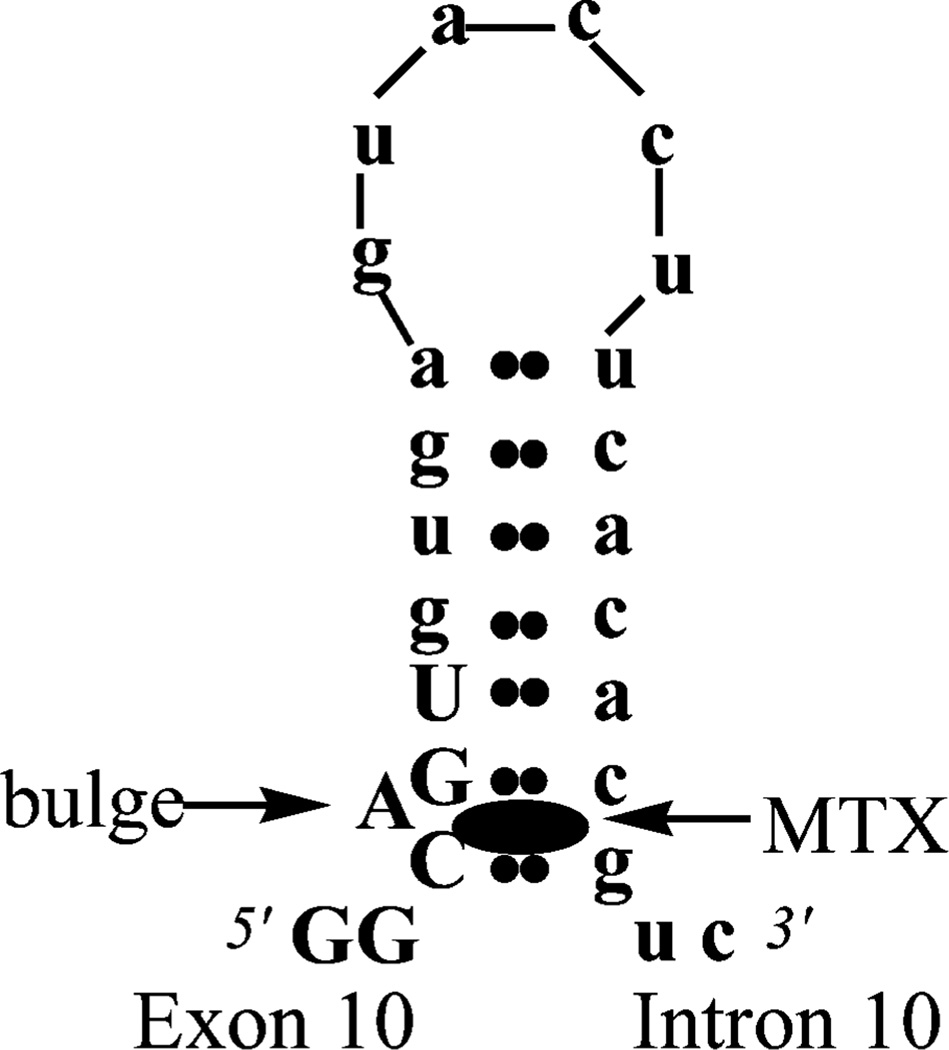Abstract
A series of mitoxantrone (MTX) analogues have been designed, synthesized, and evaluated for binding to and stabilizing a stem–loop structure that serves as a splicing regulatory element in the pre-mRNA of tau, which is involved in Alzheimer’s and other neurodegenerative diseases. Several compounds showed significantly improved binding activity relative to the original screening hit mitoxantrone. These findings establish essential structure–activity relationships to further optimize the activity of this promising class of compounds.
Neuronal filaments of the microtubule-associated protein tau are pathological features in Alzheimer’s and other neurodegenerative diseases, and evidence supports a role for aberrant tau in pathogenesis.1,2 Dominant mutations in the tau gene are associated with familial frontotemporal dementia displaying typical tau pathology.3 Many of these mutations are silent or intronic, occur near the exon 10-intron 10 border of the tau gene, and increase the inclusion of exon 10 during pre-mRNA splicing to increase the proportion of tau protein containing four microtubule-binding domain repeats (4R tau) to that containing three repeats (3R tau). These mutations were originally postulated to destabilize a stem–loop structure at this exon–intron boundary to regulate exon 10 splicing (Figure 1).4,5 Our laboratory previously validated this stem–loop as a bona fide structure that can regulate the alternative splicing of exon 10 in cells.6 Therefore, the tau stem–loop could represent a therapeutic target for treating “tauopathies” such as frontotemporal dementia and Alzheimer’s disease: small molecules that could bind to and stabilize the tau pre-mRNA stem–loop would have the opposite effect of disease-causing tau mutations.
Figure 1.
The tau stem–loop and site of MTX binding.
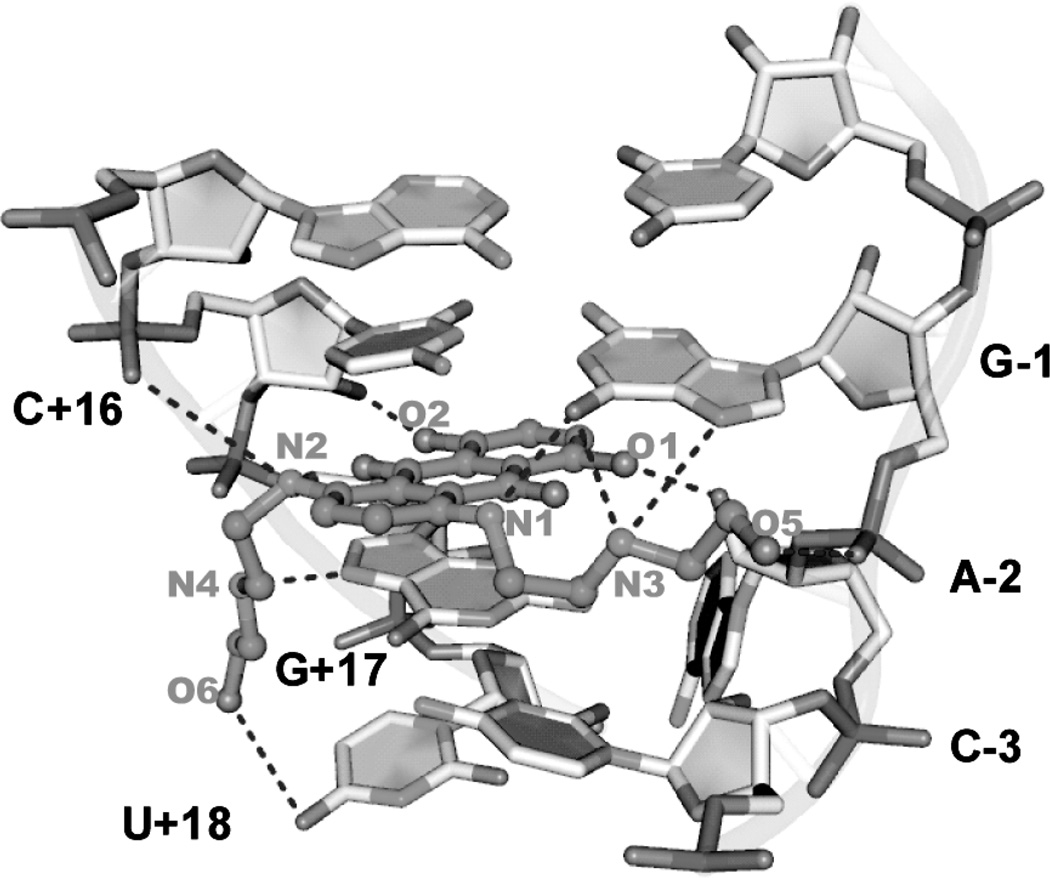
Via high-throughput screening, our laboratory recently identified the anticancer drug mitoxantrone as a small molecule that binds to and stabilizes the tau stem–loop (EC50 = 0.71 µM).7 Aminoglycosides such as neomycin are known to bind to duplex RNA and to the tau splicing regulatory element,8,9 and MTX could effectively compete with fluorescence-labeled aminoglycosides for binding to an oligonucleotide representing the stem–loop RNA. Moreover, MTX substantially increases the melting temperature of the stem–loop RNA, demonstrating its stabilizing effect upon binding. Most recently, we reported the structure of MTX bound to the tau stem–loop as determined by NMR and found that MTX interacts at the base of the stem (Figure 1), intercalating between two G–C base pairs that flank an unpaired (“bulged”) adenosine (Figure 2).10 (Previous biophysical studies have demonstrated that MTX and analogues can also intercalate DNA.11,12) The structure also suggested an important role for hydrogen bonding and electrostatic interactions between the side chains and the major groove. Herein, we report MTX analogues that are varied in the aromatic core and the number and type of side chains to better understand the nature of the interaction of MTX with the tau stem–loop, comparing the findings with expectations based on the NMR structure of the complex. These results provide initial structure–activity relationships to direct the further design of this promising class of ligands.
Figure 2.
Interaction between MTX and the tau stem–loop structure as deduced by NMR.10 MTX intercalates between two G–C base pairs that flank the unpaired (“bulged”) A-2. The carbon atoms of MTX are shown in light-blue.
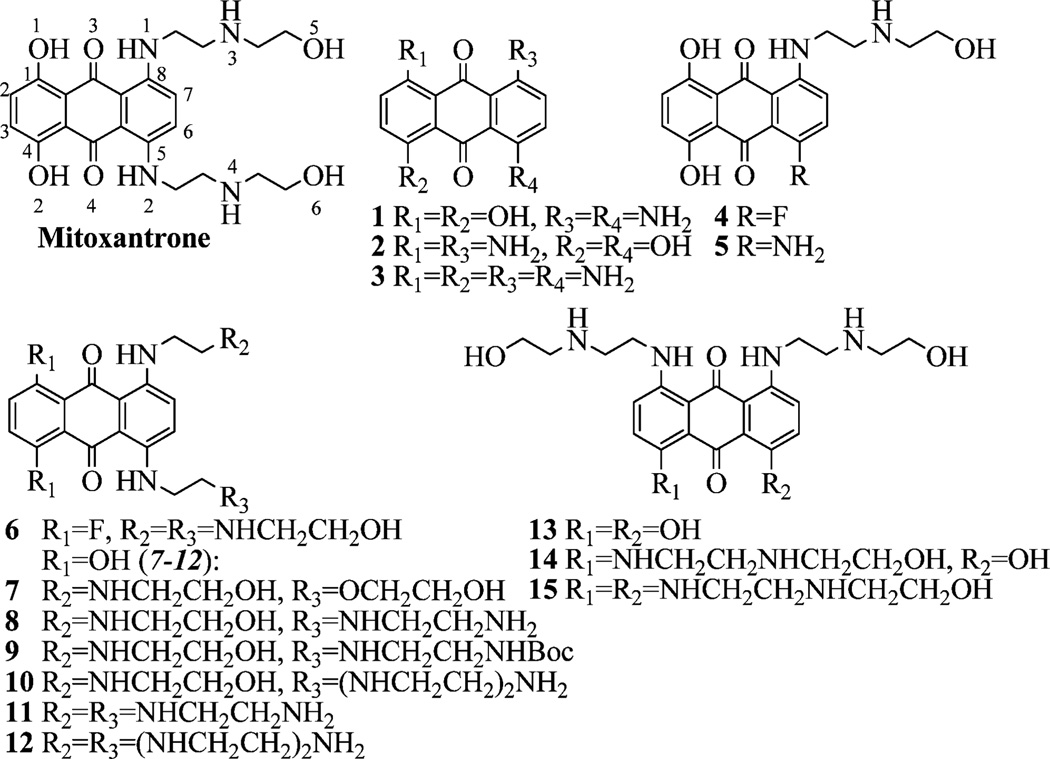
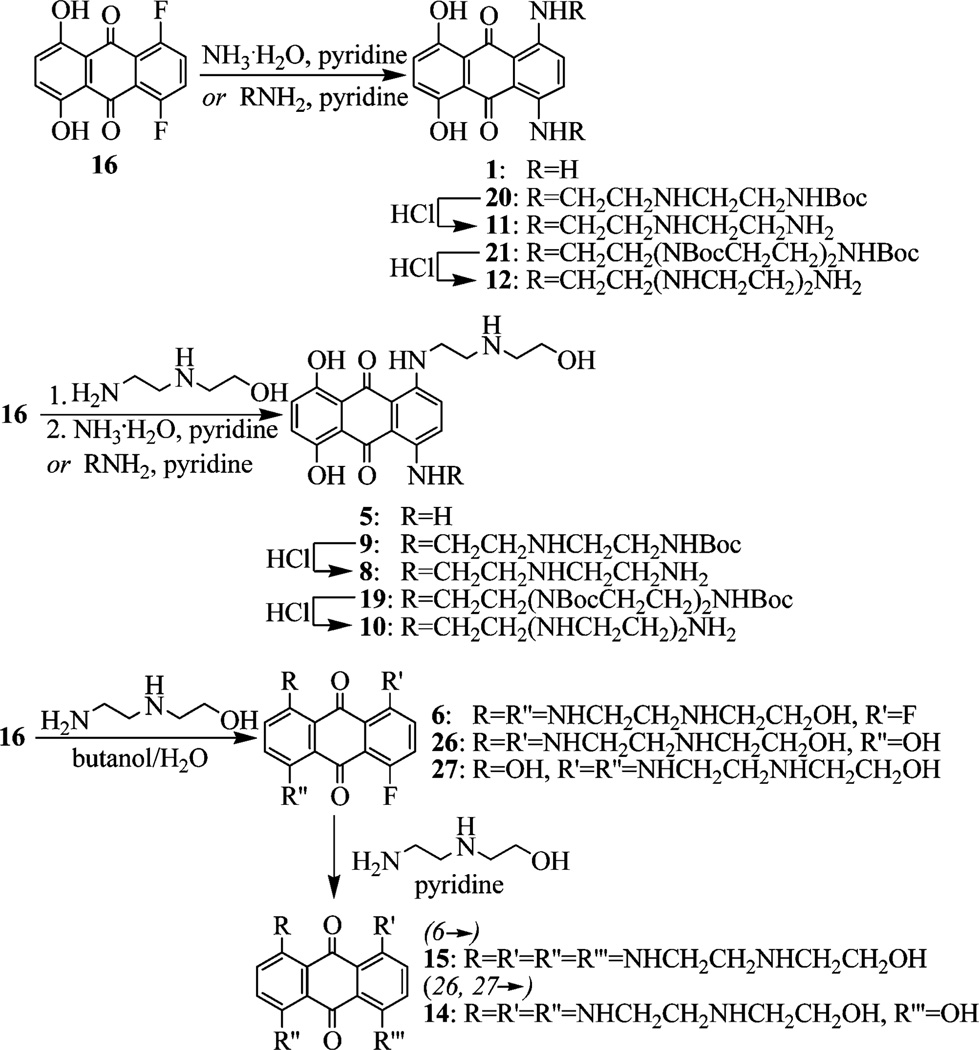
We carried out systematic dissection of or substitutions within MTX to modified compounds that consist of the anthraquinone scaffold with no side chains (1–3), one side chain (4, 5), two side chains (6–13), three side chains (14), and four side chains (15) (Figure 3). These changes also included specific heteroatom replacement (e.g., 7, 8) and altered polyamine length (e.g., 10, 12). Straightforward amination13,14 of difluoro-substituted anthraquinone 16 was applied to prepare these various MTX analogues (Scheme 1). Treatment of 16 with an excess of various amines assembled 1, 20, and 21. Boc deprotection of 20 and 21 provided 11 and 12, respectively. Successive aminations of 16 were carried out to give 5, 9, and 19. Compound 7 was synthesized via a similar method (see Supporting Information). Deprotection of 9 and 19 afforded 8 and 10, respectively. 2-(2-Aminoethylamino) ethanol was treated with 16 in aqueous butanol to mainly give 6 or mixture 26/27 depending on the solvent butanol/water ratio. Mixture 26/27 was transformed to 14, while further amination of 6 afforded 15. Toward MTX regioisomer 13 (Scheme 2), isopropyl protection and regioselective bromination15 provided key intermediate 24, which was followed by amination16,17 and AlCl3 deprotection.
Figure 3.
Structures of mitoxantrone and analogues 1–15.
Scheme 1.
Synthesis of MTX Analogues 1, 4–12, 14, and 15 by Amination
Scheme 2.
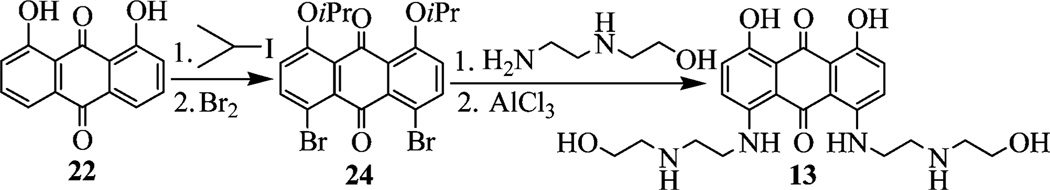
Synthesis of MTX Analogue 13
The capacity for the synthesized MTX analogues to bind the stem–loop was assessed in two ways: a competitive binding assay and an RNA stability assay. The data from these experiments are summarized in Table 1. The EC50 values represent the half-maximal effective concentrations for the previously reported competitive binding assay; active compounds increase fluorescence by increasing the level of unbound fluorescent aminoglycoside probe.7 The IC50 values represent the half maximal inhibitory concentrations for the stability assay; active compounds decrease fluorescence by preventing the unfolding of the RNA stem–loop, keeping the fluorophore and quencher on the oligonucleotide termini in proximity. These two complementary assays gave similar overall results: compounds that show higher binding affinity also result in better stabilization of the stem–loop secondary structure. Consistently higher EC50 values from the binding assay compared to IC50 data from the stability assay likely represent the higher concentration of MTX analogues required to compete with aminoglycoside probe to effectively intercalate into RNA.
Table 1.
Binding and Stabilizing Activity of MTX Analogues
| compd | side chain | EC50 (µM) | IC50 (µM) |
|---|---|---|---|
| MTX | 2 | 0.89 | 0.46 |
| 1 | 0 | >100a | >100a |
| 2 | 0 | >100a | >100a |
| 3 | 0 | >100 | >100 |
| 4 | 1 | 75 | 5.7 |
| 5 | 1 | 29a | 5.8a |
| 6 | 2 | 16a | 2.3a |
| 7 | 2 | 14a | 5.7a |
| 8 | 2 | 0.31 | 0.18 |
| 9 | 2 | 12a | 2.4a |
| 10 | 2 | 0.38 | 0.23 |
| 11 | 2 | 0.16 | 0.13 |
| 12 | 2 | 0.13 | 0.13 |
| 13 | 2 | 4.4a | 1.5a |
| 14 | 3 | 0.80 | 0.42 |
| 15 | 4 | 1.2 | 0.34 |
Stock solution of compound in DMSO. The presence of DMSO(2% final concentration) had no effect on background fluorescence.
No binding or stabilization activity was observed for aliphatic polyamine side chains alone (data not shown; see Supporting Information for structures of side chains tested). This finding indicates an essential role of aromatic stacking for tau stem–loop binding.
On the other hand, without any aliphatic amine side chains, aromatic anthraquinones 1–3 alone cannot bind RNA either. The interaction between these aromatic rings and the two GC base pairs (at the bulge region; see Figure 2) is apparently too weak to allow the aromatic system to effectively intercalate. These data suggest that the aromatic core must cooperate with the aliphatic side chains for binding to the RNA.
Introduction of only one NHCH2CH2NHCH2CH2OH substituent into the aromatic core in 4 and 5 results in measurable activity in the binding and stability assays, although the potency is much weaker than MTX. Thus, the second side chain in MTX contributes substantially to binding (Figure 2). MTX regioisomer 13 is nearly 5 times less potent than MTX in the binding assay and 3 times less potent in the stability assay, indicating that the 5,8 arrangement of the two side chains in MTX is preferable. The substitution of the 1,4 aromatic hydroxyl groups in MTX with NHCH2CH2NHCH2CH2OH to create structures with three or four side chains (14 and 15, respectively) might have led to enhanced binding potency. However, these additional substitutions had little effect on tau stem–loop binding or stabilization. Apparently, one or two additional NHCH2CH2NHCH2CH2OH substituents installed on the other side of MTX neither sterically hinder formation of the ligand–RNA complex nor build more hydrogen bonds or electrostatic interactions with RNA to enhance binding. However, the retention of binding potency by 14 and 15 suggests that substitution of the aromatic hydroxyl groups at position 1 or 4 of the anthraquinone ring system can be well tolerated in the complex and that attaching additional functionality on MTX in these positions could be advantageous (e.g., to build in additional interaction with the unpaired adenosine located nearby10).
MTX analogues 6–12 were built with two identical or different side chains. Strengthened by two side chains, 6 shows 2-fold better binding affinity and stabilization activity than 5 (containing one side chain), even though the two electron-withdrawing fluoro atoms of 6 have considerable negative effect on intercalation. This result confirms the importance of having two side chains together interacting with RNA. Compared to MTX, the binding of 6 is decreased ~18-fold and the stabilization effect decreased 5-fold because of the change from two hydroxyls to two fluoro atoms. Besides the decreased aromatic stacking force, possible loss of the suggested hydrogen bonding of aromatic hydroxyls with the 2′ hydroxyl groups of the RNA10 might also offer some explanation for the low potency of 6. Replacement of one NHCH2CH2NHCH2CH2OH substituent in MTX with the ether NHCH2CH2OCH2CH2OH in 7 results in a 15-fold increase in EC50 and a 12-fold increase in IC50. The substantial decrease in binding and stabilization of the stem–loop suggests that the substitution of ether for secondary amine may lead to loss of a critical hydrogen bond or electrostatic interaction and emphasizes that a suitable side chain is a critical factor for binding.
We also replaced the aliphatic OH to NH2 because NH2 is likewise a good hydrogen bond donor and could potentially add a new electrostatic interaction with the phosphodiester backbone of the RNA. First, only one hydroxyl was changed to a primary amine (8). This modification led to a nearly 3-fold increase in binding potency and stem–loop stabilization for 8 relative to MTX, likely due to a new electrostatic interaction. In contrast, the bulky and uncharged Boc protection group in 9 leads to dramatically decreased potency in the binding and stability assays. We considered that extension of the side chain of 8 by an additional ethylamine (10) might further enhance binding and stabilization of the stem–loop, but this change had little effect.
On the basis of the positive results with 8, both aliphatic hydroxyls of MTX were substituted by NH2 (11), which gave a more than 5-fold improvement in the binding assay and 3-fold improvement in the stability assay compared to MTX. To amplify the electrostatic interaction, two lengthened tetra-amines were incorporated into the aromatic core (12), which resulted in a 7-fold increase in binding potency and a more than 3-fold increase in stem–loop stabilization relative to MTX. Additionally, the double tetra-amine substitution in 12 led to a 3-fold improvement in binding in comparison with 10. Interestingly, the overall scaffolds of 8–12, composed of an aromatic moiety and aliphatic polyamine, reflect some similar structural features as “In-PRiNts” (inhibitor of protein–ribonucleotide sequences) reported by Hamy and co-workers.18
In summary, a series of MTX analogues were designed, synthesized, and evaluated in vitro for their ability to bind to and stabilize the tau pre-mRNA stem–loop structure, and some of these compounds show substantially higher binding affinity than MTX. The results are largely consistent with the recently reported structure of the MTX–RNA complex elucidated by NMR. We propose a highly synergistic binding mode in which the aromatic ring system and two side chains work together, through intercalation and major groove interaction, respectively, to give strong binding to the tau stem–loop near the bulged adenosine. The structure–activity validation and improved activity provide a design platform for next-generation ligands targeting the tau stem loop. Through iterative design, synthesis, and evaluation, we hope to build in greater potency and specificity to eliminate the cytotoxicity of MTX and identify compounds that work in cells to alter tau pre-mRNA splicing. Such efforts are underway in our laboratory.
Supplementary Material
Acknowledgment
This work was supported by a Zenith Fellows Award from the Alzheimer’s Association to M.S.W.
Footnotes
Supporting Information Available: Experimental details and compound characterization. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 2.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neuro-degeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe MS. Tau mutations in neurodegenerative diseases. J. Biol. Chem. 2009;284:6021–6025. doi: 10.1074/jbc.R800013200. [DOI] [PubMed] [Google Scholar]
- 4.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 5.Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donahue CP, Muratore C, Wu JY, Kosik KS, Wolfe MS. Stabilization of the tau exon 10 stem loop alters pre-mRNA splicing. J. Biol. Chem. 2006;281:23302–23306. doi: 10.1074/jbc.C600143200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donahue CP, Ni J, Rozners E, Glicksman MA, Wolfe MS. Identification of tau stem loop RNA stabilizers. J. Biomol. Screening. 2007;12:789–799. doi: 10.1177/1087057107302676. [DOI] [PubMed] [Google Scholar]
- 8.Varani L, Spillantini MG, Goedert M, Varani G. Structural basis for recognition of the RNA major groove in the tau exon 10 splicing regulatory element by aminoglycoside antibiotics. Nucleic Acids Res. 2000;28:710–719. doi: 10.1093/nar/28.3.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas JR, Hergenrother PJ. Targeting RNA with small molecules. Chem. Rev. 2008;108:1171–1224. doi: 10.1021/cr0681546. [DOI] [PubMed] [Google Scholar]
- 10.Zheng S, Chen Y, Donahue CP, Wolfe MS, Varani G. Structural basis for stabilization of the tau pre-mRNA splicing regulatory element by novantrone (mitoxantrone) Chem. Biol. 2009;16:557–566. doi: 10.1016/j.chembiol.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lown JW, Hanstock CC. High field proton NMR analysis of the 1:1 intercalation complex of the antitumor agent mitoxantrone and the DNA duplex [d(CpGpCpG)]2. J. Biomol. Struct. Dyn. 1985;2:1097–1106. doi: 10.1080/07391102.1985.10507626. [DOI] [PubMed] [Google Scholar]
- 12.Yang X-L, Robinson H, Gao Y-G, Wang AH-J. Binding of a macrocyclic bisacridine and ametantrone to CGTACG involves similar unusual intercalation platforms. Biochemistry. 2000;39:10950–10957. [PubMed] [Google Scholar]
- 13.Zee-Cheng RK, Mathew AE, Xu PL, Northcutt RV, Cheng CC. Structural modification study of mitoxantrone (DHAQ). Chloro-substituted mono- and bis[(aminoalkyl)amino]-anthraquinones. J. Med. Chem. 1987;30:1682–1686. doi: 10.1021/jm00392a028. [DOI] [PubMed] [Google Scholar]
- 14.Khanapure SP, Han W, Swartling DJ, Biehl ER. Synthesis of fluorine-substituted anthraquinones and aza-anthraquinones. J. Fluorine Chem. 1994;68:131–134. [Google Scholar]
- 15.Bringmann G, Menche D. Atropo-enantioselective total synthesis of the axially chiral phenylanthraquinone natural products knipholone and 6′-O-methylknipholone. Angew. Chem., Int. Ed. 2001;40:1687–1690. [PubMed] [Google Scholar]
- 16.Hall RH, Hey DH. Methyl derivatives of 1,4,5,8-tetraamino-anthraquinone. J. Chem. Soc. 1948:736–740. [Google Scholar]
- 17.Lindley J. Copper-assisted nucleophilic substitution of aryl halo-gen. Tetrahedron. 1984;40:1433–1456. [Google Scholar]
- 18.Hamy F, Brondani V, Florsheimer A, Stark W, Blommers MJ, Klimkait T. A new class of HIV-1 Tat antagonist acting through Tat-TAR inhibition. Biochemistry. 1998;37:5086–5095. doi: 10.1021/bi972947s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.