This review surveys current progress toward the ex vivo expansion of human umbilical cord blood-derived hematopoietic stem cells (HSCs) and hematopoietic progenitor cells. Recent studies suggest that HSCs may also give rise to nonhematopoietic cells; this plasticity and the possibility of producing nonhematopoietic cells at the clinical scale could bring new alternatives for the treatment of neural, metabolic, orthopedic, cardiac, and neoplastic disorders.
Keywords: Hematopoietic stem cells, Hematopoietic progenitors, Stem cell expansion, Umbilical cord blood
Abstract
Hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) play key roles in the production of mature blood cells and in the biology and clinical outcomes of hematopoietic transplants. The numbers of these cells, however, are extremely low, particularly in umbilical cord blood (UCB); thus, ex vivo expansion of human UCB-derived HSCs and HPCs has become a priority in the biomedical field. Expansion of progenitor cells can be achieved by culturing such cells in the presence of different combinations of recombinant stimulatory cytokines; in contrast, expansion of actual HSCs has proved to be more difficult because, in addition to needing recombinant cytokines, HSCs seem to deeply depend on the presence of stromal cells and/or elements that promote the activation of particular self-renewal signaling pathways. Hence, there is still controversy regarding the optimal culture conditions that should be used to achieve this. To date, UCB transplants using ex vivo-expanded cells have already been performed for the treatment of different hematological disorders, and although results are still far from being optimal, the advances are encouraging. Recent studies suggest that HSCs may also give rise to nonhematopoietic cells, such as neural, cardiac, mesenchymal, and muscle cells. Such plasticity and the possibility of producing nonhematopoietic cells at the clinical scale could bring new alternatives for the treatment of neural, metabolic, orthopedic, cardiac, and neoplastic disorders. Once standardized, ex vivo expansion of human HSCs/HPCs will surely have a positive impact in regenerative medicine.
Introduction
During the last two decades, umbilical cord blood (UCB) has become an important source of hematopoietic cells for both research and transplantation [1–3]. Indeed, 25 years after the first UCB transplant (UCBT), more than 20,000 UCBTs have been performed worldwide, and more than half a million UCB units are being stored in several public UCB banks throughout the world [1]. For certain pediatric hematologic disorders—including hematologic malignancies, bone marrow failure, and hemoglobinopathies—UCBTs have been shown to be as good as bone marrow (BM) or mobilized peripheral blood (MPB) transplants. In adult patients, however, UCBT usually results in delayed engraftment, which is a major cause of early morbidity and mortality. This latter observation seems to be due to the fact that the absolute number of hematopoietic cells contained in a UCB unit is significantly lower than the numbers found in a BM or a MPB unit. Thus, trying to increase these numbers is now a priority and a real challenge for those working in the UCB biology and transplantation arenas [1].
Ex vivo expansion of hematopoietic cells is one of the ways in which the number of UCB cells can be increased for transplantation. To date, many different experimental approaches have been followed, and although there have been encouraging results, several laboratories around the world are still working on the culture conditions that should be used for optimal expansion. The purpose of the present article is to give an overview of the different approaches that have been used to expand hematopoietic cells from UCB and to review the impact that such procedures have had in the clinic. Some of the implications that expanding and manipulating hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) from UCB may have in regenerative medicine are also discussed.
UCB Is a Rich Source of Primitive Hematopoietic Cells
The presence of HSCs and HPCs in UCB was first reported by Knudtzon in 1974, who described the presence of relatively mature myeloid progenitors [4]. About a decade later, Leary and Ogawa documented the presence of more primitive hematopoietic cells [5], and in the late 1980s, Broxmeyer et al. showed that UCB contains vast amounts of both primitive and mature hematopoietic cells that could be used for hematopoietic cell transplants [6].
As compared with cells from adult subjects, UCB-derived hematopoietic cells possess higher proliferation and expansion potentials, and their capacity to self-renew is also superior to that of adult cells. Differences in telomere dynamics and cell cycle progression seem to explain, at least in part, the functional differences observed between neonatal and adult cells. UCB cells possess longer telomeres and express higher levels of certain cell cycle regulators compared with hematopoietic cells from adult sources. Other mechanisms that have been implicated include differences at the level of certain transcription factor pathways, differential gene expression profiles, and the autocrine production of particular cytokines [7].
One milliliter of UCB contains approximately 25,000 hematopoietic progenitors [8, 9]. Interestingly, whereas the levels of relatively mature progenitors are similar in UCB and in adult sources, the frequency of primitive progenitors, including multipotent, erythroid, and bipotent granulo-monocytic cells, is significantly higher in UCB than in adult marrow [8–13]. The levels of more primitive hematopoietic cells, including long-term culture initiating cells (LTC-ICs) and SCID-repopulating cells (SRCs), have also been found to be significantly higher in UCB compared with adult sources [14, 15]. Such numbers, however, remain insufficient when UCB units are used to transplant subjects whose weight is >60 kg. Indeed, it is known that the absolute number of hematopoietic cells in a cord blood unit is significantly lower than the absolute number of hematopoietic cells obtained from a BM or MPB unit; furthermore, it has been well documented that the number of total nucleated cells (TNCs) or the HSC/HPC dose transplanted per kilogram of body weight of the recipient correlates with outcome [16, 17]. Accordingly, after UCBT in an adult patient, there is usually a significant delay in engraftment of all cell lines, as compared with BM or MPB transplants; thus, UCBTs remain more successful in children [16].
In trying to overcome the barriers to successful UCBT, different approaches have been assessed. Some of them have focused on improving engraftment, whereas others have focused on increasing the number of cells being transplanted. The former approach has been explored via either modulating homing of HSCs and HPCs to BM (e.g., inhibition of CD26 by pretreatment of hematopoietic stem and progenitor cells with diprotin A enhances homing and engraftment of limiting numbers of HSCs) [18, 19], or by injecting UCB cells directly into the medullary cavity (i.e., intrabone injection of UCB cells results in faster hematopoietic recovery, especially for platelets, and lower incidence of acute graft-versus-host disease) [20].
The reduced cell dose that characterizes UCBTs can be increased by at least two ways: on the one hand, by performing a double cord blood transplant, and on the other hand, by infusing ex vivo-expanded UCB cells. Hematopoietic cell transplants using two unrelated UCB units—infused sequentially one after the other—have been a major step in optimizing UCBTs [21, 22]. Through this procedure it is possible to increase the cell dose infused, thus making UCBTs available to a larger number of adult patients. Interestingly, 1 month after transplant, only one of the two units infused remains and is responsible for sustaining hematopoiesis, and the other unit disappears. The reason for this is still not fully understood; however, two recent reports have given important insights into this issue. On the one hand, it has been demonstrated that immunological factors are involved, since a CD8+ T-cell subset producing and secreting interferon-γ is generated by the engrafting unit, affecting the survival of the second unit [23]. On the other hand, evidence has been presented indicating that total cell dose and content of colony-forming cells (CFCs) and CD34+ cells in the engrafting unit also play a role [24]. Regardless of the actual mechanisms behind the dominance of only one unit, double-unit UCBT has shown advantages over single-unit transplants. Current evidence, involving a large series of patients, shows that the incidence of graft-versus-host disease and overall survival are comparable to those reported for single-unit UCBT; however, the vast majority (approximately 90%) of patients achieve neutrophil recovery at a median time of only 12–15 days. Interestingly, in patients with acute leukemia, recipients of two UCB units have a significantly lower relapse rate than those receiving one unit.
A second approach to increasing cell dose has been the infusion of ex vivo-expanded hematopoietic cells [25, 26]. The primary goal of this procedure is to generate sufficient numbers of HSCs to optimize the graft available for transplants; however, an equally important goal is to generate higher numbers of lineage-committed HPCs that, although transient, will allow rapid recovery from pancytopenia, thus decreasing early morbidity and mortality [25]. For this purpose, a variety of experimental strategies have been documented [25, 26]. A common factor in most of them has been the use of particular combinations of recombinant stimulatory cytokines; interestingly, in some studies, other elements (such as mesenchymal stromal cells [MSCs], a copper chelator, or Notch ligands) have also been included. The results obtained so far are encouraging, although we are still far from reaching the optimal scenario in terms of expanding UCB HSCs and HPCs ex vivo and using them in clinical settings.
HSC/HPC Ex Vivo Expansion: Basic Principles
When expanding primitive hematopoietic cells, a first issue to consider is the selection of the input cell population. HSCs express CD34, CD49f, CD90, CD117, and CD133 antigens and are devoid of lineage-specific markers, thus, they are regarded as lineage-negative (Lin−) cells [27–29]. Primitive, multipotent HPCs, on the other hand, show an immunophenotype similar to that of HSCs, except that they do not express CD49f or CD90 [27–29]. Committed progenitors express CD38 and, depending on their particular lineage, acquire the expression of specific antigens. The immunophenotype of the input cells is of particular importance not only for initiating the cultures but in order to follow cell development throughout the culture period.
Functionally, HSCs are defined by their ability to long-term reconstitute the hematopoietic system of immunodeficient animals (SRCs) or their ability to initiate and sustain long-term hematopoiesis in culture (LTC-ICs) [27, 30, 31], which are considered demonstrations of their multipotentiality and their capacity to self-renew. HPCs, on the other hand, generate hematopoietic colonies when cultured in semisolid media in the presence of recombinant stimulatory cytokines; accordingly, they are also known as CFCs [32].
Under normal conditions, blood cell production (hematopoiesis) takes place within the bone marrow, in a microenvironment consisting of stromal cells and their products [33]. Such elements constitute hematopoietic niches [34–37], that is, cell-molecule networks controlling survival, self-renewal, proliferation, and differentiation of primitive hematopoietic cells. At least three distinct types of marrow niches (i.e., endosteal, reticular, and vascular) have been identified, each consisting of particular cell types (osteoblasts, endothelial cells, macrophages, reticular cells, adipocytes, etc.) that favor specific physiological activities of HSCs and HPCs. Such control is achieved via direct cell-cell contact, cell-extracellular matrix interactions, and/or the secretion of regulatory molecules (cytokines) that induce stimulatory or inhibitory signals [33]. It is clear that the hematopoietic microenvironment and the marrow niches are fundamental for HSC/HPC development.
In keeping with the above, ex vivo growth of hematopoietic cells requires that some of the niche elements and conditions be reproduced in culture, so that HSCs and HPCs are able to perform under scenarios similar to those found in vivo. Accordingly, hematopoietic cell proliferation, expansion, and differentiation can take place in culture when the right conditions are present. Cell proliferation can be defined as the production of new cells from a particular cell population, regardless of the lineage and maturation stage of the cells being produced. Thus, the ex vivo proliferation of a cell population can be determined simply by quantifying the total number of cells generated in culture. The expansion potential of a particular cell population can be defined as its capacity to generate new cells that possess immunophenotypic and/or functional characteristics present in the input population; for instance, expansion of progenitor cells can be determined by assessing the generation of new CD34+ cells or CFCs; expansion of more primitive cells can be determined by assessing the generation of LTC-ICs or SRCs. Finally, the differentiation potential of a primitive cell population can be defined as its capacity to generate fully mature cells.
HSC and HPC growth, both in vivo and ex vivo, will depend on both intrinsic and extrinsic elements. The former include a variety of regulatory molecules present in a cell according to its differentiation stage and the lineage to which it belongs; the latter, on the other hand, include all the different cell types and cell products that form part of the microenvironment in which the cell develops. In other words, stem/progenitor cell function depends on intrinsic cell regulators that are modulated by external signals.
HSC/HPC Ex Vivo Expansion: Experimental Approaches
During the last two decades, different culture conditions have been used in protocols aimed at expanding HSCs/HPCs ex vivo. Two important points to consider in such protocols are the input cell population and the particular culture conditions that should be used [38]. Regarding the first point, both unselected mononuclear cells (MNCs) and particular cell fractions—enriched for primitive cells and selected by different means—have been used by several groups [3, 25, 26, 39–41]. Although some expansion has been observed when culturing MNCs, current consensus suggests that best results are obtained when the input cell population shows some degree of enrichment for primitive (CD34+ or CD133+) hematopoietic cells [42, 43].
The selection of the input cell population is a key aspect in ex vivo expansion protocols, since it has been demonstrated that the developmental stage of a hematopoietic cell dictates its biological behavior. Primitive subpopulations of CD34+ cells (e.g., CD34+ Rhlow [44], CD34+ CD38− [45, 46], CD34+ CD45RA− CD71− [47], and CD34+ CD45RA− CD71− CD90+ cells [48]) possess greater expansion potentials than their more mature counterparts. This has been shown both in bulk cultures of purified cells and in cultures containing one cell per well. Indeed, at the single-cell level, it has been shown that 1 primitive multipotent progenitor from UCB can give rise up to 70 × 106 nucleated cells and more than 90,000 CD34+ cells, whereas a committed erythroid progenitor produces up to 9 × 106 nucleated cells and no more than 5,000 CD34+ cells [49].
In terms of culture supplements and conditions, the common denominator in all of the protocols reported so far has been the presence of molecules that favor hematopoietic cell growth, particularly early- and late-acting cytokines; although, as shown below, other molecules have also been assessed. The presence of different types of stromal cells, including primary MSCs, has also been shown to result in significant expansion, especially of more primitive cells. Table 1 summarizes the results obtained in some representative studies in which UCB-derived hematopoietic stem and progenitor cells were expanded under different culture conditions.
Table 1.
Ex vivo expansion of UCB-derived HSCs and HPCs as reported in some representative studies
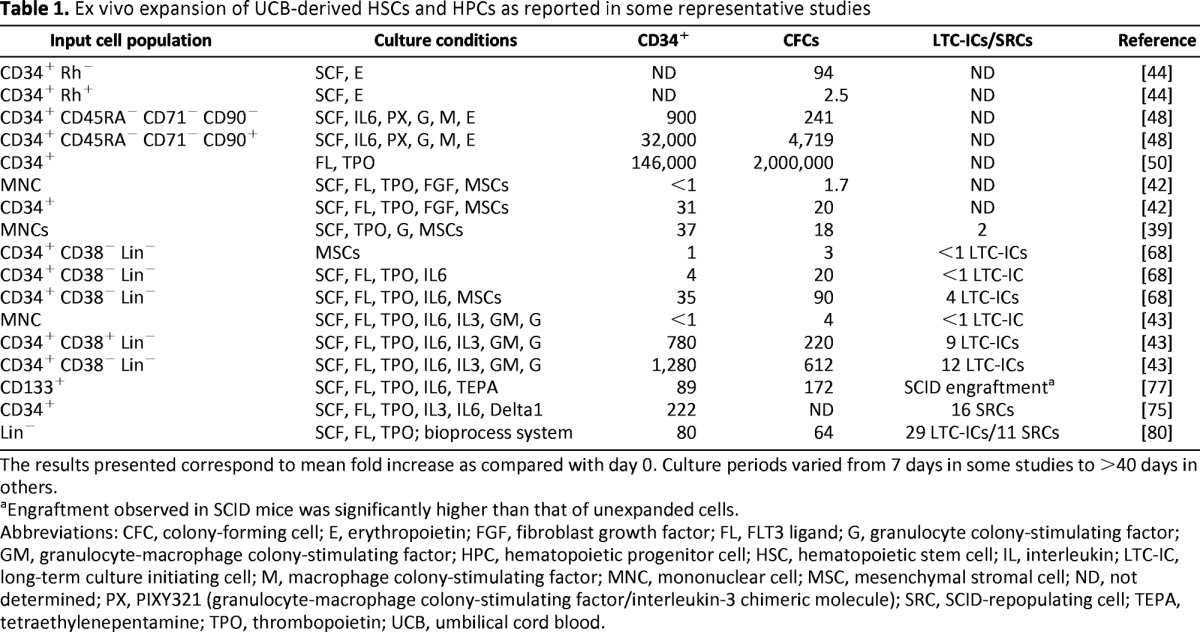
The results presented correspond to mean fold increase as compared with day 0. Culture periods varied from 7 days in some studies to >40 days in others.
aEngraftment observed in SCID mice was significantly higher than that of unexpanded cells.
Abbreviations: CFC, colony-forming cell; E, erythropoietin; FGF, fibroblast growth factor; FL, FLT3 ligand; G, granulocyte colony-stimulating factor; GM, granulocyte-macrophage colony-stimulating factor; HPC, hematopoietic progenitor cell; HSC, hematopoietic stem cell; IL, interleukin; LTC-IC, long-term culture initiating cell; M, macrophage colony-stimulating factor; MNC, mononuclear cell; MSC, mesenchymal stromal cell; ND, not determined; PX, PIXY321 (granulocyte-macrophage colony-stimulating factor/interleukin-3 chimeric molecule); SRC, SCID-repopulating cell; TEPA, tetraethylenepentamine; TPO, thrombopoietin; UCB, umbilical cord blood.
Hematopoietic Cytokines
The first attempts to expand primitive hematopoietic cells from UCB, reported in the 1990s, were based on the use of different combinations of recombinant hematopoietic cytokines and were focused on the expansion of HPCs, assessing increments in CFC and CD34+ cell numbers [3]. Those studies demonstrated that the presence of cytokines is required for optimal growth of HPCs in culture; thus, current protocols aimed at expanding primitive hematopoietic cells from UCB include recombinant stimulatory cytokines as part of the culture conditions, regardless of the presence of other molecules and/or adjuvant cells. Among the different cytokines that participate in hematopoiesis, those acting at the early stages of the hematopoietic hierarchy (inducing HSC self-renewal) (i.e., stem cell factor [SCF], FLT3 ligand [FL], and thrombopoietin [TPO]) have been found to be essential in favoring the expansion of primitive hematopoietic cells in vitro [50–52]. For instance, Piacibello et al. reported that culture of UCB CD34+ cells in the presence of FL and TPO resulted in a 146,000-fold increase in CD34+ cell number and a 2 × 106-fold increase in the number of CFCs [50]. Addition of intermediate-acting cytokines, such as interleukin-3 (IL-3) and granulocyte-macrophage colony-stimulating factor (GM-CSF), seems to have a positive effect in the generation of committed HPCs, whereas late-acting factors, such as erythropoietin (EPO) and macrophage colony-stimulating factor (M-CSF), usually contribute to the production of large numbers of mature cells; however, such late-acting cytokines do not seem to play a key role in HSC or HPC expansion [53, 54].
Interestingly, particular combinations of intermediate- and late-acting cytokines have been found to selectively induce the generation of committed HPCs from specific lineages. For instance, IL-3 and EPO favor the production of erythroid HPCs, whereas GM-CSF, granulocyte colony-stimulating factor (G-CSF), and/or M-CSF favor production of myeloid progenitors [53–55]. It is noteworthy that some controversy still exists regarding the way in which cytokines selectively favor a particular cell lineage over the others; both permissive and instructive models have been proposed, and evidence supporting each one of them has been presented [56].
On the basis of the above, it is clear that recombinant hematopoietic cytokines are key players in the ex vivo expansion of multipotent and committed HPCs, as well as the massive production of precursor and mature cells. To date, however, it has been clearly demonstrated that, besides early-acting cytokines, the presence of niche elements—that is to say, stromal cells and/or molecules involved in cell-cell interactions—is required for significant generation of HSCs ex vivo [38, 57–59].
Cocultures With Stromal Cells
In keeping with the fact that the in vivo development of HSCs and HPCs takes place in close association with microenvironment cells [33–37], ex vivo systems have been established in which stromal cells are used as feeder layers, to allow the expansion of primitive hematopoietic cells. For this purpose, different types of stromal cells have been assessed, including primary whole bone marrow stroma, endothelial cells, stroma cell lines, and MSCs, the latter from different tissues [39–41, 60–63]. These studies have demonstrated that stromal cells, particularly MSCs, are capable of promoting the ex vivo expansion of primitive cells in a process that may involve both cell-to-cell contact and cytokine secretion.
When UCB CD34+ cells are cultured in the presence of MSCs alone, the increments observed in CFC and CD34+ cell numbers are significant but usually modest [39, 64–68]. It has been shown that MSCs produce and secrete a variety of hematopoietic cytokines, including some colony-stimulating factors, several interleukins, and a few chemokines [66, 69]; thus, it seems that the stimulatory effect of MSCs on hematopoietic cells occurs, at least in part, via production and secretion of cytokines. It is noteworthy, however, that under these conditions, better results are obtained when direct contact between MSCs and CD34+ cells is allowed, suggesting that participation of cell-associated molecules and membrane-bound cytokines is also important. Indeed, Wagner et al. [66] and Flores-Guzmán et al. [68] found significantly higher increments in total cell numbers and HPC expansion in cultures of UCB CD34+ cells with human MSCs in which contact conditions were favored, as compared with noncontact conditions. Such a contact-dependent growth has been shown to be more pronounced in cultures of more primitive (e.g., CD34+ CD38− Lin−) cells, as compared with more mature (e.g., CD34+ CD38+ Lin−) cells, which is in keeping with the fact that the in vivo development of stem cells and primitive progenitors occurs in close association with bone marrow stromal cells, and the idea that HSCs are more dependent on the niche than committed HPCs [34–36].
When MSC-based cultures are supplemented with recombinant cytokines the increments observed in CFCs and CD34+ cells are even higher, particularly when early-acting cytokines are present in culture [58, 59, 68]. Interestingly, evidence indicates that when cultures are supplemented with early-, intermediate-, and late-acting cytokines the presence of MSCs is not necessary for the production of committed HPCs or mature cells [68]. However, MSCs are still required for the ex vivo generation of HSCs. Besides MSCs, other types of stromal cells, including endothelial cells, as well as the OP9 and AFT024 cell lines, have been used in experimental protocols aimed at the ex vivo expansion of primitive hematopoietic cells [58, 70, 71]. These studies have confirmed the importance of stromal cells for generation of HSCs in culture.
Notch Signaling Pathway
Self-renewal and multilineage differentiation are fundamental processes that define stem cells in functional terms [27]. Both functions depend on the expression and activity of complex signaling pathways, including those involving key molecules such as Wnt, β-catenin, and Notch. Among them, the Notch pathway has been shown to be of particular importance, since it plays important roles in mediating cell fate decisions [72]. The family of Notch receptors has been identified and characterized throughout the animal scale, from flies and worms to mice and humans [72]. In mammals, four Notch receptors (Notch 1–4) and five ligands (Delta 1, 3, and 4 and Jagged 1 and 2) have been described. Within the hematopoietic system, Notch signaling has been shown to regulate the development of multiple cell types, including T and B cells, monocytes and macrophages, dendritic cells, natural killer cells, and osteoclasts [73]. There is also evidence for activation of the Notch pathway in HSCs located in the bone marrow niche [74], suggesting a definitive role for Notch signaling in HSC maintenance and differentiation.
On the basis of the above observations, two different experimental approaches have recently been taken, demonstrating that the Notch pathway can play a role in the expansion of UCB-derived HSCs/HPCs. On the one hand, Delaney et al. cultured UCB CD34+ cells in the presence of IL-3, IL-6, TPO, FL, SCF, and the Notch ligand Delta1 and observed a 222-fold increase in CD34+ cell numbers and a 16-fold increase in SRC frequency; in contrast, in control cultures there was a 68-fold increase in CD34+ cell numbers, and no expansion was observed for SRC [75]. Fernández-Sánchez et al., on the other hand, cultured UCB CD34+ CD38− Lin− cells in the presence of SCF, FL, TPO, IL-3, IL-6, GM-CSF, and G-CSF in the presence of the OP9 cell line transduced with the Delta1 gene [71]. Under such conditions, the authors found a 21-fold increase in CD34+ CD38− Lin− cell numbers, whereas in cultures established in the presence of cytokines and MSCs, or in the presence of cytokines only, the numbers of such cells were increased 2.4- and 0.9-fold, respectively [71].
A Copper Chelator
Cellular copper has been found to participate in the regulation of HSC/HPC proliferation and differentiation [76]; therefore, some investigators have suggested that chelating such a metal could help improve HSC/HPC growth in culture. On the basis of this notion, Peled et al. purified CD133+ hematopoietic cells from human UCB and cultured them for 3 weeks, under large-scale conditions, in liquid cultures supplemented with SCF, FL, TPO, and IL-6 in the presence of the copper chelator tetraethylenepentamine (TEPA) [77]. The authors observed significant increments in the numbers of CD34+ cells (89-fold), CD34+ CD38− cells (30-fold), and CFCs (172-fold) [77]. Interestingly, the engraftment potential of the expanded cells in NOD-SCID mice was significantly higher (60% CD45+ cells; 11% CD45+ CD34+ cells) than the one of unexpanded cells (21% CD45+ cells; 4% CD45+ CD34+ cells), thus demonstrating a clear benefit with the use of TEPA and suggesting that such a molecule could be included in clinical protocols.
Bioprocess Approach
An important limitation when culturing primitive hematopoietic cells is the fact that differentiating (Lin+) cells are rapidly generated, which, in turn, produce and secrete a wide array of molecules, many of which exert inhibitory signals that affect the growth of HSCs and HPCs [78]. In trying to overcome this limitation, Zandstra and his group have developed an automated closed-system process in which a controlled fed-batch medium dilution approach is used [79, 80]. A key aspect of this experimental approach is the continuous removal from the culture of Lin+ cells, so that accumulation of negative regulators is prevented. Primitive, Lin− cells, on the other hand, are reselected and cultured throughout several days. Importantly, HSC density in culture is maintained. This particular strategy, in fact, has also been used by other groups [81, 82].
By using such a system, Zandstra and colleagues reported significant increments, as compared with day 0, in the numbers of total nucleated cells (179-fold), CFCs (64-fold), CD34+ cells (80-fold), and LTC-ICs (29-fold). Importantly, SRC were also significantly expanded (11-fold) [80], and they are capable of multilineage engraftment when transplanted into secondary animals [79]. These results clearly suggest that such a bioprocess approach may have clinical relevance in the near future.
UCBT With Expanded Cells
The results obtained in the laboratory prompted the translation of some of the experimental in vitro conditions into clinical protocols aimed at increasing the numbers of primitive UCB cells for transplantation. Two initial studies were conducted to assess the feasibility of using UCB expanded cells in patients with hematological diseases, breast cancer, and some other metabolic disorders. In such studies, UCB cells were cultured for 10–12 days in liquid media in the presence of recombinant cytokines, including FL, SCF, TPO, and EPO. Expansion of CFCs and CD34+ cells was observed in all cases, although the numbers were quite variable. Infusion of expanded cells into patients did not significantly alter myeloid, erythroid, or platelet engraftment; however, both studies concluded that this procedure was feasible and safe [83, 84].
In a further study, de Lima et al. used recombinant cytokines and TEPA to expand a fraction of UCB units and infused such cells together with the remaining of the unmanipulated fractions into patients [85]. Once again, expanded cells did engraft (median time to neutrophil and platelet engraftment was 30 and 48 days, respectively); however, no changes were observed in the time to neutrophil or platelet engraftment, as compared with transplantation of unexpanded cells. Interestingly, no grade 3–4 acute graft-versus-host disease was observed, and 100-day survival was 90% [85]. In a preliminary report, the same group of investigators transplanted UCB cells expanded ex vivo using a MSC-based system. As a result of the expansion protocol, increments in the numbers of TNCs and CD34+ cells were modest (12-fold), and after transplant, median times to neutrophil and platelet engraftment were 15 and 30 days, respectively [86].
Delaney et al. described the use of the Notch ligand Delta1 as a means of inducing the expansion of HSCs and HPCs in a clinical protocol [75]. They enrolled 10 subjects with acute leukemia in a phase I clinical trial consisting of infusing two UCB units in each patient, one unmanipulated and one ex vivo-expanded. Sixteen days before the transplant, the UCB unit chosen for ex vivo expansion was thawed, CD34+ cells were enriched, and Delta1 cultures were established. On the day of transplant, cultures were collected and cells were infused into patients 4 hours after infusion of the unmanipulated unit. At collection time, there was a 164 ± 48-fold expansion in CD34+ cells and an average fold increase of total cell numbers of 562 ± 134. The infused CD34+ cell dose derived from expanded cord blood grafts averaged 6 × 106 CD34+ cells per kilogram of body weight of the patient; this compared very favorably to the number observed in unmanipulated cord blood grafts (0.24 × 106 CD34+ cells per kilogram). Two major findings were observed. First, there was a significant reduction in the time to myeloid engraftment in those patients who received one unmanipulated and one expanded unit (16 days; range, 7–34 days), as compared with a cohort of 20 patients undergoing double cord blood transplantation with two unmanipulated units (26 days; range, 16–48 days). Second, engraftment of the expanded unit seemed to be only transient, since in the majority of the evaluable cases, the expanded unit was undetectable after 20–40 days post-transplant. The above study was the first one showing, in a most evident way, the actual benefits of taking ex vivo-expanded cells into the clinical arena [87].
More recently, de Lima et al. reported a study in which 31 patients with hematologic malignanices received two UCB units, one unmanipulated and one ex vivo-expanded in cocultures with allogeneic MSCs (14 days in the presence of SCF, FL, TPO, and G-CSF) [88]. Coculture with MSCs resulted in a 12-fold increase in TNCs and a 31-fold expansion in CD34+ cells. In patients who received one unexpanded and one expanded unit, median times to neutrophil and platelet engraftment were 15 and 42 days, respectively; in contrast, in patients who received two unmanipulated units, median times to neutrophil and platelet engraftment were 24 and 49 days, respectively. On day 26, the cumulative incidence of neutrophil engraftment was 88% in patients who received expanded cells versus 53% in patients who did not receive expanded cells; on day 60, the cumulative incidences of platelet engraftment were 71% and 31%, respectively [88]. This study gave further support to the notion of using ex vivo expansion as an efficient way to improve UCBT.
It is noteworthy that in all of the studies mentioned above, expanded cells were always infused into patients together with unmanipulated cells. The reasons for this are twofold: on the one hand, it has been shown that accessory cells are required to help HSCs/HPCs to engraft; on the other hand, preclinical results have shown that ex vivo expansion protocols clearly favor expansion of HPCs; however, actual expansion of HSCs is not a consistent finding. In fact, some investigators fear that expansion protocols may induce increments in HPC numbers at the expense of HSC levels (i.e., HSC levels decrease in culture). Thus, the unmanipulated cells are infused to help cell engraftment and to make sure that sufficient HSCs are included in the transplanted fraction. To date, no study has been reported in which UCBT were performed using solely expanded cells.
HSC Plasticity and Regenerative Medicine
A general concept in somatic stem cell biology was that such cells were restricted in their differentiation potential to an individual organ or system. Accordingly, HSCs would produce blood cells only. However, during the last 15 years, a great deal of evidence has been generated from a vast number of studies, both in mice and humans, indicating that this concept may not be true. Although it is still a controversial issue [89–91], it seems that HSC differentiation plasticity may be actually wider than previously envisioned.
By using in vivo animal models, several groups presented evidence indicating that hematopoietic cells from bone marrow were able to generate nonhematopoietic cells, such as muscle cells (including cardiomyocytes), hepatic cells, and neural cells [92–96]. Interestingly, such HSC plasticity has been demonstrated at the single-cell level, since intravenous injection of a single HSC resulted in progeny that differentiated into epithelial cells in liver, lung, skin, kidney, and intestine [97]. More recently, the differentiation of individual HSCs into mesenchymal cells has been reported [98]. Taken together, the evidence presented so far strongly supports the notion of the existence of HSCs with nonhematopoietic potential. Whether all HSCs possess such a potential or only a small subpopulation within the HSC pool does is an issue that remains to be clarified. Also, the potential mechanisms responsible for such plasticity—direct or indirect transdifferentiation—need to be elucidated.
In keeping with these studies, the generation of nonhematopoietic cells from human UCB cells has recently been reported. Functional neural cells have been derived in vitro from both CD34+ cells and CD133+ cells from cord blood [99, 100]. Such plasticity can be induced either by manipulation of the culture conditions or by enforcing the ectopic expression of particular transcription factors, such as Sox2. Importantly, UCB hematopoietic cells have also been used to generate induced pluripotent stem cells [101].
Thus, it is clear that similar to their adult marrow counterparts, UCB hematopoietic cells possess the capacity to give rise to nonhematopoietic cells. It is noteworthy, however, that evidence has been presented indicating that nonhematopoietic, multipotent cells present in UCB copurify with CD45+ Lin− hematopoietic cells [102, 103]; thus, it is important to rule out the presence of such nonhematopoietic stem cells when performing plasticity studies. Evidently, the production of nonhematopoietic cells from UCB-derived HSCs/HPCs may have important implications in regenerative medicine, since such cells—once properly manipulated and expanded ex vivo—could be used in clinical protocols aimed at treating a variety of neural, metabolic, neoplastic, orthopedic, and cardiac disorders.
Conclusion
Considering their intrinsic expansion, proliferation, and differentiation potentials [7], UCB cells are, without any doubt, excellent candidates for cellular and gene therapy protocols [104]. Accordingly, ex vivo expansion and manipulation of HSCs and HPCs from UCB have become a priority in the biomedical field because of their potential impact in regenerative medicine. Early attempts to expand primitive hematopoietic cells—based solely on the use of recombinant stimulatory cytokines—were focused on the ex vivo generation of HPCs (as determined by assessing CD34+ cells and/or CFCs). Such studies were important since they established the conceptual and experimental basis for the expansion of progenitor cells; however, they showed little or no evidence for the expansion of actual HSCs. Thus, these procedures were considered of limited relevance. It was not until the use of experimental strategies that included the presence of stromal cells, the presence of elements that promote the activation of particular self-renewal signaling pathways (including the Notch pathway), or the use of a bioprocess system that expansion of actual HSCs was achieved (as demonstrated by assessing SRCs in limiting-dilution assays and secondary engraftment in mice).
Proliferation of HSCs without differentiation (i.e., HSC expansion) is considered by many as the ultimate goal of ex vivo expansion protocols. Today, however, ex vivo generation of increased numbers of progenitor cells is also an important goal. Indeed, it is clear that expansion of both HPCs and HSCs has clinical relevance, since both types of cells play important, yet different and complementary, roles during hematopoietic reconstitution after transplant.
At least two of the clinical trials conducted to date (one at the Fred Hutchinson Cancer Center [Seattle, WA] and the other at the M.D. Anderson Cancer Center [Houston, TX]) have given encouraging results, demonstrating feasibility, safety, and improved engraftment when using ex vivo-expanded cells for UCBT. It is noteworthy, however, that in spite of all the efforts that have been made and the experience generated throughout the years, important issues remain to be solved in terms of developing optimal laboratory conditions for producing such cells at clinical scale. Peter Zandstra and his group (Toronto, ON, Canada) have developed laboratory conditions in which many of the current limitations for UCB cell expansion can be overcome [105]. Such an engineered system is more complex than the standard culture conditions used by most groups working on cell expansion; thus, it may not be easily established. However, at least some of the principles involved in such a bioprocess system (e.g., continuous dilution of inhibitory signaling factors while maintaining HSC density) may be adapted and applied to the particular ex vivo systems used in most laboratories.
In vivo, the HSC niche has been shown to be extremely complex, including not only soluble and cell-associated cytokines, but a wide variety of cells and their products. Accordingly, novel strategies, such as culturing stromal cells with other niche elements (such as extracellular matrix proteins [106, 107] and morphogens [108]) or the enforced expression of specific genes playing key roles in self-renewal, such as HOXB4 or SALL4, in HSCs/HPCs [109, 110], may help to improve culture conditions and make ex vivo expansion a more efficient method to increase hematopoietic cell numbers for clinical application.
Recent evidence indicates that HSCs not only may be the source of all the different types of mature blood cells but also may be able to give rise to a variety of nonhematopoietic cells [111]. This is, of course, still a controversial issue that needs to be clarified through significant laboratory studies, both in vivo and in vitro. The evidence of a pluripotent HSC, however, is robust. Thus, besides the production in the laboratory of increased numbers of HSCs and HPCs, the fact that it may be possible to generate neural, muscle, cardiac, and mesenchymal cells from UCB hematopoietic cells may have important implications in the future for the treatment of a wide variety of diseases. Finally, as long as we are able to develop reliable, safe, and large-scale conditions to expand and manipulate HSCs/HPCs in culture, clinical application of such UCB-derived cells will be a readily and standard practice in the not too distant future.
Acknowledgments
Research in the authors' laboratory is supported by grants from the Mexican Institute of Social Security (IMSS) and the National Council of Science and Technology (CONACYT, Mexico). H.M. is a scholar of Fundación IMSS.
Author Contributions
P.F.-G. and V.F.-S.: manuscript writing, final approval of manuscript; H.M.: conception and design, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Mayani H. Umbilical cord blood: Lessons learned and lingering challenges after more than 20 years of basic and clinical research. Arch Med Res. 2011;42:645–651. doi: 10.1016/j.arcmed.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Cairo MS, Wagner JE. Placental and/or umbilical cord blood: An alternative source of hematopoietic stem cells for transplantation. Blood. 1997;90:4665–4678. [PubMed] [Google Scholar]
- 3.Mayani H, Lansdorp PM. Biology of human umbilical cord blood-derived hematopoietic stem/progenitor cells. Stem Cells. 1998;16:153–165. doi: 10.1002/stem.160153. [DOI] [PubMed] [Google Scholar]
- 4.Knudtzon S. In vitro growth of granulocyte colonies from circulating cells in human cord blood. Blood. 1974;43:357–361. [PubMed] [Google Scholar]
- 5.Leary AG, Ogawa M. Blast cell colony assay from umbilical cord blood and adult bone marrow progenitors. Blood. 1987;69:953–956. [PubMed] [Google Scholar]
- 6.Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989;86:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayani H. Biological differences between neonatal and adult human hematopoietic stem/progenitor cells. Stem Cells Dev. 2010;19:285–298. doi: 10.1089/scd.2009.0327. [DOI] [PubMed] [Google Scholar]
- 8.Abboud M, Xu F, LaVia M, et al. Study of early hematopoietic precursors in human cord blood. Exp Hematol. 1992;20:1043–1047. [PubMed] [Google Scholar]
- 9.Traycoff CM, Abboud MR, Laver J, et al. Evaluation of the in vitro behavior of phenotypically defined populations of umbilical cord blood hematopoietic progenitor cells. Exp Hematol. 1994;22:215–222. [PubMed] [Google Scholar]
- 10.Broxmeyer HE, Hangoc G, Cooper S, et al. Growth characteristics and expansion of human umbilical cord blood and estimation of its potential for transplantation in adults. Proc Natl Acad Sci USA. 1992;89:4109–4113. doi: 10.1073/pnas.89.9.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hows JM, Bradley BA, Marsh JCW, et al. Growth of human umbilical cord blood in longterm haemopoietic cultures. Lancet. 1992;340:73–76. doi: 10.1016/0140-6736(92)90396-k. [DOI] [PubMed] [Google Scholar]
- 12.Steen R, Tjonnfjord GE, Egeland T. Comparison of the phenotype and clonogenicity of normal CD34+ cells from umbilical cord blood, granulocyte colony-stimulating factor-mobilized peripheral blood, and adult human bone marrow. J Hematother. 1994;3:253–262. doi: 10.1089/scd.1.1994.3.253. [DOI] [PubMed] [Google Scholar]
- 13.Mayani H, Gutiérrez-Rodríguez M, Espinoza L, et al. Kinetics of hematopoiesis in Dexter-type long-term cultures established from human umbilical cord blood cells. Stem Cells. 1998;16:127–135. doi: 10.1002/stem.160127. [DOI] [PubMed] [Google Scholar]
- 14.Pettengell R, Luft T, Henschler R, et al. Direct comparison by limiting dilution analysis of long-term culture-initiating cells in human bone marrow, umbilical cord blood and blood stem cells. Blood. 1994;84:3653–3659. [PubMed] [Google Scholar]
- 15.Wang JCY, Doedens JCY, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. 1997;89:3919–3924. [PubMed] [Google Scholar]
- 16.Gluckman E, Rocha V, Arcese W, et al. Factors associated with outcomes of unrelated cord blood transplant: Guidelines for donor choice. Exp Hematol. 2004;32:397–407. doi: 10.1016/j.exphem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Migliaccio AR, Adamson JW, Stevens CE, et al. Cell dose and speed of engraftment in placental/umbilical cord blood transplantation: Graft progenitor cell content is a better predictor than nucleated cell quantity. Blood. 2000;96:2717–2722. [PubMed] [Google Scholar]
- 18.Christopherson KW, Hangoc G, Mantel CR, et al. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 19.Farag SS, Broxmeyer HE. CD26 inhibition to enhance cord blood engraftment. In: Broxmeyer HE, editor. Cord Blood: Biology, Transplantation, Banking, and Regulation. Bethesda, MD: AABB Press; 2011. pp. 203–213. [Google Scholar]
- 20.Frassoni F, Podesta M, Varaldo R, et al. Intrabone transplantation of cord blood cells and the journey of hematopoietic cells. In: Broxmeyer HE, editor. Cord Blood: Biology, Transplantation, Banking, and Regulation. Bethesda, MD: AABB Press; 2011. pp. 239–252. [Google Scholar]
- 21.Brunstein CG, Setubal DC, Wagner JE. Expanding the role of umbilical cord blood transplantation. Br J Haematol. 2007;137:20–35. doi: 10.1111/j.1365-2141.2007.06521.x. [DOI] [PubMed] [Google Scholar]
- 22.Haspel RL, Ballen KK. Double cord blood transplants: Filling a niche? Stem Cell Rev. 2006;2:81–86. doi: 10.1007/s12015-006-0013-z. [DOI] [PubMed] [Google Scholar]
- 23.Gutman JA, Turtle CJ, Manley TJ, et al. Single-unit dominance after double-unit umbilical cord blood transplantation coincides with a specific CD8+ T cell response against the nonengrafted unit. Blood. 2010;115:757–765. doi: 10.1182/blood-2009-07-228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avery S, Shi W, Lubin M, et al. Influence of infused cell dose and HLA on engraftment after double-unit cord blood allografts. Blood. 2011;117:3277–3285. doi: 10.1182/blood-2010-08-300491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly SS, Parmar S, De Lima M, et al. Overcoming the barriers to umbilical cord blood transplantation. Cytotherapy. 2010;12:121–130. doi: 10.3109/14653240903440111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahlberg A, Delaney C, Bernstein I. Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood. 2011;117:6083–6090. doi: 10.1182/blood-2011-01-283606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szilvassy SJ. The biology of hematopoietic stem cells. Arch Med Res. 2003;34:446–460. doi: 10.1016/j.arcmed.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Wognum AW, Eaves AC, Thomas TE. Identification and isolation of hematopoietic stem cells. Arch Med Res. 2003;34:461–475. doi: 10.1016/j.arcmed.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Notta F, Doulatov S, Laurenti E, et al. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218–221. doi: 10.1126/science.1201219. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia M, Wang JC, Kapp U, et al. Purification of primitive human hematopoietic cells capable of repopulating immunodeficient mice. Proc Natl Acad Sci USA. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kollet O, Peled A, Byk T, et al. β2-microglobulin-deficient (β2mnull) NOD-SCID mice are excellent recipients for studying human hematopoietic stem cell function. Blood. 2000;95:3102–3105. [PubMed] [Google Scholar]
- 32.Coutinho LH, Gilleece MH, De Wynter EA, et al. Clonal and long-term cultures using human bone marrow. In: Testa NG, Molineux G, editors. Haemopoiesis: A Practical Approach. Oxford, U.K.: Oxford University Press; 1993. pp. 75–106. [Google Scholar]
- 33.Mayani H, Guilbert LJ, Janowska-Wieczorek A. Biology of the hemopoietic microenvironment. Eur J Haematol. 1992;49:225–233. doi: 10.1111/j.1600-0609.1992.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 34.Scadden DT. The stem cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 35.Morrison SJ, Spradling AC. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagasawa T, Omatsu Y, Sugiyama T. Control of hematopoietic stem cells by the bone marrow stromal niche: The role of reticular cells. Trends Immunol. 2011;32:315–320. doi: 10.1016/j.it.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Douay L. Experimental culture conditions are critical for the ex-vivo expansion of hematopoietic cells. J Hematother Stem Cell Res. 2001;10:341–346. doi: 10.1089/152581601750288948. [DOI] [PubMed] [Google Scholar]
- 39.McNiece I, Harrington J, Turney J, et al. Ex vivo expansion of cord blood mononuclear cells on mesenchymal stem cells. Cytotherapy. 2004;6:311–317. doi: 10.1080/14653240410004871. [DOI] [PubMed] [Google Scholar]
- 40.Robinson SN, Ng J, Niu T, et al. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;37:359–366. doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madkaikar M, Ghosh K, Gupta M, et al. Ex vivo expansion of umbilical cord blood stem cells using different combinations of cytokines and stromal cells. Acta Haematol. 2007;118:153–159. doi: 10.1159/000108630. [DOI] [PubMed] [Google Scholar]
- 42.Andrade PZ, Lobato da Silva C, dos Santos F, et al. Initial CD34+ cell-enrichment determines hematopoietic stem/progenitor cell yield upon ex vivo expansion. J Cell Biochem. 2011;112:1822–1831. doi: 10.1002/jcb.23099. [DOI] [PubMed] [Google Scholar]
- 43.Flores-Guzman P, Fernandez-Sanchez V, Valencia-Plata I, et al. Comparative in vitro analysis of different hematopoietic cell populations from human cord blood: In search of the best option for clinically-oriented ex vivo cell expansion. Transfusion. 2013;53:668–678. doi: 10.1111/j.1537-2995.2012.03799.x. [DOI] [PubMed] [Google Scholar]
- 44.Cicuttini FM, Welch KL, Boyd AW. The effect of cytokines on CD34+ Rh-123high and low progenitor cells from human umbilical cord blood. Exp Hematol. 1994;22:1244–1251. [PubMed] [Google Scholar]
- 45.Cardoso A, Li ML, Batard P. Release from quiescence of CD34+ CD38− human umbilical cord blood cells reveals their potentiality to engraft adults. Proc Natl Acad Sci USA. 1993;90:8707–8711. doi: 10.1073/pnas.90.18.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Wynter EA, Nadali G, Coutinho L, et al. Extensive amplification of single cells from CD34+ subpopulations in umbilical cord blood and identification of long-term culture-initiating cells present in two subsets. Stem Cells. 1996;14:566–576. doi: 10.1002/stem.140566. [DOI] [PubMed] [Google Scholar]
- 47.Mayani H, Dragowska W, Lansdorp PM. Characterization of functionally distinct subpopulations of CD34+ cord blood cells in serum-free long-term cultures supplemented with hematopoietic cytokines. Blood. 1993;82:2664–2672. [PubMed] [Google Scholar]
- 48.Mayani H, Lansdorp PM. Thy-1 expression is linked to functional properties of primitive hematopoietic progenitor cells from human umbilical cord blood. Blood. 1994;83:2410–2417. [PubMed] [Google Scholar]
- 49.Mayani H, Lansdorp PM. Proliferation of individual hematopoietic progenitors purified from umbilical cord blood. Exp Hematol. 1995;23:1453–1462. [PubMed] [Google Scholar]
- 50.Piacibello W, Sanavio F, Garetto L, et al. Extensive amplification and self renewal of human primitive hematopoietic stem cells from cord blood. Blood. 1997;89:2644–2653. [PubMed] [Google Scholar]
- 51.Gilmore GL, DePasquale DK, Lister J, et al. Ex vivo expansion of human umbilical cord blood and peripheral blood CD34+ hematopoietic stem cells. Exp Hematol. 2000;28:1297–1305. doi: 10.1016/s0301-472x(00)00531-2. [DOI] [PubMed] [Google Scholar]
- 52.Tanavde VM, Malehorn MT, Lumkul R, et al. Human stem-progenitor cells from neonatal cord blood have greater hematopoietic expansion capacity than those from mobilized adult blood. Exp Hematol. 2002;30:816–823. doi: 10.1016/s0301-472x(02)00818-4. [DOI] [PubMed] [Google Scholar]
- 53.Mayani H, Dragowska W, Lansdorp PM. Cytokine-induced selective expansion and maturation of erythroid versus myeloid progenitors from purified cord blood precursor cells. Blood. 1993;81:3252–3258. [PubMed] [Google Scholar]
- 54.Flores-Guzmán P, Gutiérrez-Rodríguez M, Mayani H. In vitro proliferation, expansion and differentiation of a CD34+ cell-enriched hematopoietic cell population from human umbilical cord blood in response to recombinant cytokines. Arch Med Res. 2002;33:107–114. doi: 10.1016/s0188-4409(01)00368-x. [DOI] [PubMed] [Google Scholar]
- 55.Metcalf D. Hematopoietic cytokines. Blood. 2008;111:485–491. doi: 10.1182/blood-2007-03-079681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogawa M. Hemopoietic stem cells: Stochastic differentiation and humoral control of proliferation. Environ Health Perspect. 1989;80:199–207. doi: 10.1289/ehp.8980199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dazzi F, Ramasamy R, Glennie S, et al. The role of mesenchymal stem cells in haematopoiesis. Blood Rev. 2006;20:161–171. doi: 10.1016/j.blre.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Kirouac DC, Madlambayan GJ, Yu M, et al. Cell-cell interaction networks regulate blood stem and progenitor cell fate. Mol Syst Biol. 2009;5:293. doi: 10.1038/msb.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peerani R, Zandstra PW. Enabling stem cell therapies through stem cell-niche engineering. J Clin Invest. 2010;120:60–70. doi: 10.1172/JCI41158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosler E, Brandt J, Chute J, et al. Cocultivation of umbilical cord blood cells with endothelial cells leads to extensive amplification of competent CD34+ CD38− cells. Exp Hematol. 2000;28:841–852. doi: 10.1016/s0301-472x(00)00177-6. [DOI] [PubMed] [Google Scholar]
- 61.Fei XM, Wu YJ, Chang Z, et al. Co-culture of cord blood CD34+ cells with human BM mesenchymal stromal cells enhances short-term engraftment of cord blood cells in NOD/SCID mice. Cytotherapy. 2007;9:338–347. doi: 10.1080/14653240701291638. [DOI] [PubMed] [Google Scholar]
- 62.Hutton JF, Rosenkov V, Khor FSL, et al. Bone morphogenetic protein 4 contributes to the maintenance of primitive cord blood hematopoietic progenitors in an ex vivo stroma-noncontact co-culture system. Stem Cells Dev. 2006;15:805–813. doi: 10.1089/scd.2006.15.805. [DOI] [PubMed] [Google Scholar]
- 63.Ando K, Nakamura Y, Chargui J, et al. Extensive generation of human cord blood CD34+ stem cells from Lin− CD34− cells in a long-term in vitro system. Exp Hematol. 2000;28:690–699. doi: 10.1016/s0301-472x(00)00154-5. [DOI] [PubMed] [Google Scholar]
- 64.Wang J-F, Wang L-J, Wu Y-F, et al. Mesenchymal stem/progenitor cells in human umbilical cord blood as support for ex vivo expansion of CD34+ hematopoietic stem cells and for chondrogenic differentiation. Haematologica. 2004;89:837–844. [PubMed] [Google Scholar]
- 65.Xie C-G, Wang J-F, Xiang Y, et al. Cocultivation of umbilical cord blood CD34+ cells with retro-transduced hMSC leads to effective amplification of long-term culture initiating cells. World J Gastroenterol. 2006;12:393–402. doi: 10.3748/wjg.v12.i3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner W, Roderburg C, Wein F, et al. Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells. 2007;25:2638–2647. doi: 10.1634/stemcells.2007-0280. [DOI] [PubMed] [Google Scholar]
- 67.Jang YK, Jung DH, Jung MH, et al. Mesenchymal stem cells feeder layer from human umbilical cord blood for ex vivo expanded growth and proliferation of hematopoietic progenitor cells. Ann Hematol. 2006;85:212–225. doi: 10.1007/s00277-005-0047-3. [DOI] [PubMed] [Google Scholar]
- 68.Flores-Guzmán P, Flores-Figueroa E, Montesinos JJ, et al. Individual and combined effects of mesenchymal stromal cells and recombinant stimulatory cytokines on the in vitro growth of primitive hematopoietic cells from human umbilical cord blood. Cytotherapy. 2009;11:886–896. doi: 10.3109/14653240903180076. [DOI] [PubMed] [Google Scholar]
- 69.Flores-Figueroa E, Montesinos JJ, Flores-Guzmán P, et al. Functional analysis of myelodysplastic syndromes-derived mesenchymal stem cells. Leuk Res. 2008;32:1407–1416. doi: 10.1016/j.leukres.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Lewis ID, Almeida-Porada G, Du J, et al. Umbilical cord blood cells capable of engrafting in primary, secondary, and tertiary xenogeneic hosts are preserved after ex vivo culture in a noncontact system. Blood. 2001;97:3441–3449. doi: 10.1182/blood.v97.11.3441. [DOI] [PubMed] [Google Scholar]
- 71.Fernández-Sánchez V, Pelayo R, Flores-Guzman P, et al. In vitro effects of stromal cells expressing different levels of Jagged-1 and Delta-1 on the growth of primitive and intermediate CD34+ cell subsets from human cord blood. Blood Cells Mol Dis. 2011;47:205–213. doi: 10.1016/j.bcmd.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 72.Lai EC. Notch signaling: Control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 73.Ohishi K, Katayama N, Shiku H, et al. Notch signaling in hematopoiesis. Semin Cell Dev Biol. 2003;14:143–150. doi: 10.1016/s1084-9521(02)00183-0. [DOI] [PubMed] [Google Scholar]
- 74.Duncan AW, Rattis FM, DiMascio LM, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 75.Delaney C, Heimfeld S, Brashem-Stein C, et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peled T, Landau E, Prus E, et al. Cellular copper content modulates differentiation and self-renewal in cultures of cord blood derived CD34+ cells. Br J Haematol. 2002;116:655–661. doi: 10.1046/j.0007-1048.2001.03316.x. [DOI] [PubMed] [Google Scholar]
- 77.Peled T, Mandel J, Goudsmid RN, et al. Pre-clinical development of cord blood-derived progenitor cell graft expanded ex vivo with cytokines and the polyamine copper chelator tetraethylenepentamine. Cytotherapy. 2004;6:344–355. doi: 10.1080/14653240410004916. [DOI] [PubMed] [Google Scholar]
- 78.Madlambayan GJ, Rogers I, Kirouac DC, et al. Dynamic changes in cellular and microenvironmental composition can be controlled to elicit in vitro human hematopoietic stem cell expansion. Exp Hematol. 2005;33:1229–1239. doi: 10.1016/j.exphem.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 79.Madlambayan GJ, Rogers I, Purpura KA, et al. Clinically relevant expansion of hematopoietic stem cells with conserved function in a single-use, closed-system bioprocess. Biol Blood Marrow Transplant. 2006;12:1020–1030. doi: 10.1016/j.bbmt.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 80.Csaszar E, Kirouac DC, Yu M, et al. Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling. Cell Stem Cell. 2012;10:218–229. doi: 10.1016/j.stem.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 81.Flores-Guzmán P, Martinez-Jaramillo G, Montesinos JJ, et al. Growth kinetics of progenitor cell-enriched hematopoietic cell populations in long-term liquid cultures under continuous removal of mature cells. Cytotherapy. 2006;8:299–307. doi: 10.1080/14653240600735776. [DOI] [PubMed] [Google Scholar]
- 82.Rogers IM, Yamanaka N, Casper RF. A simplified procedure for hematopoietic stem cell amplification using a serum-free, feeder cell-free culture system. Biol Blood Marrow Transplant. 2008;14:927–937. doi: 10.1016/j.bbmt.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 83.Shpall EJ, Quinones R, Giller R, et al. Transplantation of ex vivo expanded cord blood cells. Biol Blood Marrow Transplant. 2002;8:368–376. doi: 10.1053/bbmt.2002.v8.pm12171483. [DOI] [PubMed] [Google Scholar]
- 84.Jaroscak J, Goltry K, Smith A, et al. Augmentation of umbilical cord blood (UCB) transplantation with ex vivo-expanded UCB cells: Results of a phase I trial using the AastromReplicell System. Blood. 2003;101:5061–5067. doi: 10.1182/blood-2001-12-0290. [DOI] [PubMed] [Google Scholar]
- 85.de Lima M, McMannis J, Gee A, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tertraethylenepentamine: A phase I/II clinical trial. Bone Marrow Transplant. 2008;41:771–778. doi: 10.1038/sj.bmt.1705979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Lima M, McNiece I, McMannis J, et al. Double cord blood transplantations (CBT) with ex vivo expansion (EXP) of one unit utilizing a mesenchymal stromal cell (MSC) platform. Biol Blood Marrow Transplant. 2009;15 Supplement Abstract 122. [Google Scholar]
- 87.Mayani H. Notch signaling: From stem cell expansion to improving cord blood transplantation. Expert Rev Hematol. 2010;3:401–404. doi: 10.1586/ehm.10.37. [DOI] [PubMed] [Google Scholar]
- 88.de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. New Engl J Med. 2012;367:2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verfaillie CM. Adult stem cells: Assessing the case for pluripotency. Trends Cell Biol. 2002;12:502–508. doi: 10.1016/s0962-8924(02)02386-3. [DOI] [PubMed] [Google Scholar]
- 90.Grove JE, Bruscia E, Krause DS. Plasticity of bone marrow-derived stem cells. Stem Cells. 2004;22:487–500. doi: 10.1634/stemcells.22-4-487. [DOI] [PubMed] [Google Scholar]
- 91.Vieyra DS, Jackson KA, Goodell MA. Plasticity and tissue regenerative potential of bone marrow-derived cells. Stem Cell Rev. 2005;1:65–70. doi: 10.1385/SCR:1:1:065. [DOI] [PubMed] [Google Scholar]
- 92.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 93.Theise ND, Nimmakayalu M, Gardner R, et al. Liver from bone marrow in humans. Hepatology. 2000;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- 94.Lagasse E, Connors H, Al-Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 95.Brazelton TR, Rossi FMV, Keshet GI, et al. From marrow to brain: Expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 96.Mezey E, Chandross KJ, Harta G, et al. Turning blood into brain: Cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 97.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 98.Ogawa M, LaRue AC, Watson PM, et al. Hematopoietic stem cell origin of connective tissues. Exp Hematol. 2010;38:540–547. doi: 10.1016/j.exphem.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 99.Singh AK, Kashyap MP, Jahan S, et al. Expression and inducibility of cytochrome P450s (CYP1A1, 2B6, 2E1, 3A4) in human cord blood CD34+ stem cell-derived differentiating neuronal cells. Toxicol Sci. 2012;129:392–410. doi: 10.1093/toxsci/kfs213. [DOI] [PubMed] [Google Scholar]
- 100.Giorgetti A, Marchetto MC, Li M, et al. Cord blood-derived neuronal cells by ectopic expression of Sox2 and c-Myc. Proc Natl Acad Sci USA. 2012;109:12556–12561. doi: 10.1073/pnas.1209523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Giorgetti A, Montserrat N, Aasen T, et al. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell. 2009;5:353–357. doi: 10.1016/j.stem.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhao Y, Wang H, Mazzone T. Identification of stem cells from human umbilical cord blood with embryonic and hematopoietic characteristics. Exp Cell Res. 2006;312:2454–2464. doi: 10.1016/j.yexcr.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 103.Rogers I, Yamanaka N, Bielecki R, et al. Identification and analysis of in vitro cultured CD45-positive cells capable of multi-lineage differentiation. Exp Cell Res. 2007;313:1839–1852. doi: 10.1016/j.yexcr.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 104.Kohn DB, Weinberg KI, Nolta JA, et al. Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency. Nat Med. 1995;1:1017–1023. doi: 10.1038/nm1095-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bernstein ID, Delaney C. Engineering stem cell expansion. Cell Stem Cell. 2012;10:113–114. doi: 10.1016/j.stem.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 106.Deutsch V, Hubel E, Kay S, et al. Mimicking the haematopoietic niche microenvironment provides a novel strategy for expansion of haematopoietic and megakaryocyte progenitor cells from cord blood. Br J Haematol. 2010;149:137–149. doi: 10.1111/j.1365-2141.2009.08041.x. [DOI] [PubMed] [Google Scholar]
- 107.Celebi B, Mantovani D, Pineault N. Effects of extracellular matrix proteins on the growth of haematopoietic progenitor cells. Biomed Mater. 2011;6 doi: 10.1088/1748-6041/6/5/055011. 055011. [DOI] [PubMed] [Google Scholar]
- 108.Bhardwaj G, Murdoch B, Wu D, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172–178. doi: 10.1038/84282. [DOI] [PubMed] [Google Scholar]
- 109.Watts KL, Delaney C, Humpries RK, et al. Combination of HOXB4 and delta-1 ligand improves expansion of cord blood cells. Blood. 2010;116:5859–5866. doi: 10.1182/blood-2010-05-286062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aguila JR, Liao W, Yang J, et al. SALL4 is a robust stimulator for the expansion of hematopoietic stem cells. Blood. 2011;118:576–585. doi: 10.1182/blood-2011-01-333641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ogawa M, LaRue AC, Mehrotra M. Hematopoietic stem cells are pluripotent and not just “hematopoietic.”. Blood Cells Mol Dis. 2013;51:3–8. doi: 10.1016/j.bcmd.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


