard radiation treatment consisted of a four-field technique, at 1.8 Gy per fraction, with a pancreas conedown (boost) of 5.4 Gy. All but six patients received 50.4 Gy or greater total dose to the pancreatic bed. Among the patients who received <50.4 Gy, two patients did not receive boosts secondary to toxicities from chemoradiation, one developed an exacerbation of ulcerative colitis, and one patient elected to skip her final treatment. Two additional patients were found to have metastatic disease during treatment: one patient received only 8 fractions of treatment before imaging showed metastatic deposits to the liver and peritoneum, and the other patient received a total of 31 Gy when a large metastatic lesion was found in the sacrum. These two patients were included in the analysis as there was no clear evidence at the start of adjuvant therapy that they had metastatic disease. 45% of patients went on to receive gemcitabine following concurrent 5-FU and radiation and 58% of patients went on to receive some type of adjuvant chemotherapy following chemoradiation.
Table 2.
Adjuvant treatment
| Characteristic | # patients (%) | |
|---|---|---|
| Time between resection and adjuvant therapy (days) |
Median: | 55 |
| Range: | 33–119 | |
| ECOG performance status at initiation of therapy |
0 | 52 (60%) |
| 1 | 28 (33%) | |
| 2 | 2 (2%) | |
| Unknown | 4 (5%) | |
| BMI prior to adjuvant treatment* | Median: | 24.7 |
| Range: | 17.5-37.8 | |
| Follow-up from resection (months) among 20 patients still alive |
Median: | 31 |
| Range: | 21–62 | |
| RT Dose: (cGy) | <5040 | 6 (7%) |
| 5040 | 73 (85%) | |
| 5400 | 7 (8%) | |
| Concurrent 5-FU with RT | Continuous infusion | 78 (91%) |
| Bolus injection | 7 (8%) | |
| Capecitabine§ | 1 (1%) | |
| Other Adjuvant Chemotherapy# | Gemcitabine‡ | 39 (45%) |
| 5-FU + Leucovorin | 13 (15%) | |
| Continuous 5-FU post-RT | 1 (1%) | |
| FOLFOX† | 1 (1%) | |
Abbreviations: ECOG= Eastern Cooperative Oncology Group; BMI= body mass index; 5-FU= 5-fluorouracil; RT= radiation therapy
Post-operative BMI, prior to adjuvant chemoradiation
Capecitabine (XELODA®), Hoffmann-La Roche Inc, Nutley, NJ, USA
Patients who received other adjuvant chemotherapy following concurrent chemotherapy and radiation
4 patients who received gemcitabine received additional adjuvant chemotherapy (i.e. 3 received leucovorin, 1 received FOLFOX)
One patient received gemcitabine + FOLFOX [leucovorin, 5-FU, and oxaliplatin (Eloxatin®) Sanofi-Aventis, Paris, France]
Survival Analysis
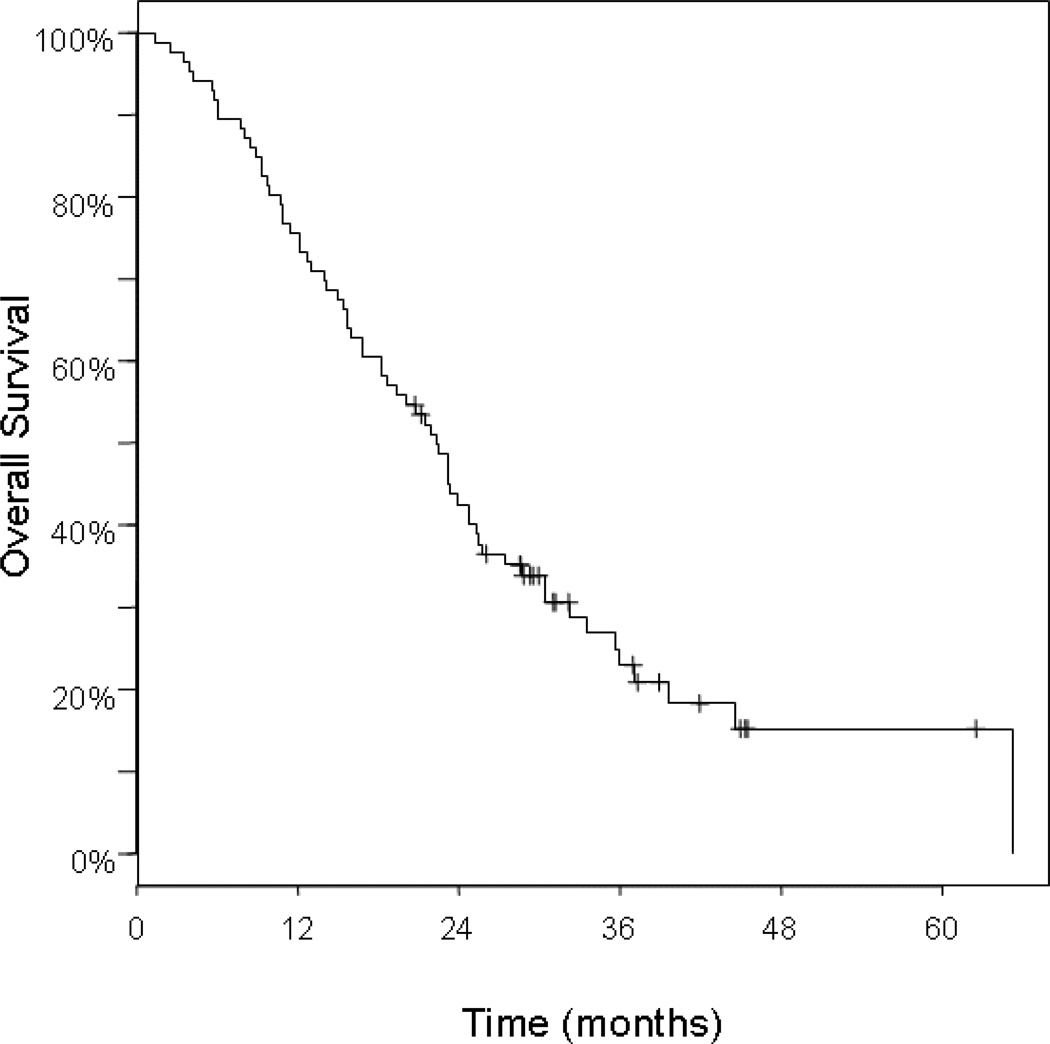
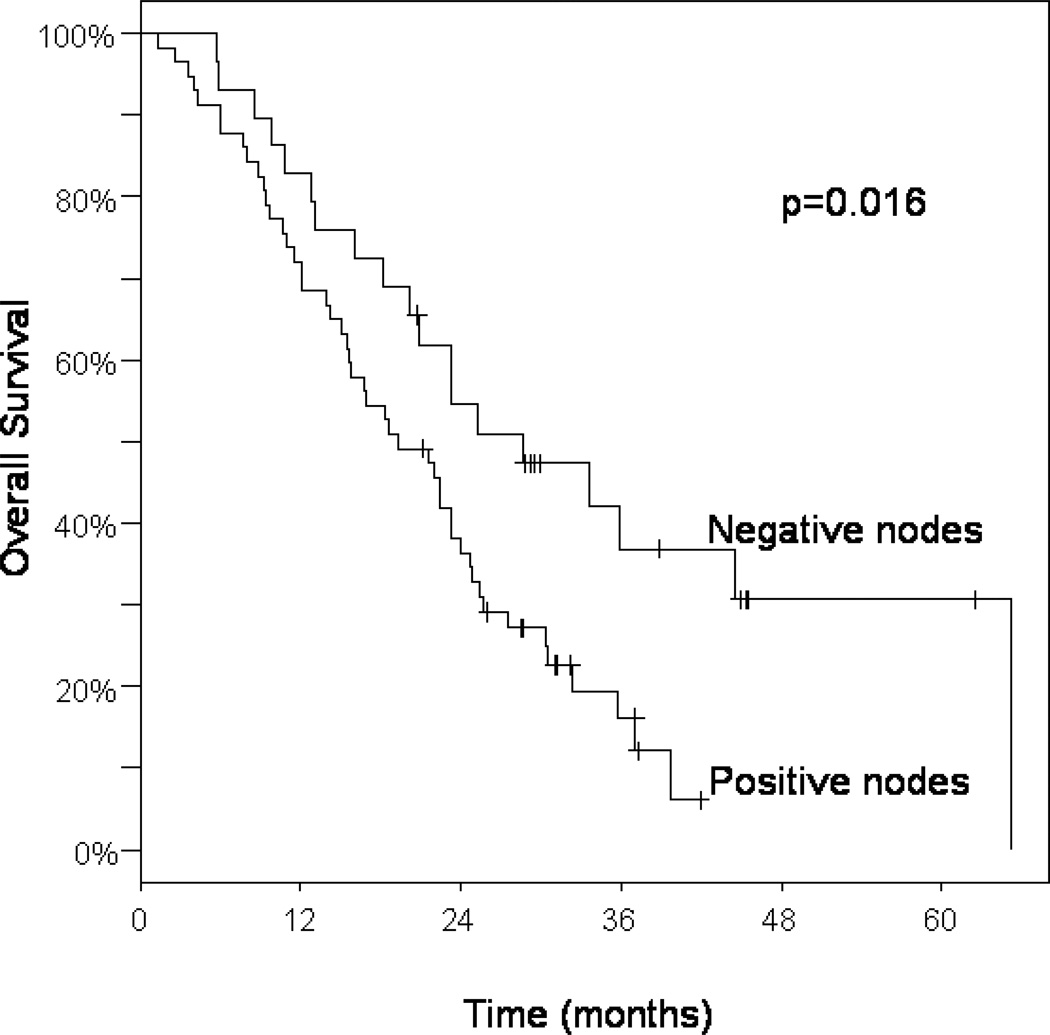
Of the 86 patients studied, 20 were alive at last follow-up, 9 with disease, and 11 with no evidence of disease. All patient deaths were due to disease. Median survival time from the start of adjuvant therapy among all patients was 22 months (95% CI: 18–25), and the 1-, 2-, and 3- year survival rates were 76%, 43%, and 23%, respectively (Table 3 and Figure 1). Regional lymph node metastasis was associated with a 16% 3-year survival, compared with 37% in patients with negative lymph nodes (p=0.016; Table 3 and Figure 2). Survival did not differ significantly by age (>70 years vs. ≤ 70), tumor grade (poor vs. moderate to well differentiated), tumor location (head vs. body or tail), tumor size (≤ 3cm vs. > 3cm), or margin status (negative vs. <1mm and positive). With regard to adjuvant treatment, we looked at whether additional chemotherapy following chemoradiation made any significant contribution to overall survival. Treatment with gemcitabine after chemoradiation trended towards improved overall survival when compared with patients who received chemoradiation alone (33% vs. 13% at 3 yrs, p=0.080; Table 3). Similarly, there was a trend towards improved survival among those who received chemoradiation followed by any adjuvant chemotherapy compared to chemoradiation alone (p=0.092).
Table 3.
Overall Survival from Adjuvant Treatment
| Factor | Median (months) |
1-year | 2-year | 3-year | p-value | |
|---|---|---|---|---|---|---|
| All patients, N=86 | 22 | 76% | 43% | 23% | ||
| Age | > 70years | 16 | 54% | 33% | 12% | 0.147 |
| ≤ 70years | 23 | 84% | 46% | 28% | ||
| Tumor grade | Well/moderately differentiated | 23 | 81% | 47% | 24% | 0.651 |
| Poorly differentiated | 20 | 63% | 37% | 21% | ||
| Tumor size | >3cm | 19 | 71% | 40% | 17% | 0.281 |
| ≤3cm | 23 | 77% | 43% | 25% | ||
| Tumor location | Head | 22 | 74% | 41% | 24% | 0.793 |
| Body/Tail | 18 | 81% | 48% | 21% | ||
| Lymph nodes | Positive | 19 | 72% | 36% | 16% | 0.016 |
| Negative | 29 | 83% | 55% | 37% | ||
| Resection margins |
Positive (including <1 mm) | 19 | 76% | 36% | 20% | 0.250 |
| Negative | 24 | 76% | 48% | 26% | ||
| Adjuvant Treatment |
Chemo-RT only | 17 | 61% | 33% | 13% | 0.080 |
| Chemo-RT + gemcitabine | 24 | 85% | 50% | 33% | ||
| Chemo-RT only | 17 | 61% | 33% | 13% | 0.552 | |
| Chemo-RT + non-gem chemo* | 18 | 91% | 45% | 18% | ||
| Chemo-RT only | 17 | 61% | 33% | 13% | 0.092 | |
| Chemo-RT + any chemo | 23 | 86% | 49% | 30% | ||
Abbreviations: Chemo-RT= Concurrent chemotherapy and radiation therapy
non-gemcitabine based chemotherapy
Figure 1.
Kaplan-Meier curve of overall survival among all patients, n=86.
Figure 2.
Kaplan-Meier curves of overall survival among all patients according to postoperative nodal status.
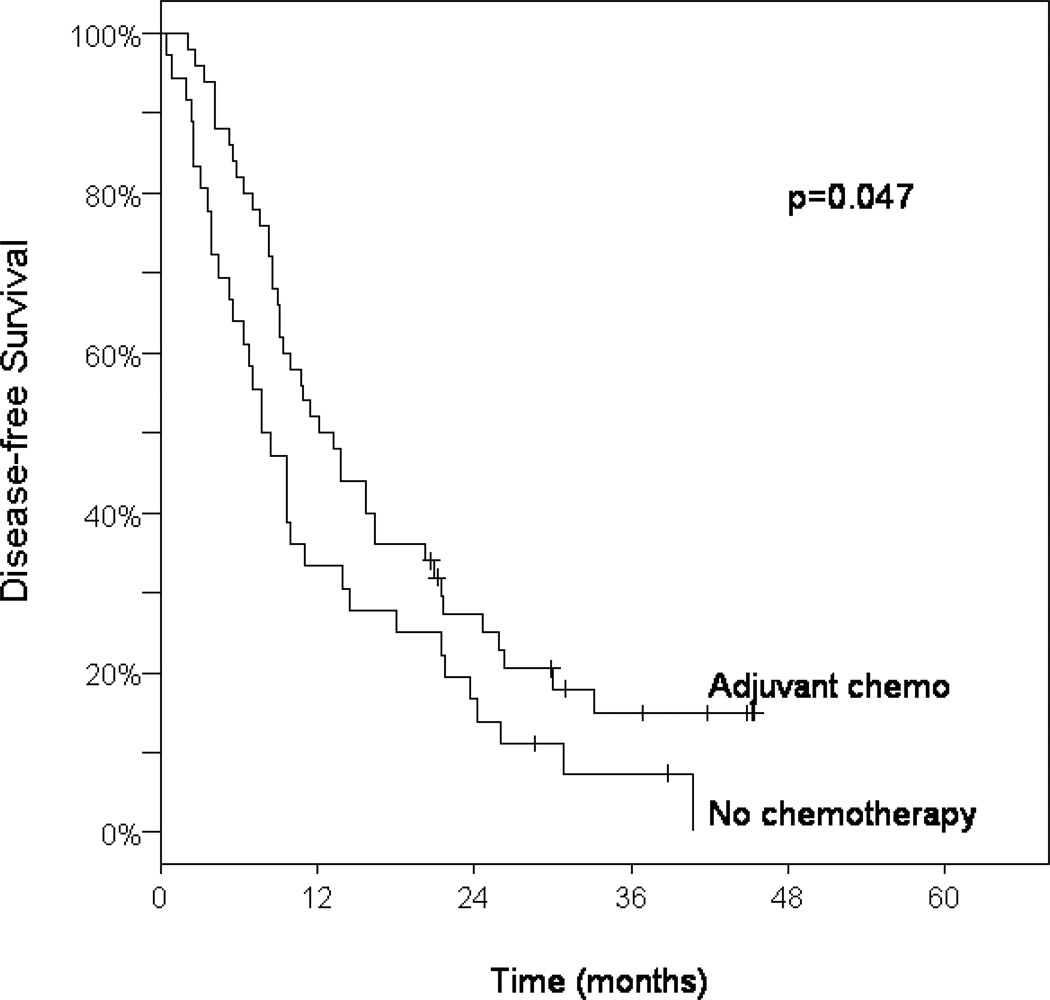
Median DFS time was 10 months (95% CI: 9–14) among the entire cohort (Table 4). Those patients with negative resection margins had a 16% 3-year DFS compared with 7% for those with close (<1 mm) or positive surgical margins (p=0.083; Table 4). Treatment with adjuvant gemcitabine was associated with a statistically significant improvement in DFS when compared with those who received chemoradiation alone, 3-year DFS 13% vs. 7%, p=0.044 (Table 4). Patients treated with any adjuvant chemotherapy following chemoradiation had a significant improvement in DFS over chemoradiation alone, p=0.047 (Table 4, Figure 3).
Table 4.
Disease-free Survival from Adjuvant Treatment
| Factor | Median (months) |
1-year | 2-year | 3-year | p-value | |
|---|---|---|---|---|---|---|
| All patients, N=86 | 10 | 44% | 23% | 12% | ||
| Age | > 70years | 9 | 38% | 13% | 13% | 0.366 |
| ≤ 70years | 11 | 47% | 27% | 12% | ||
| Tumor grade | Well/moderately differentiated | 11 | 43% | 24% | 9% | 0.886 |
| Poorly differentiated | 9 | 43% | 20% | 13% | ||
| Tumor size | >3cm | 9 | 31% | 14% | 6% | 0.102 |
| <3cm | 13 | 52% | 28% | 14% | ||
| Tumor location | Head | 11 | 49% | 24% | 14% | 0.128 |
| Body/Tail | 10 | 29% | 19% | 6% | ||
| Lymph nodes | Positive | 10 | 40% | 19% | 6% | 0.131 |
| Negative | 14 | 52% | 30% | 23% | ||
| Resection margins |
Positive (including <1 mm) | 9 | 37% | 16% | 7% | 0.083 |
| Negative | 13 | 51% | 28% | 16% | ||
| Adjuvant Chemo |
Chemo-RT only | 8 | 33% | 17% | 7% | 0.044 |
| Chemo-RT + gemcitabine | 12 | 51% | 30% | 13% | ||
| Chemo-RT only | 8 | 33% | 17% | 7% | 0.396 | |
| Chemo-RT + non-gem chemo* | 13 | 55% | 18% | 18% | ||
| Chemo-RT only | 8 | 33% | 17% | 7% | 0.047 | |
| Chemo-RT + any chemo | 13 | 52% | 27% | 15% | ||
Abbreviations: Chemo-RT= Concurrent chemotherapy and radiation therapy
non-gemcitabine based chemotherapy
Figure 3.
Kaplan-Meier curves of disease-free survival among patients treated with adjuvant chemotherapy following chemoradiation compared with patients treated with chemoradiation alone.
The remaining factors in Table 4 were not associated significantly with DFS. We additionally looked at the type of surgical resection, margin status grouped as negative and close (<1mm) vs. positive, and the presence or absence of lymphovascular invasion, none of which showed a statistically significant association with OS or DFS.
Patterns of Failure
Among the cohort, 11 patients (13%) had no documented recurrence of disease. Table 5 shows rates of locoregional recurrence; 36% of all patients had locoregional failure at 3 years. The use of adjuvant chemotherapy after chemoradiation was associated with a trend towards lower locoregional recurrence rates (p=0.080). Among all patients, 50% developed distant metastasis at 1 year and 87% at 3 years (Table 6). Positive lymph node status was associated with increased rates of distant failure, 94% vs. 73%, p=0.060. Adjuvant chemotherapy had a borderline effect on distant failure rates (p=0.100).
Table 5.
Locoregional Recurrence following Adjuvant Treatment
| Factor | 1-year | 2-year | 3-year | p-value | ||
|---|---|---|---|---|---|---|
| All patients, N=86 | 17% | 33% | 36% | |||
| Age | > 70years | 23% | 46% | 46% | 0.347 | |
| ≤ 70years | 15% | 30% | 33% | |||
| Tumor grade | Well/moderately differentiated | 18% | 32% | 36% | 0.846 | |
| Poorly differentiated | 17% | 33% | 33% | |||
| Tumor size | >3cm | 23% | 43% | 49% | 0.126 | |
| ≥3cm | 14% | 25% | 25% | |||
| Tumor location | Head | 14% | 30% | 33% | 0.361 | |
| Body/Tail | 26% | 41% | 41% | |||
| Lymph nodes | Positive | 14% | 38% | 42% | 0.384 | |
| Negative | 22% | 26% | 26% | |||
| Resection margins |
Positive (including <1 mm) | 19% | 31% | 31% | 0.965 | |
| Negative | 15% | 34% | 39% | |||
| Adjuvant Chemo |
Chemo-RT only | 30% | 39% | 45% | 0.078 | |
| Chemo-RT + gemcitabine | 11% | 25% | 25% | |||
| Chemo-RT only | 30% | 39% | 45% | 0.470 | ||
| Chemo-RT + non-gem chemo* | 0% | 42% | 42% | |||
| Chemo-RT only | 30% | 39% | 45% | 0.080 | ||
| Chemo-RT + any chemo | 9% | 29% | 29% | |||
Abbreviations: Chemo-RT= Concurrent chemotherapy and radiation therapy
non-gemcitabine based chemotherapy
Table 6.
Distant Failure following Adjuvant Treatment
| Factor | 1-year | 2-year | 3-year | p-value | |
|---|---|---|---|---|---|
| All patients, N=86 | 50% | 74% | 87% | ||
| Age | > 70years | 58% | 83% | 83% | 0.315 |
| ≤ 70years | 47% | 71% | 87% | ||
| Tumor grade | Well/moderately differentiated | 49% | 72% | 89% | 0.629 |
| Poorly differentiated | 53% | 78% | 86% | ||
| Tumor size | >3cm | 63% | 81% | 92% | 0.164 |
| <3cm | 42% | 69% | 85% | ||
| Tumor location | Head | 48% | 75% | 86% | 0.751 |
| Body/Tail | 57% | 71% | 89% | ||
| Lymph nodes | Positive | 56% | 79% | 94% | 0.060 |
| Negative | 38% | 64% | 73% | ||
| Resection margins |
Positive (including <1 mm) | 56% | 81% | 92% | 0.120 |
| Negative | 44% | 68% | 81% | ||
| Adjuvant Chemo |
Chemo-RT only | 58% | 79% | 90% | 0.114 |
| Chemo-RT + gemcitabine | 44% | 69% | 87% | ||
| Chemo-RT only | 58% | 79% | 90% | 0.342 | |
| Chemo-RT + non-gem chemo* | 45% | 77% | 77% | ||
| Chemo-RT only | 58% | 79% | 90% | 0.100 | |
| Chemo-RT + any chemo | 44% | 71% | 84% | ||
Abbreviations: Chemo-RT= Concurrent chemotherapy and radiation therapy
non-gemcitabine based chemotherapy
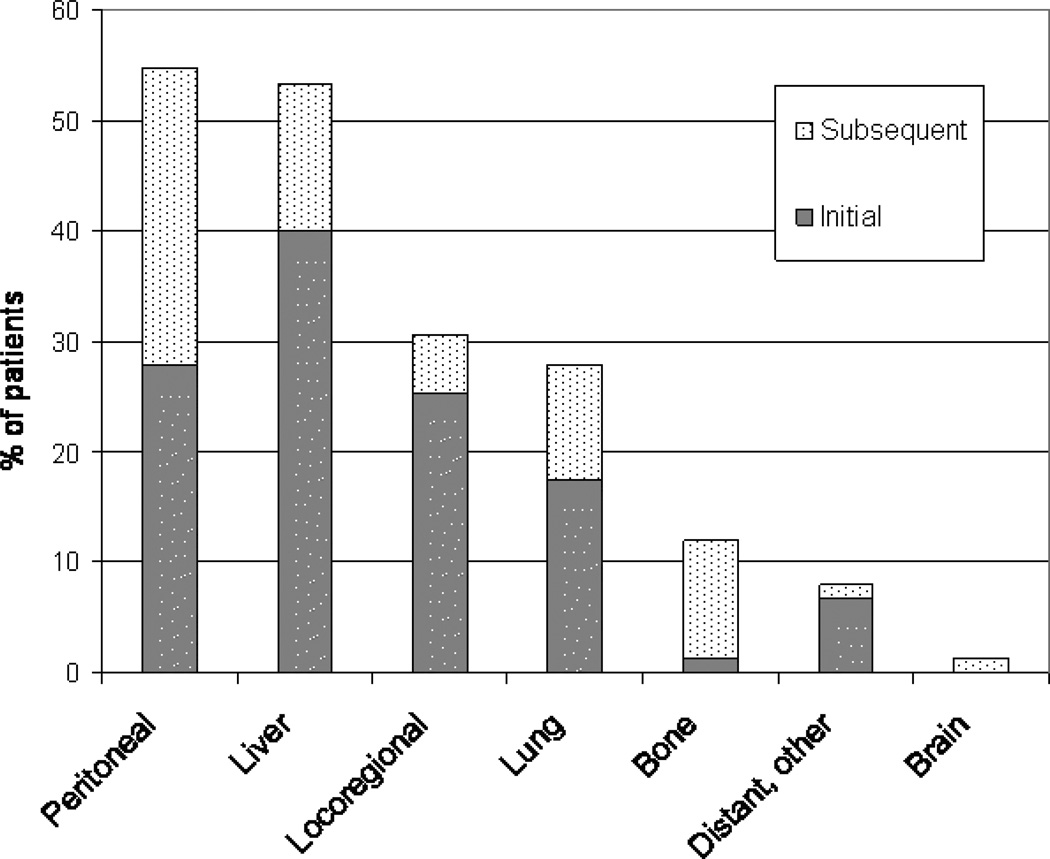
Figure 4 displays the patterns of failure among the 75 patients who did recur, stratified by initial and subsequent recurrence. Peritoneum (55%) and liver (53%) were the most common sites of recurrence. Liver was also the most common initial site of recurrence (40%). The next most common site of recurrence was locoregional (31%), which includes the pancreatic bed and regional lymph nodes. Paracardiac nodes, including paraaortic nodes, were considered to be locoregional recurrences. The lung was the 4th most common site of recurrence, followed by bone, other distant sites, and the brain. Other distant sites of recurrence included the following: supraclavicular lymph node, paratracheal node, adrenal gland, inguinal lymph node, ureter, and mediastinal lymph node.
Figure 4.
Patterns of Failure. The lower portion of each bar indicates the percentage of patients based on those who recurred who experienced their initial site of recurrence at that site; the upper portion indicates the percentage of patients who subsequently recurred in that site after an initial recurrence elsewhere.
Discussion
Pancreatic adenocarcinoma remains a relatively incurable disease with a dismal prognosis. The rationale for combined-modality adjuvant therapy is supported by the high rate of locoregional recurrence following resection.9, 20 Three major randomized controlled trials have largely shaped opinion regarding the benefit of adjuvant chemoradiation for resected pancreatic cancer. While the GITSG trial showed improved survival with adjuvant chemoradiation, the EORTC failed to show a statistically significant survival benefit, and ESPAC-1 suggested a deleterious effect of radiation.1, 3, 6 We studied a consecutive, unselected series of patients treated with adjuvant 5-FU and radiation therapy for resected pancreatic adenocarcinoma with the goal of comparing our institutional results with those in the literature.
Median overall survival in our cohort was 22 months (95% CI: 18–25), higher than that of the chemoradiation arm in the EORTC trial (17.1 months), and comparable to the survival observed on the GITSG chemoradiation arm (20 months) and to the results of RTOG 97-04.1, 3, 14 The ESPAC-1 trial showed a 15.9 month median survival in the groups that received chemoradiation vs. 17.9 months in the no chemoradiation arms.6 The median survival in the present study is higher than that of both these arms in the ESPAC-1 trial. With regard to disease recurrence, our study cohort showed similar results to the chemoradiation group in the ESPAC-1 study. In that study, known recurrence was seen in 82% of patients (vs. 87% in present study) with a median time to recurrence of 10.7 months (vs. 10 months in present study). In ESPAC-1, median time from resection to start of treatment was 46 days for patients receiving chemotherapy and 61 days for those receiving chemoradiation; in the present study, the median time between resection and adjuvant treatment was 55 days (range 33–119 days).
There may be several explanations for the higher survival seen in our cohort, compared with that of the chemoradiation arms in ESPAC-1. In our series, 93% of the patients had an ECOG performance status of 0 or 1, while this was not specified in ESPAC-1, so our cohort may have been healthier at the outset. Also, in ESPAC-1, 21% of patients in the chemoradiation arms received <40 Gy and 9% received no chemoradiation at all; these patients were included in their intent-to-treat analysis. Conversely, in our cohort, 93% of the patients were treated to 5040 Gy or more, all with 3-D conformal radiation treatment.
Hsu et al. recently presented an abstract of the Johns Hopkins-Mayo Clinic combined experience of 1045 patients with resected pancreatic adenocarcinoma, comparing those who received 5-FU based chemoradiation with surgical controls.21, 22 Median overall survival was higher among those who received chemoradiation than the observation group (22.5 months v. 16.3 months). These findings are comparable to the randomized data from GITSG and EORTC, but not consistent with ESPAC-1. Our results are very similar to this experience, though we were not able to include a post-operative observation group because the standard of care at our institutions was adjuvant chemoradiation during the years that the study patients were treated.
In the current study, the absence of nodal involvement was found to be a significant predictor of overall survival (Table 3, Figure 2). Resection margins, tumor grade, tumor location, and tumor size were not prognostic of overall survival as seen in other studies, although the power for detecting significant differences was limited by the relatively modest size of the cohort. We were unable to analyze ECOG performance status, which was found to be predictive of survival in the GITSG study, because of the relative homogeneity of our cohort (93% were PS 0–1). Negative margins trended toward improved DFS (p=0.083, Table 4) which is consistent with the literature.
Whether adjuvant chemotherapy following chemoradiation results in improved survival is an unanswered question in the treatment of resected pancreatic adenocarcinoma. Also, recent studies suggest the use of gemcitabine in the adjuvant setting represents an advance in the treatment of pancreatic cancer.12, 14 Over half of the cohort (58%) received some type of chemotherapy following chemoradiation, with 45% of patients receiving gemcitabine. Our findings show that additional chemotherapy with gemcitabine or any chemotherapy after chemoradiation is associated with improved DFS (Table 4), and a trend towards improved OS (Table 3) and lower locoregional recurrence (Table 5) as well as decreased distant failure (Table 6). Again, it is difficult to interpret whether this effect is due solely to the effect of gemcitabine, as only 11 patients received non-gemcitabine based chemotherapy, so the power is low for detecting significant differences compared to the patients who received gemcitabine or no additional chemotherapy following chemoradiation. Certainly this association is subject to inherent biases in this retrospective series; patients who responded well to chemoradiation may have been more likely to be treated with additional chemotherapy, thus selecting patients with better outcomes. A randomized trial would be required to define the role of adjuvant chemotherapy following chemoradiation.
Patterns of failure in the present study showed that liver and peritoneal metastases were the two most common sites of recurrence with locoregional sites following in third. This is consistent with the patterns of relapse reported in other studies (Table 7). These results support the idea that the microscopic burden of disease present in these patients post-operatively is frequently found in unirradiated or minimally irradiated sites (peritoneum, liver).24, 26 Even in the present cohort where 48% of patients had either close (<1 mm, 15%) or positive (33%) margins, adjuvant chemoradiation appears to improve local control, but future survival improvements should be directed to reducing intraabdominal relapse.
Table 7.
Site-specific patterns of failure among patients receiving post-operative chemoradiation
| Author | # of pts (n)* | % of pts with site-specific first failure | |||
|---|---|---|---|---|---|
| Liver | Peritoneum | Locoregional | Distant | ||
| Abrams 199923 | 21 | 43% | 19% | 48% | 5% |
| Foo 199324 | 28 | 43% | 43% | 7% | NA |
| Foo (Review of GITSG) 25 |
15 | 40% | NA | 47% | NA |
| Paulino 199926 | 19 | 79% | 37% | 21% | 37% |
| Present study | 75 | 40% | 28% | 25% | 25% |
Only includes patients who recurred and received post-operative chemoradiation
NA= data not available
The present study is limited as it is a relatively small, uncontrolled, retrospective series with a median follow-up of 31 months. However, it sheds light on the interpretation of the GITSG, EORTC, and ESPAC-1 studies, and represents the most current and widely-used treatment regimens in the United States. The patients in this study received modern 3-D conformal radiation therapy with CT-guided planning at two specialty cancer treatment centers. As all of the patients in this series received adjuvant chemoradiation, our results cannot determine the role of chemoradiation in the adjuvant treatment of pancreatic adenocarcinoma. However, the finding that our survival data are similar to the treatment arms of GITSG, EORTC and RTOG 97-04, while the ESPAC-1 results are significantly worse, do not support the hypothesis that properly delivered radiation has a negative effect on survival in the adjuvant therapy of pancreatic cancer, and we therefore continue to consider adjuvant combined modality therapy an acceptable option for patients with resected pancreatic adenocarcinoma. Ultimately, however, further randomized controlled studies are required to define the optimal adjuvant therapy for pancreatic cancer, and significant improvements await a better understanding of the biology of this disease, with development of novel agents that exploit this improved understanding.
Acknowledgments
Sources of Support/ Financial Disclosure: DF/HCC CCSG: Funding code P30 CA06516
References
- 1.Kalser MH, Ellenberg SS. Pancreatic cancer Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 985 Aug;120(8):899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 2.Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Gastrointestinal Tumor Study Group. Cancer. 1987 Jun 15;59(12):2006–2010. doi: 10.1002/1097-0142(19870615)59:12<2006::aid-cncr2820591206>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999 Dec;230(6):776–782. doi: 10.1097/00000658-199912000-00006. discussion 782–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrams RA, Lillemoe KD, Piantadosi S. Continuing controversy over adjuvant therapy of pancreatic cancer. Lancet. 2001 Nov 10;358(9293):1565–1566. doi: 10.1016/S0140-6736(01)06666-1. [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001 Nov 10;358(9293):1576–1585. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 6.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004 Mar 18;350(12):1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 7.Oya N. Chemoradiotherapy for pancreatic cancer: current status and perspectives. Int J Clin Oncol. 2004 Dec;9(6)(451-457) doi: 10.1007/s10147-004-0449-6. [DOI] [PubMed] [Google Scholar]
- 8.Koshy MC, Landry JC, Cavanaugh SX, et al. A challenge to the therapeutic nihilism of ESPAC-1. Int J Radiat Oncol Biol Phys. 2005 Mar 15;61(4):965–966. doi: 10.1016/j.ijrobp.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Pisters PW, Wolff RA, Crane CH, Evans DB. Combined-modality treatment for operable pancreatic adenocarcinoma. Oncology (Williston Park) 2005 Mar;19(3):393–404. 409. [PubMed] [Google Scholar]
- 10.Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 1997 Mar;15(3):928–937. doi: 10.1200/JCO.1997.15.3.928. [DOI] [PubMed] [Google Scholar]
- 11.Mehta VK, Fisher GA, Ford JM, et al. Adjuvant radiotherapy and concomitant 5-fluorouracil by protracted venous infusion for resected pancreatic cancer. Int J Radiat Oncol Biol Phys. 2000 Dec 1;48(5):1483–1487. doi: 10.1016/s0360-3016(00)00774-4. [DOI] [PubMed] [Google Scholar]
- 12.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. Jama. 2007 Jan 17;297(3):267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 13.Neuhaus P, Oettle H, Post S, et al. CONKO-001: Final results of the randomized, prospective, multicenter phase III trial of adjuvant chemotherapy with gemcitabine versus observation in patients with resected pancreatic cancer (PC). [Abstract] J Clin Oncol. 2008;26(15S) [Google Scholar]
- 14.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. Jama. 2008 Mar 5;299(9):1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 15.Abrams RA, Regine KAWWF, Safran H, Hoffman JP, Konski AA, Benson AB, Macdonald JS, Rich TA, Willett CG. Correlation of RTOG 9704 (adjuvant therapy (rx) of pancreatic adenocarcinoma (pan ca)) radiation therapy quality assurance scores (RTQASc) with survival (S) J Clin Oncol. 2007;25(18S):4523. [Google Scholar]
- 16.Social Security Death Index. [accessed January 25, 2008];Accessed via rootsweb.com. Available from URL: http://ssdi.rootsweb.com/
- 17.Consumer Health Ratings. [accessed January 17, 2008];Massachusetts Health Care Quality and Cost Information: Pancreatectomy, 2005. Available from URL: http://www.consumerhealthratings.com/index.php?action=showSubCats&cat_id=71.
- 18.Sobin LH, Wittekind Ch, editors. TNM Classification of malignant tumors. 5th ed. New York: John Wiley & Sons, Inc; 1997. [Google Scholar]
- 19.Greene FL, Page DL, Fleming ID, et al., editors. American Joint Committee on Cancer Staging Manual. 6th ed. Philadelphia: Springer; 2002. [Google Scholar]
- 20.Chu QD, Khushalani N, Javle MM, Douglass HO, Jr, Gibbs JF. Should adjuvant therapy remain the standard of care for patients with resected adenocarcinoma of the pancreas? Ann Surg Oncol. 2003 Jun;10(5):539–545. doi: 10.1245/aso.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 21.Hsu CCHJ, Corsini MM. Benefit of adjuvant chemoradiation therapy for pancreatic adenocarcinoma: The Johns Hopkins Hospital--Mayo Clinic collaborative study of 1,045 patients. [Abstract] In. [accessed May 12, 2008];2008 Gastrointestinal Cancers Symposium. Available from URL: http://www.asco.org/ASCO/Abstracts+%26+Virtual+Meeting/Abstracts?&vmvie w=abst_detail_view&confID=53&abstractID=10067#.
- 22.Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008 Jul 20;26(21):3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abrams RA, Grochow LB, Chakravarthy A, et al. Intensified adjuvant therapy for pancreatic and periampullary adenocarcinoma: survival results and observations regarding patterns of failure, radiotherapy dose and CA19-9 levels. Int J Radiat Oncol Biol Phys. 1039-1046 Jul 15;44(5) doi: 10.1016/s0360-3016(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 24.Foo ML, Gunderson LL, Nagorney DM, et al. Patterns of failure in grossly resected pancreatic ductal adenocarcinoma treated with adjuvant irradiation +/− 5 fluorouracil. Int J Radiat Oncol Biol Phys. 1993 Jun 15;26(3):483–489. doi: 10.1016/0360-3016(93)90967-z. [DOI] [PubMed] [Google Scholar]
- 25.Foo ML, Gunderson LL. Adjuvant postoperative radiation therapy +/− 5-FU in resected carcinoma of the pancreas. Hepatogastroenterology. 1998 May-Jun;45(21):613–623. [PubMed] [Google Scholar]
- 26.Paulino AC. Resected pancreatic cancer treated with adjuvant radiotherapy with or without 5-fluorouracil: treatment results and patterns of failure. Am J Clin Oncol. 1999 Oct;22(5):489–494. doi: 10.1097/00000421-199910000-00014. [DOI] [PubMed] [Google Scholar]