Abstract
Background
Calcium dobesilate (CaD) is a member of the synthetic veno-active drug family. Only a small number of reports are available that describe the micro-angiogenic effects of CaD in the current literature.
Material/Methods
The antiangiogenic potential of CaD was compared with bevacizumab (Bb), which is a potent angiogenesis inhibitor, in a chick chorioallantoic membrane model. Four different concentrations (10−7, 10−6, 10−5, and 10−4 M) of drug pellet were prepared for each drug. Changes in vessel formation were scored and compared for each drug according to the previous literature.
Result
The antiangiogenic behavior of CaD was lower than Bb, despite the significant dose-dependent manner of escalation. The anti-angiogenic scores of CaD were determined as 0.20, 0.47, 0.66, 1.0 in 10−7, 10−6, 10−5, and 10−4 M concentrations, respectively (average score >0.5 was significant).
Conclusions
According to the data obtained, this agent should be used carefully for cases in which angiogenesis plays an important role in healing.
Keywords: calcium dobesilate, angiogenesis, dose-dependent action, chorioallantoic membrane model
Background
Veno-active drugs are commonly utilized agents for managing the symptoms of chronic venous insufficiency [1]. Their anti-oxidant and anti-inflammatory effects are also widely described in previous reports [2,3] and the microcirculatory effects of this drug family were recently investigated [3,4]. Calcium dobesilate (CaD) is a synthetic veno-active drug that acts by increasing lymphatic drainage and venous tone. It is used for the management of chronic venous insufficiency symptoms such as edema, paresthesia, and cramps [3,5]. Previous studies have described the erythrocyte aggregation decrement effect, erythrocyte flexibility increment impact, and microcirculation regulation with decreasing blood viscosity and anti-oxidative behaviors of CaD [3]. However, angiogenic behaviors have been addressed in only a few reports [4,6].
Angiogenesis is an excellent therapeutic target for cardiovascular diseases [7]. The lack of sufficient angiogenesis might be responsible for clinical outcomes in patients with arterial circulation disorders such as peripheral artery disease and coronary artery disease [7]. Therefore, many cardiovascular drugs were investigated to explain their angiogenic behaviors [7,8]. Moreover, cardiovascular drugs were compared with well-known antiangiogenic molecules. In previous studies, Bevacizumab (Bb) (which is a monoclonal antibody against vascular endothelial growth factor) is an agent used to compare the antiangiogenic potential of drugs with the antiangiogenic behaviors of molecules [8]. Determining the angiogenic or antiangiogenic effects of therapeutic agents might be helpful for more consistent and systematic management of vascular disorders.
In this study, we compared the antiangiogenic potential of CaD with Bb in a chick embryo chorioallantoic membrane model (CAM) in vivo.
Material and Methods
Study concept
A single-blind, randomized, controlled chick embryo chorioallantoic membrane study was designed to evaluate the antiangiogenic potential of calcium dobesilate. Ethical approval was received from the Local Animal Ethics Committee in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals.
Pellet preparation
To create a negative control group, drug-free pellets were prepared and applied to a chick embryo chorioallantoic membrane model (CAM). No significant antiangiogenic behavior was observed (as expected) with the drug-free pellets. Bevacizumab (Bb) (Avastin®, Genentech, South San Francisco, CA; Roche AG, Basle, Switzerland) was used for the positive control group, creating 4 different concentrations: 10−7, 10−6, 10−5, and 10−4 M. Calcium dobesilate (CaD) (Doxium®, OM Pharma, Geneva, Switzerland) was used for the study control group by creating 4 different concentrations: 10−7, 10−6, 10−5, and 10−4 M. Agarose (Merck, Darmstadt, Germany) was mixed with distilled water to obtain a 1.5% (w/v) solution. An autoclave was used to provide dissolution and sterilization of the agarose solution at 121°C and under 1 atmospheric pressure. Subsequently, sterile containers were filled with agarose solutions and allowed to cool to 37°C. The study drug was added at this stage. All levels were applied in accordance with previous scientific explanations [7–9].
Supply of fertilized eggs
The Ross 308 strain was chosen for study and the required fertilized hens’ eggs were obtained from Yemsel Poultry Company (Kayseri, Turkey). Temperature-controlled (37.5°C) and humidity-controlled (80±5%) egg incubators were used to store the eggs during embryo development. Eggs were horizontally placed into incubators.
Study levels
Nearly 5 ml albumen was taken from the eggshell via syringe on the 5th day of incubation (Figure 1A). After that, a small piece (~2–3 cm) of eggshell was removed from the converse side of the eggs to evaluate development of the normal chick embryo (Figure 1B). Any eggs with detected deformation were excluded from the study. After the evaluation, holes in the eggshells were sealed with gelatin (to prevent contact with the external environment) and the eggs were replaced in the incubators. Eggs then took, on average, 72 hours to reach a 2-cm diameter embryo development. Drug pellets were dropped into the eggs after the removal of the gelatin seals on the 8th day. Eggs were resealed with gelatin and replaced in incubators for 24 hours following drug pellet placement (Figure 2A). At the end of the waiting time (after 24 hours), the gelatin seals were removed and any embryos that were dead, deformed, or disrupted by inflammation were excluded. The remaining embryos were scored according to the scoring system previously described by Bürgermeister et al., using a stereoscopic microscope [10]. All tests were duplicated for each drug solution for confirmation and standardization.
Figure 1.
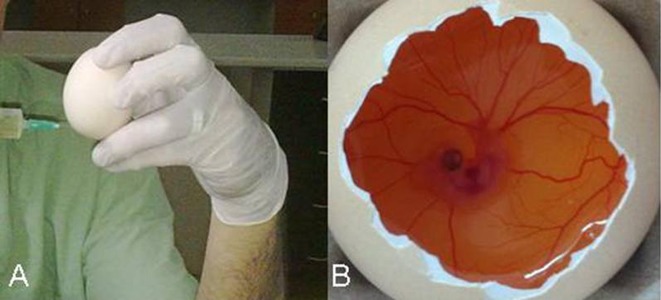
(A) Albumen aspirating (~5–6) ml via syringe. (B) Normal embryo development after the removal of eggshell.
Figure 2.

(A) Placement of drug pellet (B) Evaluation of variations in capillary density around the drug pellets [Score 2] (×8).
Antiangiogenesis scoring system
The scoring system described by Bürgermeister et al. is based on the variations in capillary density around the drug pellets (Figure 2B) and expansion area classification according to expected normal development [10]. This system has 4 scores: 0, 0.5, 1, and 2, defined as follows:
Score 0 indicates that no antiangiogenic effect can be observed (normal capillary development).
Score 0.5 means slight ambivalent differences (no marked capillary diminution, but an ambivalent zone with partial reduction of capillary density, which is smaller than the pellet sphere).
Score 1 indicates significant mid-level variations (small area with capillary lack and/or a faint area with marked capillary density decrement that does not exceed double the size of the pellet).
Score 2 reflects a definite antiangiogenic effect (a sharp area without capillaries around the pellet that is nearly the same size as or more than double the size of the pellet).
The formula of average scoring (AS) was: [Egg number (Score 2) × 2 + Egg number (Score 1) × 1]/[Total egg number (Score 0, 1, and 2)].
The average scores are interpreted as follows:
0–0.5= Significant antiangiogenic effect was not detected.
0.5–1= Slightly antiangiogenic effect was determined
1= Remarkable antiangiogenic effect was determined.
Statistical analysis
Antiangiogenic scores were compared with the Kruskal-Wallis ANOVA test and the Mann-Whitney U test for each drug, statistically. The p-value for statistical significance was defined as p<0.05.
Results
There was no significant antiangiogenic effect in the negative control (drug-free pellets) group [AS=0.0]. The positive control group (pellets with Bb) was evaluated at 4 different concentrations: 10−4, 10−5, 10−6, and 10−7 M.
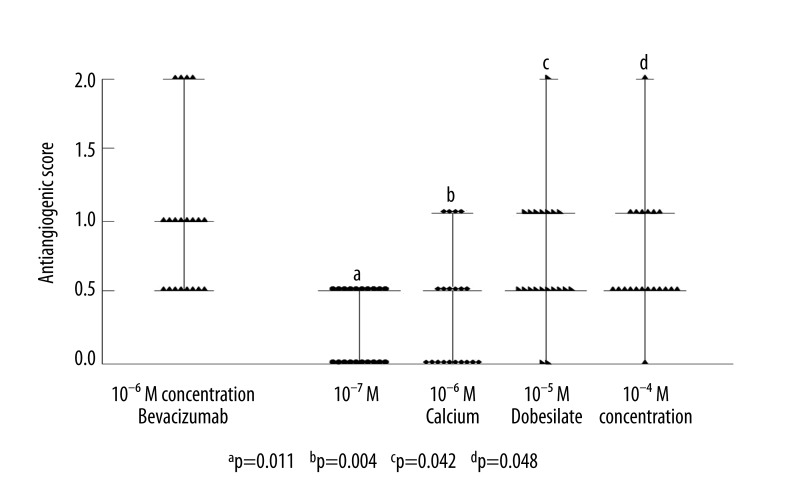
As expected, marked angiogenesis inhibition was observed for each dose of Bb. The AS for the 10−7, 10−6, 10−5, and 10−4 M of Bb were obtained as 0.82, 1.0, 1.6, and 2.1, respectively. These findings were compared with the study (pellets with CaD) group. The comparison of antiangiogenic score distribution at 10−6 M concentration of Bb and 10−7, 10−6, 10−5, and 10−4 M of CaD is shown in Figure 3.
Figure 3.
Comparison of distribution of antiangiogenic scores at 10−6 M concentration of Bevacizumab and 10−7, 10−6, 10−5, and 10−4 M concentrations of Calcium Dobesilate.
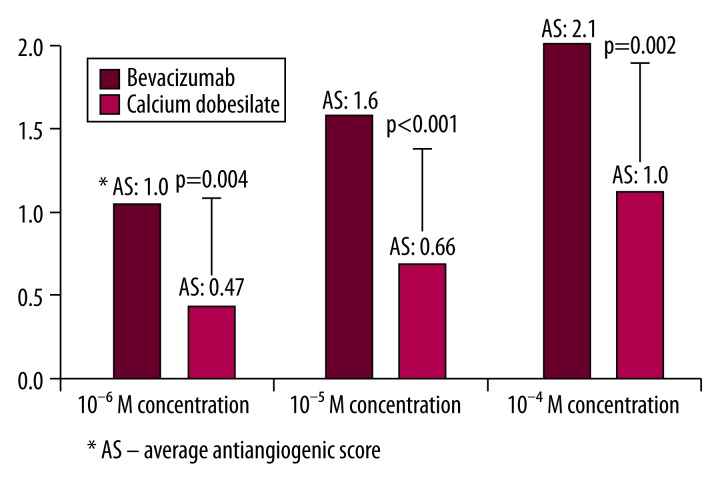
Antiangiogenic activity at 0.5 and/or over was observed in 10 eggs at 10−7 M concentrations, in 10 eggs at 10−6 M concentrations, in 18 eggs at 10−5 M concentrations, and in 19 eggs at 10−5 M concentrations of CaD (n=20 eggs for each drug concentration). The distribution and comparison of antiangiogenic scores for each CaD concentration is listed in Figure 3. The antiangiogenic scores of CaD were determined as 0.20, 0.47, 0.66, 1.0 in 10−7, 10−6, 10−5, 10−4 M concentrations, respectively (average score >0.5 was significant). Despite increasing antiangiogenic efficacy with dose escalation, the only significant antiangiogenic scores obtained were 10−5 and 10−4 M concentrations of CaD. Additionally, statistically significant differences were observed (Figure 3) between these dose-related antiangiogenic tendencies (p<0.05). Average scores (AS) of 10−6, 10−5, and 10−4 M concentrations of Bb and CaD are compared in Figure 4. The antiangiogenic potential of CaD reached underestimated values, although Bb showed statistically higher antiangiogenic behaviors than CaD.
Figure 4.
Comparison of average antiangiogenic scores between 10−6, 10−5, 10−4 M concentrations of Bevacizumab and Calcium Dobesilate in a scatter graph.
Discussion
The capillary endothelial variations from different vascular sites have previously been described. These differences facilitate the definition of differences in function [11]. The venous endothelium acts in part as a main barrier between blood and vascular tissue. Additionally, it partially prevents the physical injury of the vessel wall by external destructive factors via providing sufficient intravascular volume and maintaining required oxygen delivery [12]. Moreover, endothelial cells are clearly important for angiogenesis, because functional endothelial cell migration is essential for new vessel formation [13]. All endothelial cells are derived from mesodermal cells into hemangioblasts [13]. Thus, angiogenic stimulation can induce whole endothelial structures; for example, vascular endothelial growth factor (VEGF) stimulates both vascular angiogenesis and lymphangiogenesis [14]. Researchers have investigated new therapeutic options for diseases with angiogenesis regulation abilities [7]. Angiogenesis stimulation can be beneficial for ischemic events, but it was claimed that stimulation of angiogenesis can worsen retinopathy. Additionally, angiogenesis is not recommended in malignancies to block tumorogenesis [15].
Calcium dobesilate (CaD) is a synthetic product, marketed worldwide, that is classified as a vasculo-protector and veno-tonic drug. Safety studies of CaD commonly investigate various effects on hematological parameters and capillary impacts and have been described in several reports [16,17]. CaD exhibits its inhibitor effect on capillary permeability through the regulation of serotonin, bradykinin, and histamine [16,17]. Additionally, it reduces endothelial desquamation via nitric oxide release and synthesis [16]. The dose-dependent microcirculatory effects of CaD were found to reduce platelet aggregation via prostaglandin inhibition and also to reduce erythrocyte aggregation and their suspension viscosity [16]. In addition to these studies, the antiangiogenic potential of CaD was investigated in a few studies; for instance, Lameynardie et al. claimed that CaD inhibits VEGF over-expression in rat retinas [18]. As described earlier, stimulation of angiogenesis can worsen retinopathy and inhibition of angiogenesis can be therapeutic [15]. Lameynardie et al. reported a significant reduction of choroidal angiogenesis in diabetic Goto-Kakizaki rat retinas and suggested that CaD might be therapeutically beneficial in diabetic retinopathy, based on these data [18]. Cuevas et al. found that neovascularization was remarkably reduced with CaD through fibroblast growth factor (FGF) suppression in rats [6]. They suggested that CaD may be an effective therapeutic agent for angiogenesis-dependent disorders [6]. CaD was suggested as a therapeutic agent for rosacea, as well as reducing pathological angiogenesis in women in another study by Cuevas et al. [19]. Angulo et al. reported that CaD inhibits endothelial proliferation in human umbilical vein endothelial cells via VEGF and FGF reduction [4]. They added that CaD can be beneficial for treatment of disorders with excessive angiogenesis [4]. Conversely, we studied the antiangiogenic potential of CaD in a physiological angiogenesis model of CAM. Our results were similar to those found in the existing literature. Although with less antiangiogenic potential than antitumor agent Bb, we obtained significant angiogenesis inhibition via 10−5 and 10−4 M concentrations of CaD. Furthermore, CaD shows significant dose-dependent antiangiogenic behaviors (p<0.05).
Conclusions
Briefly, CaD seems to be a potent antiangiogenic agent with a dose-dependent action. Most of the existing literature declares that it can be beneficial for disorders with undesirable neoangiogenesis. Based on this, it may be helpful for diabetic retinopathy treatment. Furthermore, there is no qualified evidence for interaction with other diabetic vasculopathies such as diabetic peripheral arterial disease, which requires neoangiogenesis. We suggest that the antiangiogenic efficacy of CaD should be investigated in diseases that require angiogenesis in the healing process.
Footnotes
Statement
The limitation of the study concerns the study model. Subjects in each group were created with CAM. The model is also primitive and used basic methods. Therefore, this study was designed as a pilot study for further investigations.
Declaration of conflicting interests
The authors declare they have no conflicts of interest with respect to the authorship and/or publication of this article.
Source of support: Departmental sources
Funding
The authors received no financial support for the research and/or authorship of this article.
References
- 1.Rabe E, Jaeger KA, Bulitta M, Pannier F. Calcium dobesilate in patients suffering from chronic venous insufficiency: a double-blind, placebo-controlled, clinical trial. Phlebology. 2011;26:162–68. doi: 10.1258/phleb.2010.010051. [DOI] [PubMed] [Google Scholar]
- 2.Brunet J, Farine JC, Garay RP, Hannaert P. In vitro antioxidant properties of calcium dobesilate. Fundam Clin Pharmacol. 1998;12:205–12. doi: 10.1111/j.1472-8206.1998.tb00943.x. [DOI] [PubMed] [Google Scholar]
- 3.Akgun OO, Arslan C, Suzer O, Bozkurt AK. The effects of calcium dobesilate and micronized purified flavonoid fractions on myocardial protection. Turkish J Thorac Cardiovasc Surg. 2011;19:417–24. [Google Scholar]
- 4.Angulo J, Peiró C, Romacho T, et al. Inhibition of vascular endothelial growth factor (VEGF)-induced endothelial proliferation, arterial relaxation, vascular permeability and angiogenesis by dobesilate. Eur J Pharmacol. 2011;667:153–59. doi: 10.1016/j.ejphar.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Akbulut B. Calcium dobesilate and oxerutin: effectiveness of combination therapy. Phlebology. 2010;25:66–71. doi: 10.1258/phleb.2009.008085. [DOI] [PubMed] [Google Scholar]
- 6.Cuevas P, Sanchez I, Lozano RM, Gimenez-Gallego G. Dobesilate is an angiogenesis inhibitor. Eur J Med Res. 2005;10:369–72. [PubMed] [Google Scholar]
- 7.Katrancioglu N, Karahan O, Kilic AT, et al. Comparison of the antiangiogenic effects of heparin sodium, enoxaparin sodium, and tinzaparin sodium by using chorioallantoic membrane assay. Blood Coagul Fibrinolysis. 2012;23:218–21. doi: 10.1097/MBC.0b013e3283504132. [DOI] [PubMed] [Google Scholar]
- 8.Katrancioglu N, Karahan O, Kilic AT, et al. The antiangiogenic effects of levosimendan in a CAM assay. Microvasc Res. 2012;83:263–66. doi: 10.1016/j.mvr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Karahan O, Yavuz C, Demirtas S, et al. The Investigation of the Antiangiogenic Potential of Amiodarone HCl in the Chick Embryo Chorioallantoic Membrane Model. Biomedical Research. 2013;24:131–34. [Google Scholar]
- 10.Bürgermeister J, Paper DH, Vogl H, et al. LaPSvS1, a (1–>3)-beta-galactan sulfate and its effect on angiogenesis in vivo and in vitro. Carbohydr Res. 2002;337:1459–66. doi: 10.1016/s0008-6215(02)00163-5. [DOI] [PubMed] [Google Scholar]
- 11.Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–61. [PubMed] [Google Scholar]
- 12.Carrasco OF, Ranero A, Hong E, Vidrio H. Endothelial function impairment in chronic venous insufficiency: effect of some cardiovascular protectant agents. Angiology. 60:2009–2010. 763–71. doi: 10.1177/0003319709332108. [DOI] [PubMed] [Google Scholar]
- 13.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–94. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 14.Hoeben A, Landuyt B, Highley MS, et al. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–80. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 15.Tongers J, Roncalli JG, Losordo DW. Therapeutic angiogenesis for critical limb ischemia: microvascular therapies coming of age. Circulation. 2008;118:9–16. doi: 10.1161/CIRCULATIONAHA.108.784371. [DOI] [PubMed] [Google Scholar]
- 16.Allain H, Ramelet AA, Polard E, Bentué-Ferrer D. Safety of calcium dobesilate in chronic venous disease, diabetic retinopathy and haemorrhoids. Drug Saf. 2004;27:649–60. doi: 10.2165/00002018-200427090-00003. [DOI] [PubMed] [Google Scholar]
- 17.De Courten A. Doxium in capillary dysfunction. OM Digest. 1979;1:1–7. [Google Scholar]
- 18.Lameynardie S, Chiavaroli C, Travo P, et al. Inhibition of choroidal angiogenesis by calcium dobesilate in normal Wistar and diabetic GK rats. Eur J Pharmacol. 2005;510:149–56. doi: 10.1016/j.ejphar.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Cuevas P, Arrazola JM. Therapeutic response of rosacea to dobesilate. Eur J Med Res. 2005;10:454–56. [PubMed] [Google Scholar]