Abstract
Purpose
To present and evaluate a new method of integrating risk factors into the analysis of rates of visual field progression in glaucoma.
Methods
The study included 352 eyes of 250 glaucoma patients followed up for an average of 8.1 ± 3.5 years. Slopes of change over time were evaluated by the mean deviation (MD) from standard automated perimetry. For each eye, the follow-up time was divided into two equal periods: the first half was used to obtain the slopes of change and the second period was used to test the predictions. Slopes of change were calculated with two methods: the conventional approach of ordinary least squares (OLS) linear regression and a Bayesian regression model incorporating information on risk factors and presence of progressive optic disc damage on stereophotographs. The mean square error (MSE) of the predictions was used to compare the predictive performance of the different methods.
Results
Higher mean IOP, thinner central corneal thickness (CCT), and presence of progressive optic disc damage were associated with faster rates of MD change. Incorporation of risk factor information into the calculation of individual slopes of MD change with the Bayesian method resulted in better prediction of future MD values than with the OLS method (MSE: 4.31 vs. 8.03, respectively; P < 0.001).
Conclusions
A Bayesian regression model incorporating structural and risk factor information into the estimation of glaucomatous visual field progression resulted in more accurate and precise estimates of slopes of functional change than the conventional method of OLS regression. (ClinicalTrials.gov number, NCT00221897.)
A Bayesian regression model incorporating structural and risk factor information into the estimation of glaucomatous visual field progression resulted in more accurate and precise estimates of rates of functional change compared to the conventional method of ordinary least squares regression.
Introduction
Standard automated perimetry (SAP) remains the most commonly used method for monitoring functional changes in glaucoma. In addition to detecting whether progression has occurred, the rate of SAP deterioration can be estimated, which is essential for defining optimal management for individual patients.1–6
Detection of visual field progression and estimation of rates of change is made difficult by measurement variability over time. Tasked with separating true change from noise, clinicians often incorporate information on the presence of concomitant structural change and risk factors into their clinical judgment in order to better assess whether progression has occurred and whether the estimated rate of change is reliable. Several risk factors have been identified as associated with glaucoma progression, such as older age, high intraocular pressure (IOP), thinner corneas, and presence of optic disc hemorrhages.5–14 In addition, progressive optic disc damage has been shown to be highly predictive of future development of standard visual field loss.14 Therefore, the presence of high IOP or progressive optic disc damage, for example, would increase the probability that a change detected in SAP is indeed real. On the other hand, a change in SAP in the presence of low IOP and absence of optic disc changes may have a higher probability of being just fluctuation over time, and not related to progression. Such clinical assessment is often done subjectively and the relationship between SAP changes, progressive optic disc damage, and risk factors, such as IOP, is not formally taken into account in the decision-making process.
The analysis of rates of visual field loss in glaucoma is usually performed by using the method of ordinary least squares (OLS) linear regression of SAP measurements over time.15–19 The true rate of change, however, is actually an unobservable variable, and the slope of change obtained from OLS is just an imprecise estimate that is confounded by noise and influenced by the number and intervals of measurements during follow-up.20 OLS estimates are obtained by taking into account only the measurements of an individual patient, without considering the influence of other existing information. Although other assembled data are rarely used to improve the accuracy of a patient's estimated rate of visual field loss, it could be argued that improved accuracy would be possible by incorporating information on risk factors and presence of concomitant structural damage. For example, it is reasonable to assume that the best estimator of the rate of visual field change in a patient for whom there are no SAP measurements collected over time is the average rate of change in the overall population where he or she comes from, that is, a population with a similar risk profile. As measurements are acquired for this patient, however, his or her rate of SAP change will most likely deviate from the population average. For patients with fewer measurements, the accuracy and precision of the estimates can be increased by “borrowing strength” from the population with similar risk factors, whereas for patients with large number of measurements, accurate and precise estimates can be obtained by relying almost only on the individual data, and the need to borrow strength from the population decreases.20
The incorporation of risk factors into the assessment of change as outlined above can be performed with Bayesian statistics. The authors have previously used Bayesian models to integrate structural and functional information for detection of glaucomatous progression,21,22 to improve estimation of rates of visual field loss,20,22 as well as for merging event- and trend-based methods for assessment of change.23 In the current study, Bayesian models were used to incorporate risk factors, in addition to structural information, into the assessment of rates of SAP change over time. It was demonstrated that these rates can more accurately predict future observations and show increased precision than do traditional OLS estimates.
Methods
This was an observational cohort study. Participants were included from a prospective longitudinal study designed to evaluate optic nerve structure and visual function in glaucoma (DIGS, Diagnostic Innovations in Glaucoma Study) conducted at the Hamilton Glaucoma Center, University of California, San Diego (UCSD). Participants in the DIGS study were longitudinally evaluated according to a pre-established protocol that included regular follow-up visits in which patients underwent clinical examination and several other imaging and functional tests. All the data were entered in a computer database. All participants from the DIGS study who met the inclusion criteria described below were enrolled in the current study. Informed consent was obtained from each participant. The University of California San Diego Human Subjects Committee approved all protocols and the methods described adhered to the tenets of the Declaration of Helsinki.
At each visit during follow-up, subjects underwent a comprehensive ophthalmologic examination including review of medical history, best-corrected visual acuity, slit-lamp biomicroscopy, IOP measurement, gonioscopy, dilated fundoscopic examination, stereoscopic optic disc photography, and automated perimetry using Swedish Interactive Threshold Algorithm (SITA Standard 24-2). All patients had central corneal thickness (CCT) measurements obtained by a trained technician using ultrasound pachymetry (Pachette GDH 500; DGH Technology, Inc., Philadelphia, PA). Only subjects with open angles on gonioscopy were included. Subjects were excluded if they presented best-corrected visual acuity less than 20/40, spherical refraction outside ±5.0 diopters (D), and/or cylinder correction outside 3.0 D, or any other ocular or systemic disease that could affect the optic nerve or the visual field.
The study included 352 eyes from 250 patients diagnosed with glaucoma, as determined on the baseline visit. Eyes were classified as glaucomatous if they had repeatable (two consecutive) abnormal visual field test results on the baseline visits and/or a glaucomatous appearing optic disc by masked stereophotograph assessment. An abnormal visual field was defined as a pattern standard deviation (PSD) outside of the 95% normal confidence limits, or a Glaucoma Hemifield Test result outside normal limits. Signs of glaucomatous damage to the optic nerve were considered diffuse or localized neuroretinal rim loss, excavation, and retinal nerve fiber layer (RNFL) defects. During follow-up, patients were managed at the discretion of the treating ophthalmologist.
Standard Automated Perimetry
All visual fields were evaluated by the UCSD Visual Field Assessment Center (VisFACT).24 Visual fields with more than 33% fixation losses or false-negative errors, or more than 15% false-positive errors were excluded. The only exception was the inclusion of visual fields with false-negative errors of more than 33% when the field showed advanced disease. Visual fields exhibiting a learning effect were also excluded. Visual fields were further reviewed for the following artifacts: lid and rim artifacts, fatigue effects, inappropriate fixation, evidence that the visual field results were due to a disease other than glaucoma (such as homonymous hemianopia), and inattention. The VisFACT requested repeats of unreliable visual field test results, and these were obtained whenever possible.
Evaluation of rates of visual field change during follow-up was performed by using the mean deviation (MD) global index provided by the Humphrey perimeter (Carl-Zeiss Meditec, Inc., Dublin, CA).
Stereophotograph Grading
To detect progressive optic disc damage, pairs of simultaneous stereoscopic optic disc photographs (TRC-SS; Topcon Instrument Corp. of America, Paramus, NJ) obtained over time were reviewed with a stereoscopic viewer (Asahi Pentax Stereo Viewer II; Asahi Optical Co., Tokyo, Japan). Definition of change was based on focal or diffuse thinning of the neuroretinal rim, increased excavation, and appearance or enlargement of RNFL defects. Evidence of progression was based on masked (patient name, diagnosis, temporal order of photographs) comparison between the baseline and follow-up photographs, by two observers. If these observers disagreed, a third observer served as an adjudicator. Progression was graded as a yes/no variable.
The presence of optic disc hemorrhage during follow-up was also evaluated by masked assessment of optic disc stereophotographs. Disc hemorrhages had to be located within 1 disc diameter from the optic disc border and not associated with optic disc edema, papillitis, diabetic retinopathy, central or branch retinal vein occlusion, or any other retinal disease.
Data Analysis
A minimum of 5 SAP tests during a minimum of 2 years of follow-up was required. Average follow-up time was 8.1 ± 3.5 years. Slopes of MD change were calculated with two methods: the conventional approach of OLS linear regression and a Bayesian regression model (see model details below). The assessment of rates of change with the Bayesian model incorporated information on the presence of progressive optic disc damage on stereophotographs and values of risk factors.
To compare the OLS and Bayesian linear regression models, their ability to accurately predict future observations was evaluated. For each eye, the follow-up time was divided into two equal periods: the first half was used to obtain the slopes of change and the second period was used to test the predictions. That is, rates of change were obtained with only the tests available during the first half of follow-up. Subsequently, these rates of change were used to predict observations for individual eyes during the second half of the follow-up period. For the Bayesian method, the information on the presence of progressive optic disc damage and risk factors was also used only during the first follow-up period.
For each method (OLS versus Bayesian), the residual difference, or error, between the actual value and the predicted value was calculated for each observation available during the second half of the follow-up period. The mean square error (MSE) of the predictions was used to evaluate and compare the predictive performance of the different methods.
The calculation of slopes of change with Bayesian analysis proceeded in two steps. In step 1, the risk factors associated with rates of visual field progression were identified, and in step 2, the structural and risk factor information was incorporated into the assessment of rates of MD change for individual eyes.
Step 1: Identification of Risk Factors Associated with Visual Field Progression. To identify and quantify the factors associated with visual field progression, a regression model was initially built that evaluated the relationship between rates of visual field loss and putative predictive factors. The potential risk factors evaluated were age at baseline, mean IOP, CCT, and presence of optic disc hemorrhages. The relationship between progressive optic disc damage detected on stereophotographs and rates of visual field progression was also evaluated.
After the information on risk/predictive factors was obtained, the coefficients associated with these factors were subsequently used in a Bayesian regression model to influence the estimates of slopes of visual field change on individual eyes. It is important to note that estimates of model parameters were obtained with information from n−1 eyes and applied to obtain the estimates of slopes of change in the nth eye.
Step 2: Bayesian Regression Model. For the Bayesian analysis of trend in the visual fields over time, a random-intercept random-slope Bayesian hierarchical model was fitted for the MD data. In these models, the average evolution of MD values was described using a linear function of time, and eye-specific deviations from this average evolution were introduced by random intercepts and random slopes, allowing for different baseline values and different rates of change for each eye.25–27 In our application, the Bayesian models incorporated results from risk factors and presence of optic disc progression in the prior distribution for the slopes, which allowed them to influence the MD slopes.
The model can be written as:
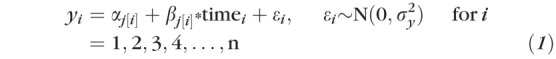 |
where yi represents the MD data and the αjs and βjs represent the intercepts and slopes for each one of the j eyes, respectively. Each eye had nj visual field tests over time. Equation (1) is called the data model or the likelihood. The αjs and βjs were modeled by using a bivariate normal distribution, that is, in Bayesian inference, the bivariate distribution was used as the “prior” distribution for the pairs of slopes and intercepts. To allow predictive factors to influence inferences made on MD slopes, a group-level regression on the slopes was added. This can be written as:
Equation (2) is called the prior model, where ukj represents the value of the kth predictive factor on the jth eye. By doing that, different prior distributions are permitted for the slope of MD change for each eye. For any particular eye, its slope βj has a prior distribution with mean 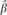 j = γ0 + γkukj. The γks represent the coefficients associated with each one of the kth predictive factors and were obtained from step 1.
j = γ0 + γkukj. The γks represent the coefficients associated with each one of the kth predictive factors and were obtained from step 1.
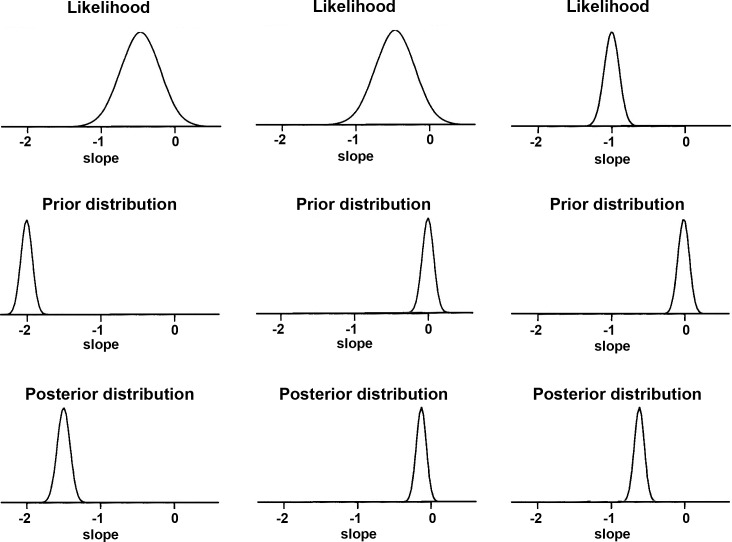
Inferences on the MD slopes were made by analyzing their posterior distributions. In Bayesian inference, the likelihood is multiplied by the prior distribution, and inferences are summarized by random draws from this product, the posterior distribution (Fig. 1).28 For each eye j, the likelihood for the MD slope indicates the range of values of βj that are most consistent with the data available for that eye. The likelihood is more informative as the sample size increases, that is, the larger the number of MD measurements available for a particular eye, the more informative the likelihood will be. The prior distribution conveys information about the distribution of the MD slopes (βjs) among the eyes in the population with similar values on the predictive factors. The posterior distribution is centered at a point between the maximum likelihood estimate and the maximum of the prior distribution—a weighted average of the likelihood and prior estimates—falling closer to the prior when the number of measurements per eye is small or have a large variance, and closer to the likelihood when the number of measurements is large or there is a small variance. Therefore, the influence of the prior is greater when there is less information to estimate the slope, based on the fact that only a few MD measurements are available over time or there is large variability. When there are many measurements available over time for a particular eye, its slope of change can usually be estimated with great precision and, therefore, the prior exerts less influence.
Figure 1.
Illustration of the influence of prior information on the estimation of the posterior distribution. In the leftmost and middle columns, the posterior distribution suffers great influence from the prior, owing to the weakly informative likelihood (i.e., wide likelihood distribution). In contrast, in the rightmost column, the posterior distribution has little influence from the prior owing to the relatively stronger likelihood.
Hierarchical models can also easily handle the correlation structure arising from data coming from both eyes of the same patient.29 However, fitting a more complex model with eyes nested within patient did not provide any overall improvement in our model and, therefore, only the results of the simpler model were reported here.
Estimates of the posterior distributions of the parameters of interest, that is, the random effects, were obtained by Markov chain Monte Carlo (MCMC) procedures. The MCMC sampler was implemented in WinBUGS software.30 Ten thousand iterations were used after discarding the initial 5000 iterations for burn-in. Convergence of the generated samples was assessed by standard tools in WinBUGS (trace plots, autocorrelation function plots) as well as Gelman-Rubin convergence diagnostics. After the posterior distributions were estimated, summary measures were calculated, such as mean and credible intervals. For the Bayesian MD slope, it was considered that progression had occurred if the upper limit of the 95% credible interval for the slope was less than zero.
Results
The study included 352 eyes of 250 participants with a mean ± SD age of 62 ± 11 years at baseline. One hundred and thirty-eight (55%) patients were female. One-hundred eighty-five (53%) eyes had glaucomatous visual field loss at baseline. For these eyes, median (first quartile, third quartile) MD and PSD of the visual field closest to the baseline imaging test date were −3.25 dB (−1.93 dB, −5.23 dB) and 3.68 dB (2.46 dB, 6.31 dB), respectively. There was a large variation in baseline MD in the 352 eyes included in the study, with values ranging from −20.1 dB to 1.81 dB.
Analysis of Risk Factors Associated with the Rate of Visual Field Loss
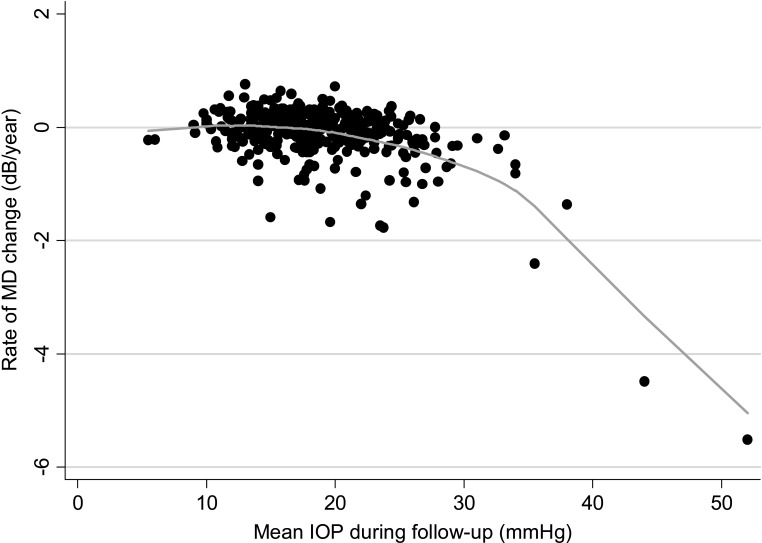
Table 1 shows the relationship between each investigated predictive factor and the rates of glaucomatous visual field loss. Coefficients shown in Table 1 are from the final multivariable model obtained by using all data available during the first follow-up period from all eyes. Eighty-five of the 352 (24%) eyes had progressive optic disc damage during follow-up. As expected, these eyes had, on average, −0.35 dB/year faster rate of MD change than those without evidence of progression on disc photographs. The effect of optic disc progression on rates of visual field loss was influenced by the duration of follow-up until progressive disc change was detected, as indicated by the interaction term optic disc progression × follow-up time in Table 1. The shorter the duration of follow-up until detection of optic disc change, the faster was the associated rate of glaucomatous visual field loss. Higher mean intraocular pressure during follow-up was also associated with faster visual field progression; however, the effect of IOP showed a quadratic relationship with rates of field loss as shown in Figure 2. For values of mean IOP above approximately 30 mm Hg, visual field losses accelerated at a faster rate than below this number. Thin corneas were also associated with faster rates of MD change, with a 0.21 dB/year faster rate for each 100 μm thinner cornea.
Table 1.
Results of the Multivariable Model Investigating the Relationship between Rates of Visual Field Loss and Putative Predictive Factors for Progression
|
Predictive Factor |
Coefficient |
Estimate |
95% CI |
P |
| Mean IOP* | γ1 | −0.028 | −0.045 to −0.011 | <0.001 |
| (Mean IOP)∧2 | γ2 | −0.0035 | −0.005 to −0.002 | <0.001 |
| CCT,† per 100 μm | γ3 | 0.21 | 0.04 to 0.38 | 0.015 |
| Optic disc progression‡ | γ4 | −0.35 | −0.51 to −0.19 | <0.001 |
| Optic disc progression × follow-up time§ | γ5 | 0.083 | 0.029 to 0.136 | 0.003 |
| Constant | γ0 | 0.08 | −0.001 to 0.162 | 0.054 |
CI, confidence interval.
IOP values were centered on their mean value in the population (19 mm Hg).
CCT measurements were centered on their mean value in the population (543 μm).
Optic disc progression was a categorical variable with value 1 if there was evidence of optic disc progression on stereophotographs and zero otherwise.
Follow-up time corresponds to the follow-up until first detection of optic disc progression.
Figure 2.
Scatterplot illustrating the relationship between rates of MD change over time and mean intraocular pressure. A locally weighted scatterplot smoothing (LOWESS) curve is fitted to the plot.
Sixty-seven of the 352 eyes (19%) had optic disc hemorrhages during follow-up time. These eyes had on average a 0.23 dB/year faster rate of MD change over time than those without disc hemorrhage (P = 0.042). However, when included along with evidence of progressive optic disc damage on stereophotographs, in a multivariable analysis, optic disc hemorrhages lost their significance (P = 0.788). Age at baseline did not significantly influence rates of progressive visual field loss in the studied population (P = 0.239). Owing to their lack of significance in the multivariable model, both age and optic disc hemorrhages were not included as factors influencing the estimation of individual slopes of change in the Bayesian method.
Comparison of Bayesian Versus OLS Methods for Estimation of Slopes of MD Change
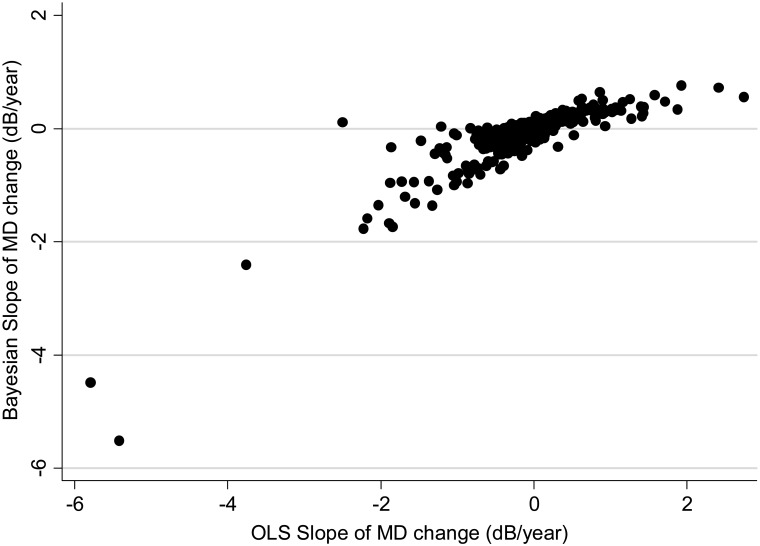
A median number of 6 visual fields (range: 4–23) was available during the first period of follow-up and used for calculation of the slopes of MD change over time. For calculation of Bayesian slopes, the model parameters were obtained from information from n−1 eyes and applied to obtain the estimate of slope of change in the nth eye. Figure 3 shows a scatterplot of slopes of MD change obtained by the Bayesian and OLS methods.
Figure 3.
Scatterplot illustrating the relationship between slopes of MD change obtained by the Bayesian regression model and by OLS linear regression.
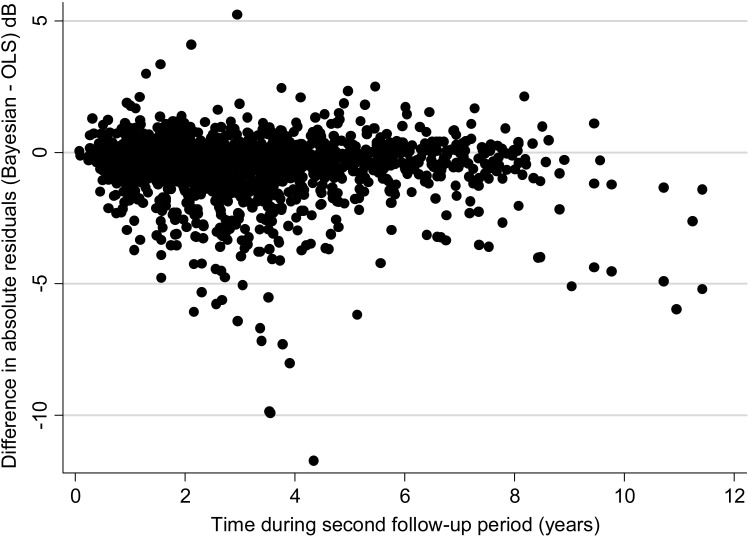
A total of 1846 visual fields were available to evaluate the predictions of the Bayesian and OLS models, with a median number of 5 visual fields per eye. For the OLS method, the average difference between actual minus predicted MD value was 0.14 dB (median: 0.01 dB; first quartile: −1.31 dB; third quartile: 1.37 dB). The mean square error of the OLS predictions was 8.03 (95% CI: 7.02–9.05). For the Bayesian method, the average difference between actual minus predicted MD value was 0.06 dB (median: 0.02 dB; first quartile: −1.03 dB; third quartile: 1.02 dB). The mean square error of the Bayesian predictions was 4.31 (95% CI: 3.75–4.86). The mean square error of the predictions was significantly lower for the Bayesian than the OLS model (P < 0.001). Figure 4 shows a scatterplot of the difference between the absolute residuals for each method versus time. In this plot, the initial time in the horizontal axis was set to zero at the first visual field available to check predictions. The plot shows that residuals for OLS estimates tended to be larger than those for the Bayesian model, that is, predictions from OLS regression were in general worse than those based on the Bayesian model.
Figure 4.
Relationship between differences in absolute values of MD residuals for Bayesian and OLS predictions and time. Negative values indicate that residuals for the OLS method were greater than those from the Bayesian method.
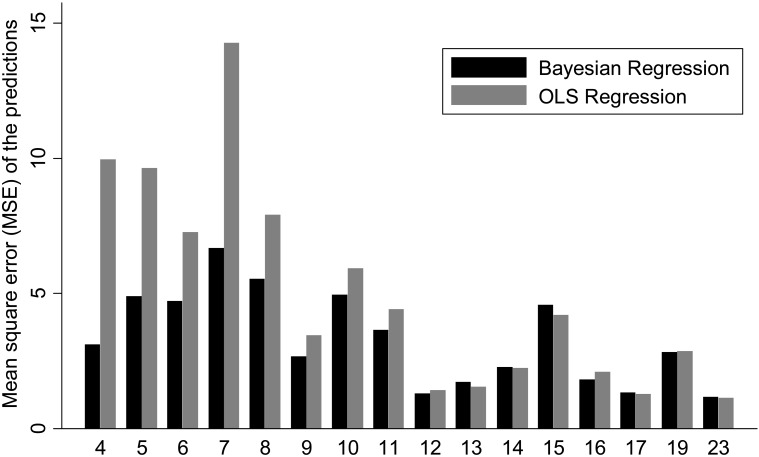
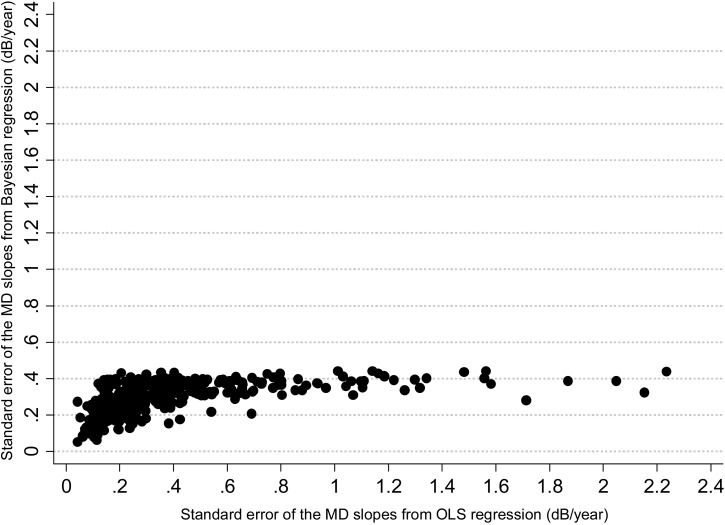
Figure 5 shows the relationship between the mean square error of the predictions for each method versus the number of visual fields available for calculation of the slopes of MD change. While there is a decrease in the difference between the two methods with an increase in the number of visual fields, the Bayesian method was in general superior or at least as good as the OLS method even when a large number of visual fields were used for calculating the slopes. In addition, the precision of the MD slopes was also evaluated by calculation with the Bayesian and OLS methods. The average standard error of the MD slopes calculated with the Bayesian method was significantly lower than that obtained by the OLS method (0.29 dB vs. 0.40 dB, respectively; P < 0.001), indicating that Bayesian slopes were in general more precise than those obtained by OLS regression. Figure 6 shows a scatterplot of the standard errors of the MD slopes obtained by the Bayesian and OLS methods.
Figure 5.
Bar plot illustrating the differences in mean square error of the predictions of MD between the Bayesian and OLS methods. The horizontal axis shows the number of visual field tests used for calculating the slopes of change.
Figure 6.
Scatterplot of the relationship between standard errors of the slopes of MD change over time, obtained by the Bayesian and OLS regression methods.
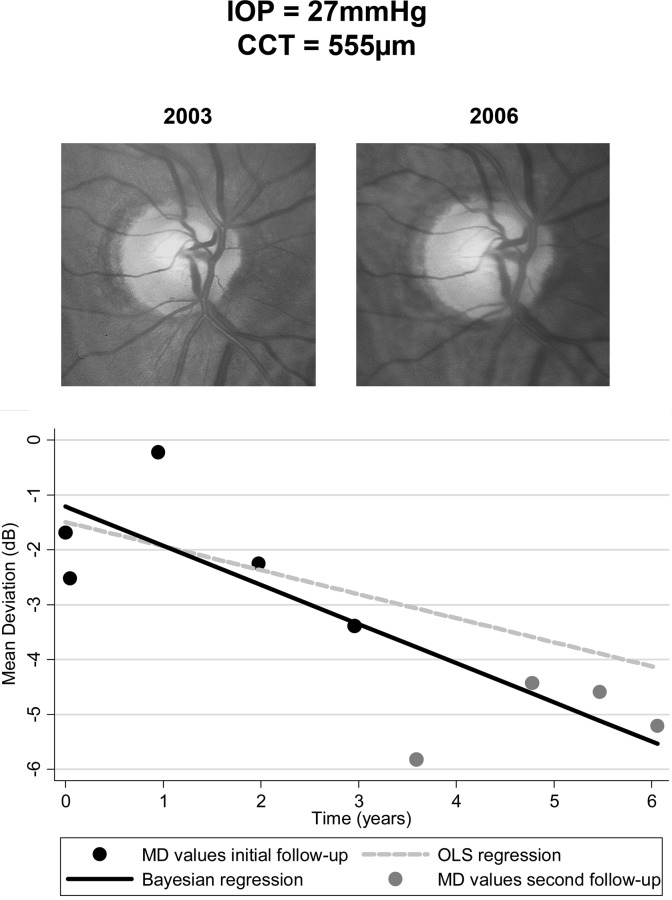
Bayesian slopes of MD change showed statistically significant negative slopes in 43 of the 85 eyes (51%) with progressive optic disc damage, compared to only 28 (33%) by the OLS method. Statistically significant positive slopes of change were found in 6 of the 352 eyes (1.7%) with the Bayesian method and 12 of the 352 eyes (3.4%) with the OLS method. Figure 7 shows an example of an eye with progressive optic disc damage during follow-up and the slopes of change calculated by the Bayesian and OLS methods.
Figure 7.
Example illustrating differences in calculated slopes of change with the Bayesian versus OLS regression methods. The eye had mean intraocular pressure of 27 mm Hg and evidence of progressive optic disc damage during the first follow-up period (5 initial visual fields). Central corneal thickness was 555 μm. Slopes of change were calculated with the Bayesian (black line) and OLS (gray dashed line) methods by using data from the first follow-up period and extrapolated to the second follow-up period. Predictions from the Bayesian model were closer to the observed MD values (gray dots) during the second follow-up period than those obtained from OLS regression.
Discussion
In the current study, a methodology was proposed for the integration of structural and risk factor information to improve the assessment of progression and rates of visual field loss in glaucoma. Our approach resulted in more precise estimates of slopes of functional change, which were also able to more accurately predict future visual field observations. To our knowledge, this is the first study to report a successful method for integrating risk factor information into the assessment of glaucomatous progression with Bayesian statistics.
An initial evaluation was performed to determine which risk factors were associated with rates of glaucomatous visual field loss in order to subsequently use the information on these factors to improve estimates of rates of change. The results confirmed previous published findings showing that high IOP, thinner CCT, and presence of optic disc hemorrhages are significantly associated with increased risk of progression.5–13 In addition, presence of progressive optic disc damage was also found to be associated with faster rates of visual field loss. This is in agreement with a report by Medeiros et al.14 showing that progressive optic disc damage is highly associated with development of future visual field loss with a hazard ratio of 25.8. This is an expected result as glaucoma is a progressive optic neuropathy and the associated visual field damage is essentially the result of retinal ganglion cell loss. In that sense, progressive optic disc damage should not be strictly considered a risk factor for the disease, as it is actually part of its definition. However, the use of information on the presence of progressive optic disc damage may help improve inferences derived from functional measurements, as shown in the current study. In contrast to some other previous studies, older age was not found to significantly influence rates of visual field loss. The relationship between age and glaucoma progression is likely complex. Although previous reports have shown that age is associated with increased risk of progression, as measured by event-based assessments, the association between age and rates of change has not been clarified.
After obtaining the coefficients associated with the effect of the different risk/predictive factors on the rates of change, this information was incorporated into the Bayesian model in order to improve inferences on the slopes of functional change. It is important to note that a leave-one-out approach was used in order to prevent model overfitting, that is, the model coefficients were obtained from n−1 eyes and applied to the estimation of the slope of the nth eye. In addition, the information on risk factors was collected only during the first half of the follow-up period and used to influence calculation of slopes obtained from MD measurements available at the same interval of time. The estimated slopes were then used to predict future observations collected during the second half of the follow-up period. Our results showed that Bayesian slopes performed significantly better than slopes calculated from the OLS method to predict future observations. Predictions obtained by extrapolating slopes of change calculated from the Bayesian model were closer to the actual MD values than those obtained by extrapolating OLS regression lines, as shown by differences in the mean square error of the predictions.
The use of Bayesian statistics helps improve the estimates of slopes of change by incorporating “prior” information that contributes to their final estimation. In our methodology, the prior is composed of a multivariate distribution relating slopes and intercepts of functional change and influenced by the values of risk factors and presence of progressive optic disc damage. For example, an eye that had mean IOP of 30 mm Hg during follow-up and evidence of progressive optic disc damage will have a prior distribution that corresponds to the expected distribution of slopes of visual field loss of eyes with the same characteristics. Such distribution will obviously be concentrated towards negative values, indicating that these eyes are in general expected to have relatively fast rates of progression. In contrast, an eye that had mean IOP of 15 mm Hg and no evidence of progressive optic disc damage during follow-up will have a prior distribution that will be concentrated towards values close to zero, indicating that on average these eyes are not expected to have much visual field change over time. The prior information will exert influence into the calculation of the final slope, but the amount of influence will depend on the degree of information that can be derived solely from the visual field measurements of the eye under consideration. If the visual fields show large variability, or only a few measurements are available over time, then the slope of change would be poorly estimated, using only the visual field data for that particular eye. In this situation, the prior will exert great influence on the estimation of the final slope of change. This is a very desirable property as it will allow better evaluation of progression in those who have large visual field variability over time, a common situation in clinical practice. A patient with high IOP and evidence of progressive disc damage may still be declared as progressing and have a fast estimated rate of change despite having relatively little information that can be derived from visual field data only. On the other hand, if a large amount of reliable visual field information is available in the eye under consideration, then its slope of change can be calculated with great precision and the prior will exert little influence on the final calculation. Again, this is also a desirable property. An eye that has sufficient visual field information to be declared as nonprogressor should not be deemed as progressing just because of the presence of risk factors. Similarly, an eye with significant evidence of progression based only on visual field data should still be declared as progressing despite the absence of apparent risk factors for progression.
Besides better accuracy, slopes of change obtained by Bayesian regression had also greater precision. The precision is a measure of how closely an estimator is expected to be to the true value of the parameter and can be measured by the standard error or confidence interval of the slopes. Large imprecision, that is, large standard errors, will confound the interpretation of the clinical relevance of an estimated slope, as there will be great uncertainty about where the true slope of change is likely to be. In the presence of imprecise slopes, more tests will increase the precision of the estimate. Although an improvement in the predictions and standard error of the slopes was seen for both the Bayesian and OLS methods with an increase in the number of visual fields used for the calculation of the slopes, Bayesian predictions still outperformed OLS predictions for large number of tests, as indicated by Figure 5. It is known that as the number of measurements increases, the OLS estimate approaches the true underlying latent variable. However, in clinical practice, there is a cost associated with obtaining more measurements over time, including the expense of the test itself, the cost in patient time, and the cost related to delaying detection of change. By providing more precise estimates of rates of change with fewer tests, the Bayesian approach potentially allows more confident clinical decision-making to be made earlier with regard to the clinical and statistical significance of the calculated slopes. It is important to emphasize, however, that when assessing the clinical relevance of an estimated slope, clinicians also need to consider other factors, such as life expectancy and the patient's expectations with regard to treatment.
In previous studies, the authors used Bayesian statistics to incorporate longitudinal information on optic disc topography and retinal nerve fiber layer change obtained from imaging devices into the assessment of rates of visual field loss.21,22 A better agreement was shown between rates of structural and functional change estimated with Bayesian regression than with the OLS method. In the current investigation, it was also shown that Bayesian slopes of change detected a significantly higher proportion of eyes, which showed progressive optic disc damage based on stereophotographs. The evaluation of the accuracy of any new method for detection of glaucoma progression is hampered by the inexistence of a perfect reference standard for progression. However, our results suggest a higher sensitivity of the Bayesian method than OLS because it detected more cases of progressive optic disc damage. Additionally, the smaller number of eyes with significant positive slopes, as compared to the OLS method, also suggests that the Bayesian method had a higher specificity for detection of progression. This conclusion is supported by the demonstration that Bayesian slopes were also better predictors of future observations.
Our proposed model provides individualized estimates of rates of change, which could potentially be used in clinical practice for individual management. The estimation of parameters of the model depends on previously acquired data in order to evaluate the relationship between rates of structural and functional change and the impact of risk factors in glaucoma. Accurate assessment of these relationships is essential in order to define how risk factors and structural information will influence rates of functional change. However, once this information is available, the model can easily be used to estimate individualized slopes by taking into account risk factors and the results of structural and functional tests performed in the eye under evaluation. Construction of built-in databases would be required to implement this methodology in currently available instruments, in a similar approach to the built-in progression packages available for some instruments. Another potential advantage of our methodology is that data from patients tested over time could be continuously incorporated into the Bayes model, leading to improved estimates that would more likely reflect the progression rates in a particular clinical setting, generating a “customized” database for comparison.
This study had limitations. A linear rate of functional change over time was assumed. Previous studies using cross-sectional data, however, suggest that functional changes over the whole course of the disease would probably not be linear.31–35 In fact, the use of decibel scale may ultimately underestimate the rate of retinal ganglion cell loss in early stages of the disease.36 However, extensions of our methodology to incorporate nonlinear change or change assessed by other metrics are also possible. Changes related only to the parameter MD were also evaluated. It is known that change in MD values can be influenced by media opacities, such as development of cataract over time. However, such effect would affect calculation of the slopes with both the Bayesian and OLS methods and would likely not interfere with the comparison between the two methods. The MD is also a global parameter that may not fully capture localized changes in the visual field. However, extension of our method for evaluation of changes in sectoral parameters should be straightforward and will be the subject of future investigations.
In conclusion, a Bayesian regression model incorporating structural and risk factor information into the estimation of glaucomatous visual field progression resulted in more accurate and precise estimates of slopes of functional change than the conventional method of OLS regression. The proposed strategy may result in better assessment of the global risk for development of functional impairment in individual patients.
Footnotes
Supported in part by National Institutes of Health/National Eye Institute grants EY021818 (FAM), EY11008 (LMZ), EY14267 (LMZ); grant from Research to Prevent Blindness; grant from Velux Foundation, Zurich, Switzerland (KM); grant from CAPES BEX 1066/11-0 (RL); and grants for participants' glaucoma medications from Alcon, Allergan, Pfizer, Merck, and Santen.
Disclosure: F.A. Medeiros, Carl-Zeiss Meditec (F); L.M. Zangwill, Carl-Zeiss Meditec (F); K. Mansouri, None; R. Lisboa, None; A. Tafreshi, None; R.N. Weinreb, Carl-Zeiss Meditec (C)
References
- 1. Anderson DR, Drance SM, Schulzer M. Natural history of normal-tension glaucoma. Ophthalmology. 2001;108:247–253. [DOI] [PubMed] [Google Scholar]
- 2. Heijl A, Bengtsson B, Hyman L, Leske MC. Natural history of open-angle glaucoma. Ophthalmology. 2009;116:2271–2276. [DOI] [PubMed] [Google Scholar]
- 3. Medeiros FA, Zangwill LM, Alencar LM, et al. Rates of progressive retinal nerve fiber layer loss in glaucoma measured by scanning laser polarimetry. Am J Ophthalmol. 2010;149:908–915. [DOI] [PubMed] [Google Scholar]
- 4. Medeiros FA, Susanna R, Jr, Singh K. Who should be treated? In: Weinreb RN, Liebmann J. eds Medical Treatment of Glaucoma. The Hague, The Netherlands: Kugler Publications; 2010:1–15. [Google Scholar]
- 5. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. [DOI] [PubMed] [Google Scholar]
- 6. Boland MV, Quigley HA. Risk factors and open-angle glaucoma: classification and application. J Glaucoma. 2007;16:406–418. [DOI] [PubMed] [Google Scholar]
- 7. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration: the AGIS Investigators. Am J Ophthalmol. 2000;130:429–440. [DOI] [PubMed] [Google Scholar]
- 8. Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma [discussion in Arch Ophthalmol. 2002;120:829–830]. Arch Ophthalmol. 2002;120: 714–720. [DOI] [PubMed] [Google Scholar]
- 9. Miglior S, Pfeiffer N, Torri V, et al. Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology. 2007;114:3–9. [DOI] [PubMed] [Google Scholar]
- 10. Medeiros FA, Sample PA, Zangwill LM, et al. Corneal thickness as a risk factor for visual field loss in patients with preperimetric glaucomatous optic neuropathy. Am J Ophthalmol. 2003;136:805–813. [DOI] [PubMed] [Google Scholar]
- 11. Leske MC, Heijl A, Hyman L, et al. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972. [DOI] [PubMed] [Google Scholar]
- 12. Medeiros FA, Alencar LM, Zangwill LM, et al. The relationship between intraocular pressure and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology. 2009;116:1125–1133.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Medeiros FA, Weinreb RN, Sample PA, et al. Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol. 2005;123:1351–1360. [DOI] [PubMed] [Google Scholar]
- 14. Medeiros FA, Alencar LM, Zangwill LM, et al. Prediction of functional loss in glaucoma from progressive optic disc damage. Arch Ophthalmol. 2009;127:1250–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bengtsson B, Heijl A. A visual field index for calculation of glaucoma rate of progression. Am J Ophthalmol. 2008;145:343–353. [DOI] [PubMed] [Google Scholar]
- 16. Wild JM, Hutchings N, Hussey MK, et al. Pointwise univariate linear regression of perimetric sensitivity against follow-up time in glaucoma. Ophthalmology. 1997;104:808–815. [DOI] [PubMed] [Google Scholar]
- 17. Viswanathan AC, Fitzke FW, Hitchings RA. Early detection of visual field progression in glaucoma: a comparison of PROGRESSOR and STATPAC 2. Br J Ophthalmol. 1997;81:1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nouri-Mahdavi K, Hoffman D, Ralli M, Caprioli J. Comparison of methods to predict visual field progression in glaucoma. Arch Ophthalmol. 2007;125:1176–1181. [DOI] [PubMed] [Google Scholar]
- 19. Wollstein G, Schuman JS, Price LL, et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol. 2005;123:464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Medeiros FA, Zangwill LM, Weinreb RN. Improved prediction of rates of visual field loss in glaucoma using empirical Bayes estimates of slopes of change. J Glaucoma. 2012;21:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Medeiros FA, Leite MT, Zangwill LM, Weinreb RN. Combining structural and functional measurements to improve detection of glaucoma progression using bayesian hierarchical models. Invest Ophthalmol Vis Sci. 2011;52:5794–5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Medeiros FA, Zangwill LM, Girkin C, et al. Combining structural and functional measurements to improve estimates of rates of glaucomatous progression. Am J Ophthalmol. 2012. February 6 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Medeiros FA, Weinreb RN, Moore G, et al. Integrating event- and trend-based analyses to improve detection of glaucomatous visual field progression. Ophthalmology. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Racette L, Liebmann JM, Girkin CA, et al. African Descent and Glaucoma Evaluation Study (ADAGES) III: ancestry differences in visual function in healthy eyes. Arch Ophthalmol. 2010;128:551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 26. Goldstein H, Browne W, Rasbash J. Multilevel modelling of medical data. Stat Med. 2002;21:3291–3315. [DOI] [PubMed] [Google Scholar]
- 27. Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16:2349–2380. [DOI] [PubMed] [Google Scholar]
- 28. Berry DA. Statistics: A Bayesian Perspective. Belmont, CA: Duxbury Press; 1995. [Google Scholar]
- 29. Medeiros FA, Alencar LM, Zangwill LM, et al. Detection of progressive retinal nerve fiber layer loss in glaucoma using scanning laser polarimetry with variable corneal compensation. Invest Ophthalmol Vis Sci. 2009;50:1675–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–337. [Google Scholar]
- 31. Caprioli J, Miller JM. Correlation of structure and function in glaucoma: quantitative measurements of disc and field. Ophthalmology. 1988;95:723–727. [DOI] [PubMed] [Google Scholar]
- 32. Reus NJ, Lemij HG. Relationships between standard automated perimetry, HRT confocal scanning laser ophthalmoscopy, and GDx VCC scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2005;46:4182–4188. [DOI] [PubMed] [Google Scholar]
- 33. Schlottmann PG, De Cilla S, Greenfield DS, et al. Relationship between visual field sensitivity and retinal nerve fiber layer thickness as measured by scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2004;45:1823–1829. [DOI] [PubMed] [Google Scholar]
- 34. Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26:688–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garway-Heath DF, Holder GE, Fitzke FW, Hitchings RA. Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Invest Ophthalmol Vis Sci. 2002;43:2213–2220. [PubMed] [Google Scholar]
- 36. Medeiros FA, Lisboa R, Weinreb RN, et al. A combined index of structure and function for staging glaucomatous damage. Arch Ophthalmol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]