Abstract
Background
Renalase is a recently discovered secretory protein involved in regulation of arterial blood pressure in humans and animals. Results of animal experiments from independent laboratories indicate that administration of human recombinant renalase decreases blood pressure and some genetically predisposed hypertensive rats have lowered renalase levels.
Material/Methods
The levels of renalase mRNA expression in brain hemispheres, heart, and kidneys of spontaneously hypertensive rats (SHR) with moderate (140–180 mm Hg) or high (>180 mm Hg) hypertension and of control Wistar-Kyoto (WKY) rats were analyzed using real-time PCR.
Results
Spontaneously hypertensive rats with high hypertension (>180 mm Hg) had a lower renalase mRNA level in brain hemispheres, and higher heart and kidney renalase mRNA levels compared with control WKY rats. In SHR with a moderate increase in arterial blood pressure (140–180 mm Hg), the tissue renalase mRNA changed in the same direction but did not reach the level of statistical significance as compared with control rats.
Conclusions
The results indicate that the development of hypertension in SHR is accompanied by altered expression of the renalase gene in the examined organs as compared with control WKY rats. The brain and peripheral tissues renalase mRNA levels demonstrate opposite trends, which are obviously crucial for impaired regulation of blood pressure in SHR.
Keywords: renalase mRNA, real-time PCR, spontaneously hypertensive rats, Wistar-Kyoto rats, tissue specific expression pattern
Background
Renalase is a recently discovered secretory protein involved in regulation of arterial blood pressure in humans and animals [1–4]. Although mechanisms responsible for the renalase-mediated normalization of blood pressure remain unknown and pilot studies on the involvement of renalase in degradation of circulating catecholamines [2,5] have not been confirmed by other laboratories [1,6], good evidence exists that renalase is a novel and important regulator of blood pressure [1,3]. Although major attention is currently focused on kidney renalase expression (and secretion) altered in patients with renal pathology (particularly end-stage renal disease) [2,3,7,8], human renalase gene expression has been also found in cardiomyocytes, liver, and skeletal muscles [5]. More recently, renalase mRNA has been detected in human peripheral nerves, and in adrenal and adipose tissue [6].
Results of animal experiments from independent laboratories indicate that administration of human recombinant renalase decreases blood pressure [5,7]. Renalase knockout mice have higher blood pressure and susceptibility to myocardial ischemia [8]. Dahl rats with inherited predisposition to salt-sensitive arterial hypertension have renalase deficiency [9]. Recently, authors [10,11] have reported the decreased content of the renalase protein in plasma and kidneys of SHR (with mean systolic blood pressure of 198±29 mm Hg) as compared with WKY rats, and renal denervation caused a temporary increase in renal and plasma renalase and a decrease in blood pressure. This suggests involvement of renalase in regulation of blood pressure in this animal model. Therefore there is a clear need for analysis of tissue expression of renalase in SHR, which has not been performed so far.
In this preliminary study we investigated renalase mRNA in the brain, heart, and kidneys of control WKY rats with normal blood pressure of 110–120 mm Hg and of 2 groups of SHR with moderate (about 140–180 mm Hg) and high (more than 180 mm Hg) levels of blood pressure. Our results indicate that only the rats with high hypertension are characterized by statistically significant changes in the tissues levels of renalase mRNA as compared with control WKY rats, and brain and peripheral tissue renalase mRNA levels demonstrate opposite trends.
Material and Methods
Male 12–14 week old WKY (n=6; blood pressure of 110–120 mm Hg) and SHR rats with moderate (n=5; blood pressure of 140–180 mm Hg) and high (n=5, blood pressure exceeding 180 mm Hg) hypertension were purchased from the Animal Breeding Center (a branch of Shemyakin and Ovchinnikov Institute of Bio-organic Chemistry, Russian Academy of Sciences, Pushchino, Russia).
After decapitation under light anesthesia, brains were rapidly removed, and pooled samples of left and right cortex (5–10 mg), as well as samples of left ventricles and kidneys (5–10 mg each) were used for isolation of total RNA.
Total RNA was isolated using standard kits for total RNA isolation “SV Total RNA Isolation System” (Promega) as recommended by the supplier; 3–5 μg were used for cDNA synthesis using Primer Extension System-AMV Revertase Transcriptase Kit (Promega) and oligo d(T)10 primers. Custom synthesized primers (Table 1) purchased from Sintol (Russia) were used for amplification of selected genes encoding rat renalase mRNA 1825 bp (NM_001014167) and beta-actin mRNA 849 bp (EF156276).
Table 1.
Oligonucleotide primers for real-time PCR of rat renalase and β-actin.
| Gene | Oligonucleotide primer sequence 5′→3′ | Fragment size (bp) | |
|---|---|---|---|
| Renalase | Forward primer | AGCGCAACACAGAGTCATCA | 71 |
| Reverse primer | TGTGACTCCAAATGGGACGG | ||
| Dual-labeled fluorescent probe | R6G-ATGTGGCCCATTGCTGGTGGTCCATAC-BHQ2 | ||
|
| |||
| β-actin | Forward primer | AGCCATGTACGTAGCCATCCA | 81 |
| Revers primer | TCTCCGGAGTCCATCACAATG | ||
| Dual-labeled fluorescent probe | FAM-TGTCCCTGTATGCCTCTGGTCGTACCAC-BHQ1 | ||
PCR cycles were optimized for each gene for the log phase amplification [12]. No template control (NTC) samples (without DNA added) were amplified under our experimental conditions (Figure 1). Numbers of mRNA copies were determined by the method of real-time TaqMan PCR detection, using a CFX96 real-time PCR detection system (BioRad).
Figure 1.
Real-Time PCR amplification plot of β-actin mRNA from brain hemispheres, heart, and kidney of control WKY rats. An arrow indicates lack of amplification of no-template control (NTC).
Details of the real-time TaqMan PCR detection have been described previously [12]. Results were also normalized vs. β-actin gene expression and treated using a program provided by the supplier of the real-time PCR instrument (BioRad).
Statistical difference was evaluated by unpaired t test. Differences were considered as statistically significant at p≤0.05.
Results
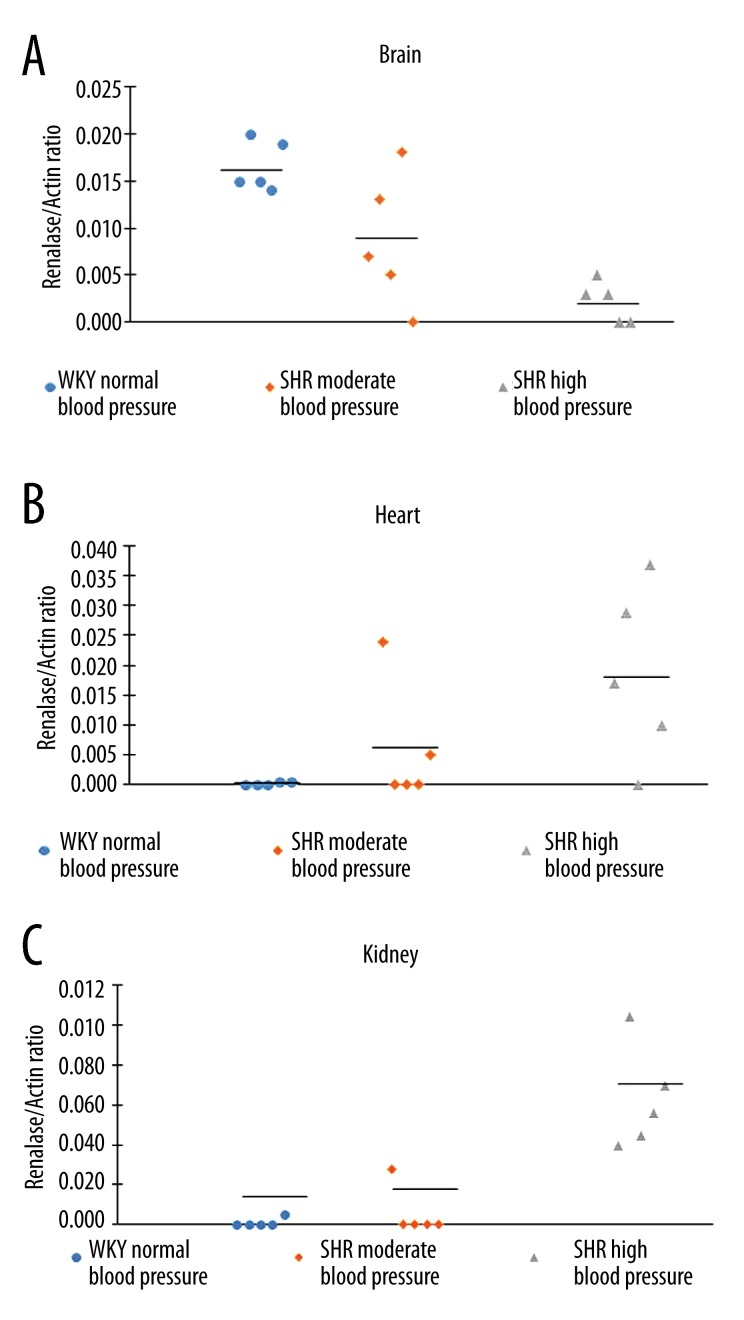
In brain, heart, and kidney samples of WKY and both groups of SHR rats, the mRNA transcripts for the β-actin gene gave similar (and basically indistinguishable) signals (Figure 1) and all such renalase expression results were therefore normalized vs. β-actin gene expression. Analysis of renalase mRNA in 3 tissues of WKY and SHR rats showed different trends. In brain, the highest levels of renalase mRNA were found in WKY rats. In SHR with the moderate increase in blood pressure (120–140 mm Hg), the renalase mRNA level was somewhat lower than in control WKY rats (with normal blood pressure of 110–1120 mm Hg), while in SHR with the highest level of blood pressure (>180 mm Hg) it demonstrated further decrease, which reached the level of statistically significant difference (p<0.01, vs. WKY) (Figure 2A). In heart, no changes were found in mRNA expression between WKY and SHR rats with moderate blood pressure, while in SHR with the highest level of blood pressure it significantly exceeded control level (Figure 2B; p<0.05). In kidneys of WKY rats, the mRNA level of renalase was near the detectable limit, as well as in SHR with moderate hypertension. However, SHR with the highest level of blood pressure (>180 mm Hg) had significantly higher level of renalase mRNA (p<0.001) (Figure 2C).
Figure 2.
Renalase mRNA expression in brain hemispheres (A), heart (B), and kidney (C) of control WKY rats, SHR with moderate (140–180 mm Hg) and high (>180 mm Hg) hypertension. Data represent individual values of the renalase/actin mRNA ratio for each animal. Other explanations are given in the text.
Discussion
Spontaneously hypertensive rats were originally inbred from Wistar rats and their Wistar-Kyoto non-hypertensive controls (for a review, see Tamura et al. [13]). They are characterized by the development of stable spontaneous hypertension at the age of 4–6 weeks. Our results indicate that although mRNA renalase levels gradually changed from normotensive WKY rats to SHR with moderate hypertension and further to SHR with high hypertension, only the adult SHR with high hypertension (>180 mm Hg) are characterized by marked and statistically significant changes in the mRNA levels in brain hemispheres (p<0.01), heart (p<0.05), and kidneys (p<0.001) compared with WKY rats (Figure 2A–2C). In the brain tissues, there was a clear decrease in renalase mRNA, while in myocardium and especially in kidney parenchyma significant increases in renalase mRNA were detected. These opposite changes suggest differential regulation of renalase gene expression in the brain and peripheral tissues during the development of hypertension. Earlier tissue-specific regulation has been reported for angiotensin gene expression in SHR vs. WKY [14].
Recent studies [10,11] have reported decreased content of the renalase protein in plasma and kidneys of SHR (with mean systolic blood pressure of 198±29 mm Hg) as compared with WKY rats, and renal denervation caused a temporal increase in renal and plasma renalase and a decrease in blood pressure. Although tissue renalase mRNA levels have not been investigated, this suggests that renalase is an important player in this model of hypertension. In this context, our results indicate that altered expression of the renalase gene is associated with development of hypertension in SHR. It is reasonable to suggest that altered transcription of the renalase gene in adult male SHR is not accompanied by corresponding changes in the tissue/plasma levels of the renalase protein due to impairments in posttranscriptional regulatory mechanisms of renalase protein synthesis. Such a scenario is quite possible if we take into consideration data [8] that indicate renalase inhibition by antisense RNA (which usually blocks translation of the sense mRNA [15]) results in increased basal blood pressure and hyperergic response to adrenergic stress. Study of this problem is among our future plans.
Conclusions
The results of this study indicate that SHR with high hypertension (>180 mm Hg) are characterized by lower levels of renalase mRNA in brain hemispheres and decreased levels in heart and kidneys as compared with control WKY rats. The opposite changes in renalase mRNA expression suggest involvement of tissue-specific regulation of renalase gene transcription, which is crucial for impaired regulation of blood pressure.
Footnotes
Source of support: This work was supported by a grant from the Russian Foundation for Basic Research (project no. 11-04-01181a)
References
- 1.Baroni S, Milani M, Pandini V, et al. Is renalase a novel player in catecholaminergic signaling? The mystery of the catalytic activity of an intriguing new flavoenzyme. Curr Pharm Des. 2013;19:2540–51. doi: 10.2174/1381612811319140005. [DOI] [PubMed] [Google Scholar]
- 2.Desir GV, Wang L, Peixoto AJ. Human renalase: A review of its biology, function, and implications for hypertension. J Am Soc Hypertens. 2012;6:417–26. doi: 10.1016/j.jash.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Malyszko J, Malyszko JS, Rysz J, et al. Renalase, Hypertension, and Kidney – The Discussion Continues. Angiology. 2013;64:181–87. doi: 10.1177/0003319712459212. [DOI] [PubMed] [Google Scholar]
- 4.Medvedev AE, Veselovsky AV, Fedchenko VI. Renalase, a new secretory enzyme responsible for selective degradation of catecholamines: Achievements and unsolved problems. Biochemistry. 2010;75:951–58. doi: 10.1134/s0006297910080018. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Li G, Wang P, et al. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest. 2005;115:1275–80. doi: 10.1172/JCI24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hennebry SC, Eikelis N, Socratous F, et al. Renalase, a novel soluble FAD-dependent protein, is synthesized in the brain and peripheral nerves. Mol Psychiatry. 2010;15:234–36. doi: 10.1038/mp.2009.74. [DOI] [PubMed] [Google Scholar]
- 7.Pandini V, Ciriello F, Tedeschi G, et al. Synthesis of human renalase1 in Escherichia coli and its purification as a FAD-containing holoprotein. Protein Expr Purif. 2010;72:244–53. doi: 10.1016/j.pep.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Desir GV. Renalase, a new renal hormone: its role in health and disease. Curr Opin Nephrol Hypertens. 2007;16(4):373–78. doi: 10.1097/MNH.0b013e3281bd8877. [DOI] [PubMed] [Google Scholar]
- 9.Desir GV. Renalase deficiency in chronic kidney disease, and its contribution to hypertension and cardiovascular disease. Curr Opin Nephrol Hypertens. 2008;17(2):181–85. doi: 10.1097/MNH.0b013e3282f521ba. [DOI] [PubMed] [Google Scholar]
- 10.Guo Y, Li L, Tan L, et al. Impact of renal denervation on expression of renalase and tyrosine hydroxylase in adult rats with spontaneous hypertension. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012;37(8):829–33. doi: 10.3969/j.issn.1672-7347.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Jiang W, Guo Y, Tan L, et al. Impact of renal denervation on renalase expression in adult rats with spontaneous hypertension. Exper Ther Med. 2012;4:493–96. doi: 10.3892/etm.2012.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedchenko V, Globa A, Kaloshin A, et al. The effect of short-term administration of (–)-deprenyl and isatin on expression of some genes in the mouse brain cortex. Med Sci Monit. 2008;14(12):BR269–73. [PubMed] [Google Scholar]
- 13.Dornas WC, Silva ME. Animal models for the study of arterial hypertension. J Biosci. 2011;36(4):731–37. doi: 10.1007/s12038-011-9097-y. [DOI] [PubMed] [Google Scholar]
- 14.Tamura K, Umemura S, Nyui N, et al. Tissue-specific regulation of angiotensinogen gene expression in spontaneously hypertensive rats. Hypertension. 1996;7:1216–23. doi: 10.1161/01.hyp.27.6.1216. [DOI] [PubMed] [Google Scholar]
- 15.Melton DA. Injected anti-sense RNAs specifically block messenger RNA translation in vivo. Proc Natl Acad Sci USA. 1985;82:144–48. doi: 10.1073/pnas.82.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]